

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50174269 (1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208258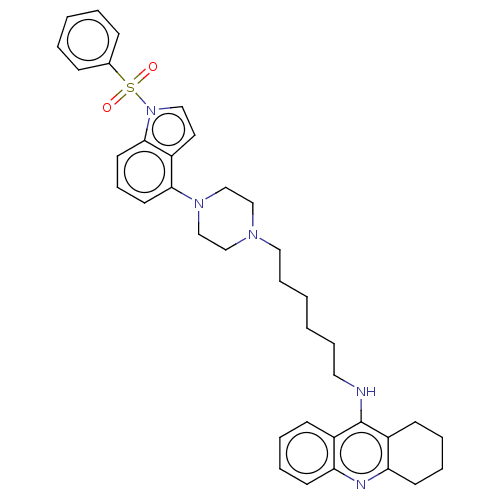 (CHEMBL3885186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208263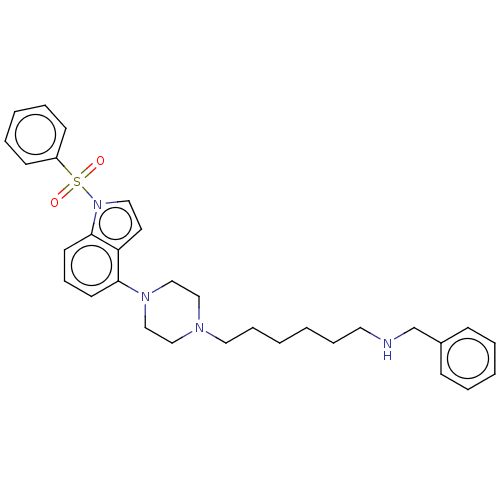 (CHEMBL3884227) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208217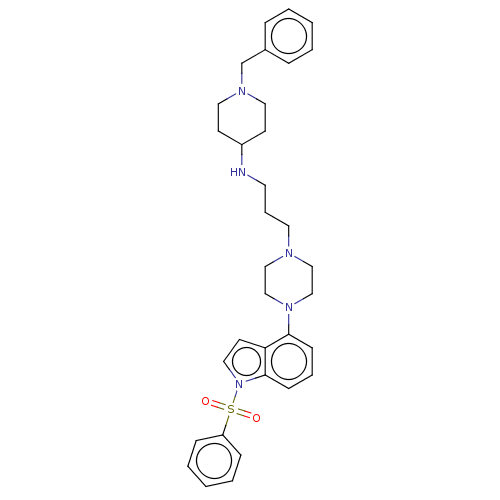 (CHEMBL3884195) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208254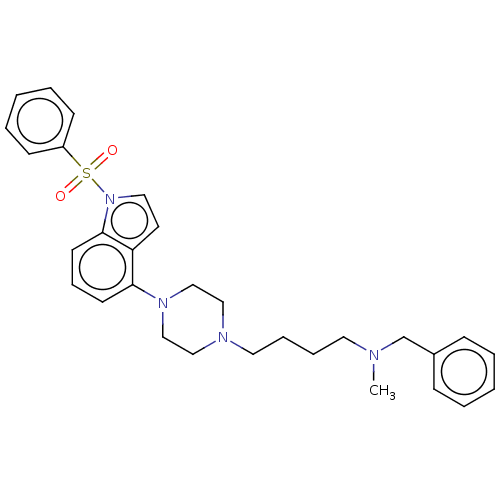 (CHEMBL3883921) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208256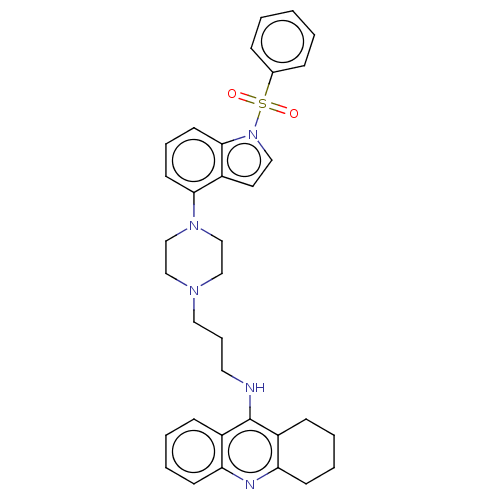 (CHEMBL3884618) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208214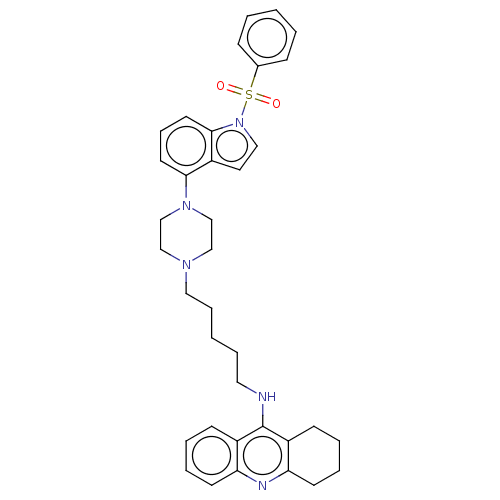 (CHEMBL3884858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208259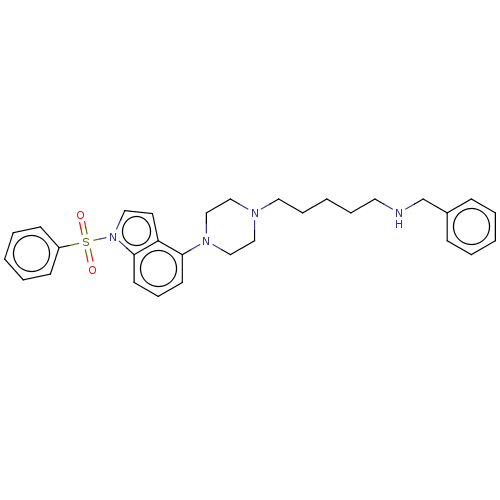 (CHEMBL3884704) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208262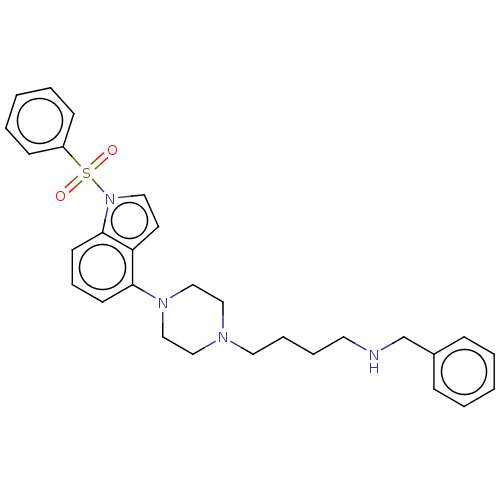 (CHEMBL3884312) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208253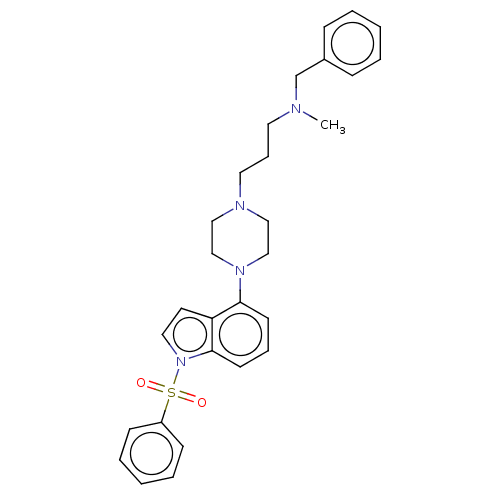 (CHEMBL3884867) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208257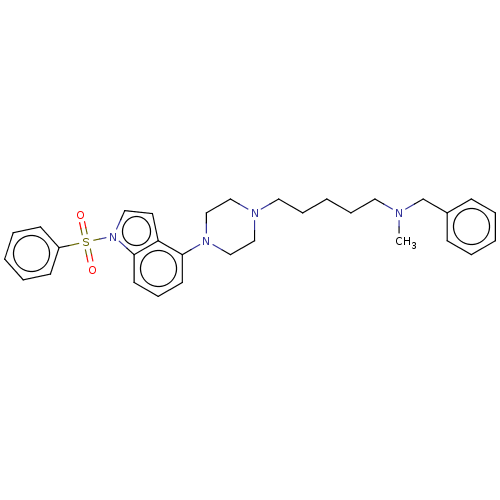 (CHEMBL3884709) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208260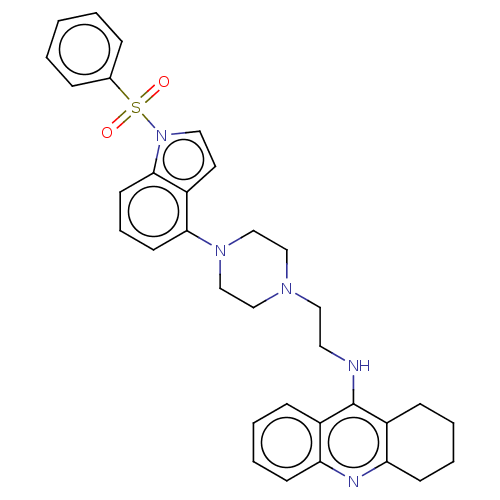 (CHEMBL3884988) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208261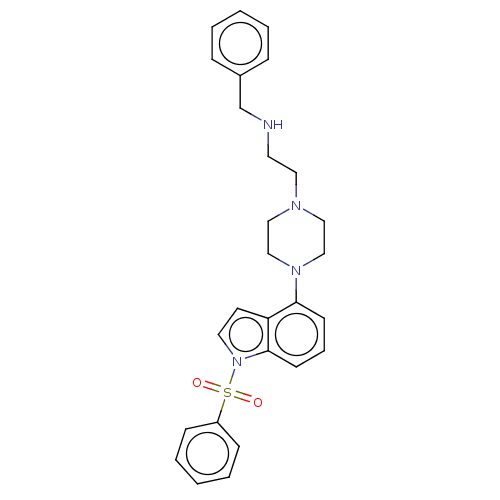 (CHEMBL3885452) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208255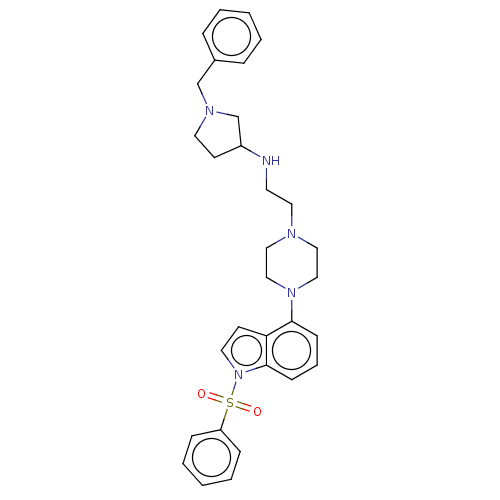 (CHEMBL3885238) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208212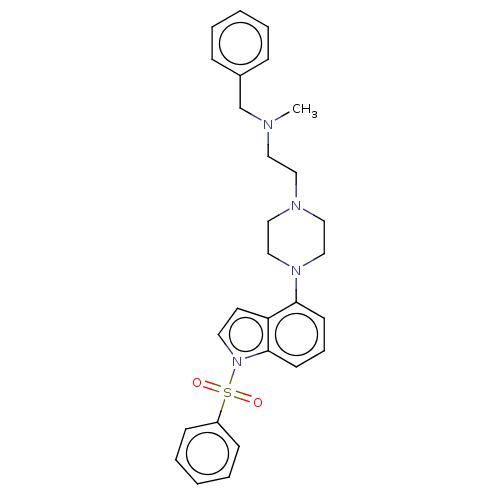 (CHEMBL3883443) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208252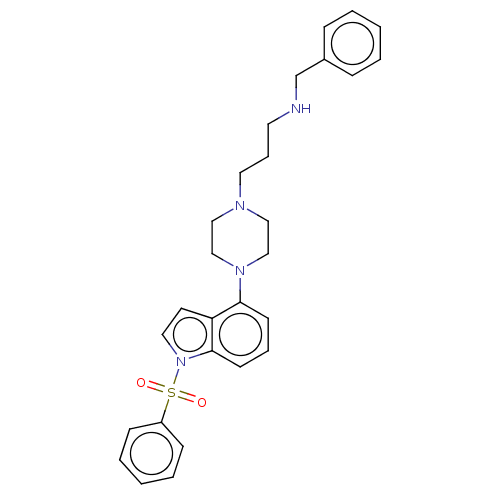 (CHEMBL3884154) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208213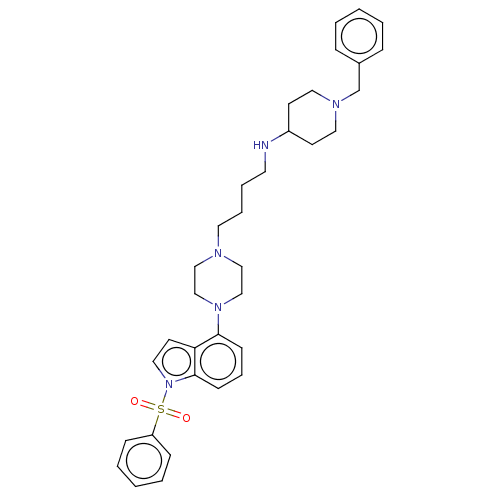 (CHEMBL3884254) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208216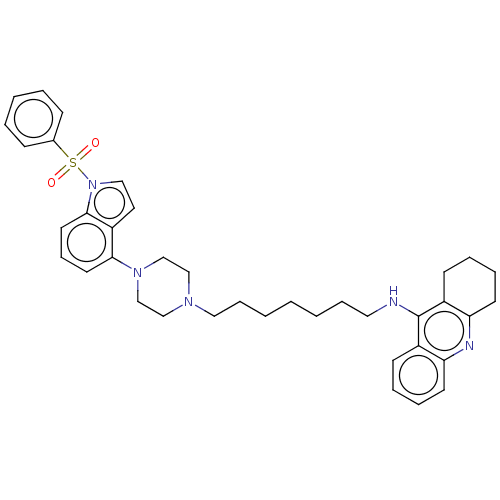 (CHEMBL3884690) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208218 (CHEMBL3883432) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50208215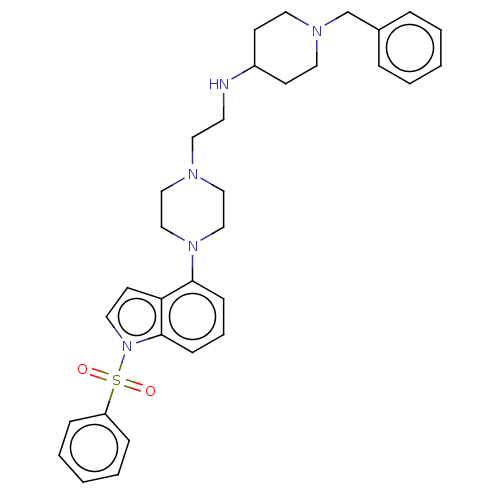 (CHEMBL3883620) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human recombinant 5-HT6 receptor expressed in CHOK1 cell membranes measured after 60 mins by scintillation counter | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50234769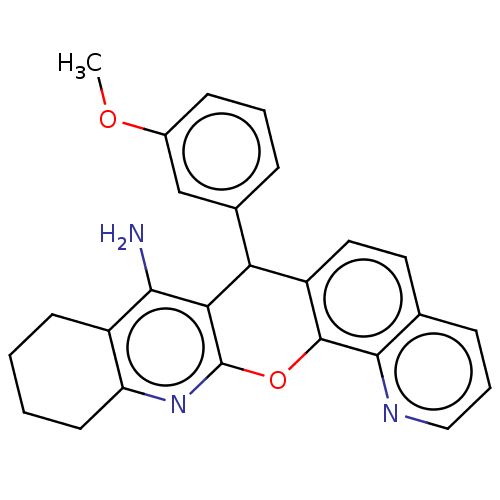 (CHEMBL4095908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sfax Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant human AChE expressed in HEK293 cells using varying levels of acetylthiocholine iodide as substrate pretreat... | Eur J Med Chem 126: 576-589 (2017) Article DOI: 10.1016/j.ejmech.2016.11.050 BindingDB Entry DOI: 10.7270/Q21R6SR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate pretreated for 10 mins followed by substrate addition measured after 15 mins by... | Eur J Med Chem 126: 576-589 (2017) Article DOI: 10.1016/j.ejmech.2016.11.050 BindingDB Entry DOI: 10.7270/Q21R6SR5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50208218 (CHEMBL3883432) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman'... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 5 mins followed by butyrylthiocholine iodide substrate addition measured after 5 mins by Ellman's m... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5... | Eur J Med Chem 125: 676-695 (2017) Article DOI: 10.1016/j.ejmech.2016.09.078 BindingDB Entry DOI: 10.7270/Q22Z17QK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman'... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50208216 (CHEMBL3884690) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman'... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50208218 (CHEMBL3883432) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman's met... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50371473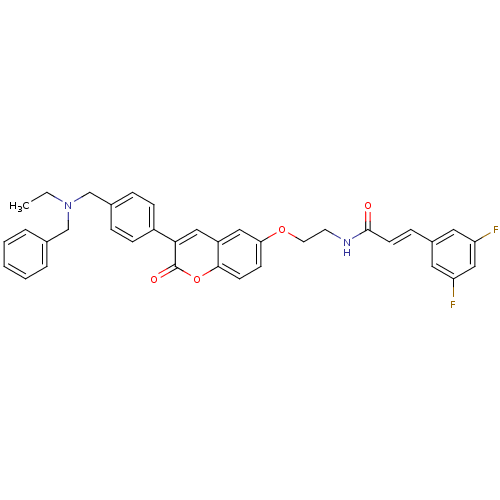 (CHEMBL239046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 125: 676-695 (2017) Article DOI: 10.1016/j.ejmech.2016.09.078 BindingDB Entry DOI: 10.7270/Q22Z17QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50208260 (CHEMBL3884988) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman's met... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50208258 (CHEMBL3885186) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 5 mins followed by butyrylthiocholine iodide substrate addition measured after 5 mins by Ellman's m... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50208256 (CHEMBL3884618) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman'... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50208216 (CHEMBL3884690) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 5 mins followed by butyrylthiocholine iodide substrate addition measured after 5 mins by Ellman's m... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman's met... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 125: 676-695 (2017) Article DOI: 10.1016/j.ejmech.2016.09.078 BindingDB Entry DOI: 10.7270/Q22Z17QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50208256 (CHEMBL3884618) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman's met... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50208218 (CHEMBL3883432) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 5 mins followed by butyrylthiocholine iodide substrate addition measured after 5 mins by Ellman's m... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50208258 (CHEMBL3885186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman'... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50208256 (CHEMBL3884618) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 5 mins followed by butyrylthiocholine iodide substrate addition measured after 5 mins by Ellman's m... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50208216 (CHEMBL3884690) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman's met... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50208260 (CHEMBL3884988) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 5 mins followed by butyrylthiocholine iodide substrate addition measured after 5 mins by Ellman's m... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50208214 (CHEMBL3884858) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman'... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50208214 (CHEMBL3884858) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 5 mins followed by butyrylthiocholine iodide substrate addition measured after 5 mins by Ellman's m... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of Electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 125: 676-695 (2017) Article DOI: 10.1016/j.ejmech.2016.09.078 BindingDB Entry DOI: 10.7270/Q22Z17QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 5 mins followed by acetylthiocholine iodide substrate addition measured after 5 mins by Ellman's met... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50208258 (CHEMBL3885186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE preincubated for 5 mins followed by butyrylthiocholine iodide substrate addition measured after 5 mins by Ellma... | Eur J Med Chem 124: 63-81 (2016) Article DOI: 10.1016/j.ejmech.2016.08.016 BindingDB Entry DOI: 10.7270/Q2PZ5BT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measure... | Eur J Med Chem 126: 576-589 (2017) Article DOI: 10.1016/j.ejmech.2016.11.050 BindingDB Entry DOI: 10.7270/Q21R6SR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50210827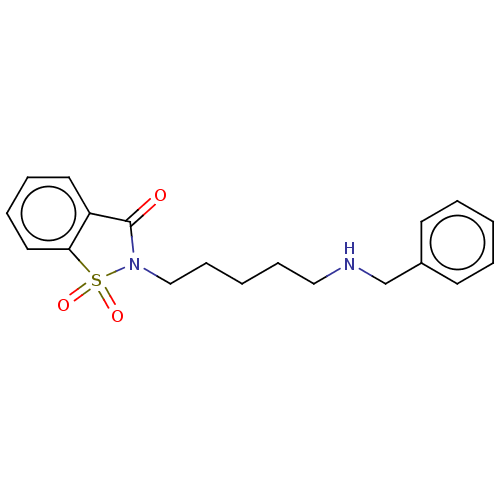 (CHEMBL3922423) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured afte... | Eur J Med Chem 125: 676-695 (2017) Article DOI: 10.1016/j.ejmech.2016.09.078 BindingDB Entry DOI: 10.7270/Q22Z17QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234775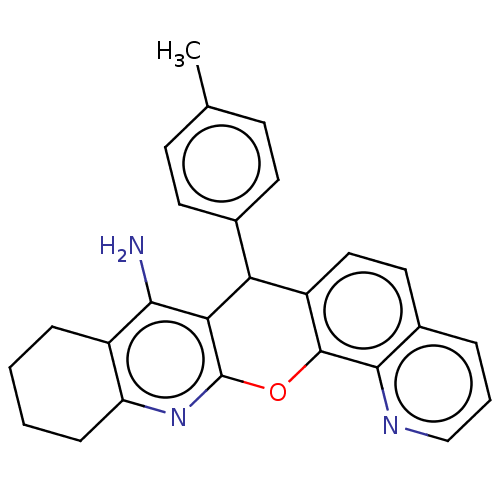 (CHEMBL4103664) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measure... | Eur J Med Chem 126: 576-589 (2017) Article DOI: 10.1016/j.ejmech.2016.11.050 BindingDB Entry DOI: 10.7270/Q21R6SR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234769 (CHEMBL4095908) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measure... | Eur J Med Chem 126: 576-589 (2017) Article DOI: 10.1016/j.ejmech.2016.11.050 BindingDB Entry DOI: 10.7270/Q21R6SR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 105 total ) | Next | Last >> |