 Found 6661 hits with Last Name = 'hill' and Initial = 'j'
Found 6661 hits with Last Name = 'hill' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine deaminase
(Homo sapiens (Human)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase. |
J Med Chem 26: 1478-82 (1983)
Checked by Author
BindingDB Entry DOI: 10.7270/Q29Z95GT |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50367032

(COFORMYCIN)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |r,c:18| Show InChI InChI=1S/C11H16N4O5/c16-2-6-8(18)9(19)11(20-6)15-4-14-7-5(17)1-12-3-13-10(7)15/h3-6,8-9,11,16-19H,1-2H2,(H,12,13)/t5-,6-,8-,9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase |
J Med Chem 26: 1478-82 (1983)
Checked by Author
BindingDB Entry DOI: 10.7270/Q29Z95GT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM81541
(CAS_195530 | NSC_195530 | S-Beta-Dimethylhistamine...)Show InChI InChI=1S/C7H13N3/c1-5(6(2)8)7-3-9-4-10-7/h3-6H,8H2,1-2H3,(H,9,10) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Medical Centre
Curated by PDSP Ki Database
| |
Biochem Soc Trans 20: 122-5 (1992)
Article DOI: 10.1042/bst0200122
BindingDB Entry DOI: 10.7270/Q2RX99KH |
More data for this
Ligand-Target Pair | |
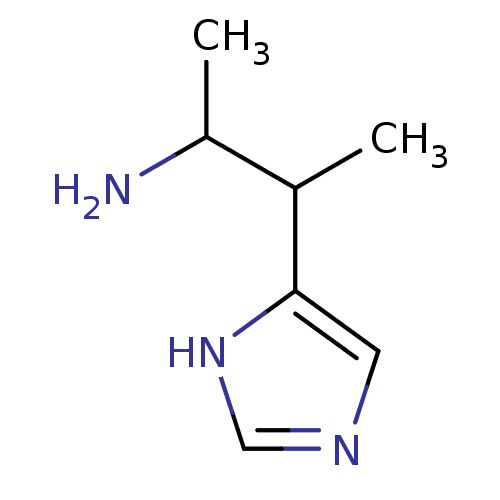
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50074629
(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. |
J Med Chem 42: 903-9 (1999)
Article DOI: 10.1021/jm980310g
BindingDB Entry DOI: 10.7270/Q2ST7P1D |
More data for this
Ligand-Target Pair | |
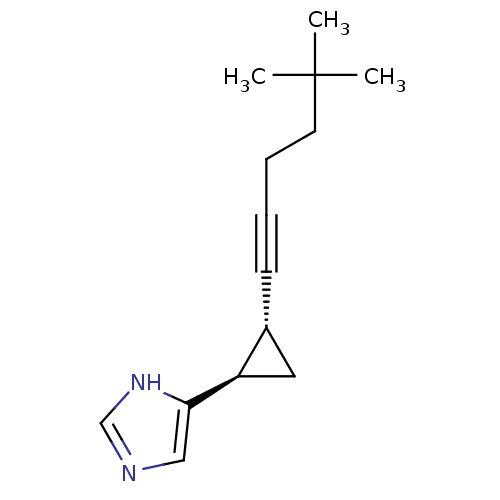
Promotilin
(Homo sapiens (Human)) | BDBM50143037
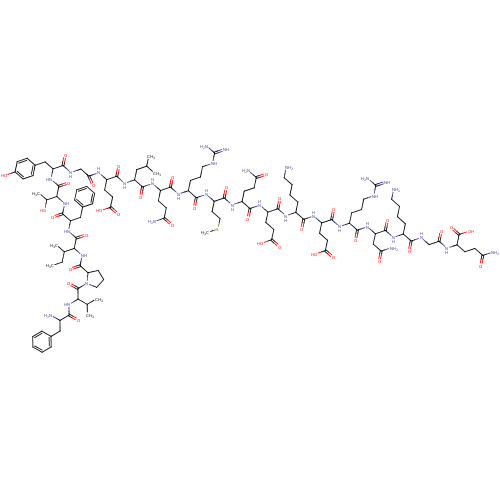
(CHEMBL411576 | MOTILIN)Show SMILES CCC(C)C(NC(=O)C1CCCN1C(=O)C(NC(=O)C(N)Cc1ccccc1)C(C)C)C(=O)NC(Cc1ccccc1)C(=O)NC(C(C)O)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(N)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CCSC)C(=O)NC(CCC(N)=O)C(=O)NC(CCC(O)=O)C(=O)NC(CCCCN)C(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CC(N)=O)C(=O)NC(CCCCN)C(=O)NCC(=O)NC(CCC(N)=O)C(O)=O Show InChI InChI=1S/C120H188N34O35S/c1-9-64(6)97(152-114(184)86-31-22-53-154(86)117(187)96(63(4)5)151-99(169)70(123)56-66-23-12-10-13-24-66)115(185)150-84(57-67-25-14-11-15-26-67)113(183)153-98(65(7)155)116(186)149-83(58-68-32-34-69(156)35-33-68)101(171)135-60-91(161)136-75(39-45-93(163)164)105(175)147-82(55-62(2)3)111(181)145-77(37-43-88(125)158)106(176)140-73(29-20-51-132-119(128)129)103(173)146-80(48-54-190-8)110(180)142-76(36-42-87(124)157)107(177)144-79(41-47-95(167)168)108(178)139-72(28-17-19-50-122)102(172)143-78(40-46-94(165)166)109(179)141-74(30-21-52-133-120(130)131)104(174)148-85(59-90(127)160)112(182)138-71(27-16-18-49-121)100(170)134-61-92(162)137-81(118(188)189)38-44-89(126)159/h10-15,23-26,32-35,62-65,70-86,96-98,155-156H,9,16-22,27-31,36-61,121-123H2,1-8H3,(H2,124,157)(H2,125,158)(H2,126,159)(H2,127,160)(H,134,170)(H,135,171)(H,136,161)(H,137,162)(H,138,182)(H,139,178)(H,140,176)(H,141,179)(H,142,180)(H,143,172)(H,144,177)(H,145,181)(H,146,173)(H,147,175)(H,148,174)(H,149,186)(H,150,185)(H,151,169)(H,152,184)(H,153,183)(H,163,164)(H,165,166)(H,167,168)(H,188,189)(H4,128,129,132)(H4,130,131,133) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by PDSP Ki Database
| |
Br J Pharmacol 140: 948-54 (2003)
Article DOI: 10.1038/sj.bjp.0705505
BindingDB Entry DOI: 10.7270/Q2N58JZF |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM112780
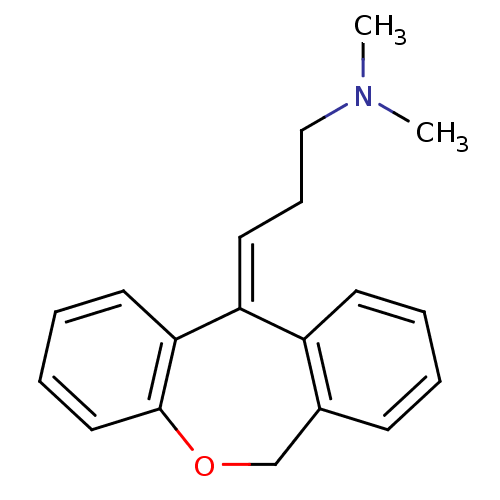
(US8629135, SW-07)Show InChI InChI=1S/C19H21NO/c1-20(2)13-7-11-17-16-9-4-3-8-15(16)14-21-19-12-6-5-10-18(17)19/h3-6,8-12H,7,13-14H2,1-2H3/b17-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... |
Bioorg Med Chem Lett 7: 3017-3022 (1997)
Article DOI: 10.1016/S0960-894X(97)10137-8
BindingDB Entry DOI: 10.7270/Q2280843 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor |
Bioorg Med Chem Lett 8: 1133-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GF0SPX |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM81543

(Alpha-Dimethylhistane-alpha | CAS_195960 | NSC_195...)Show InChI InChI=1S/C7H13N3/c1-6(8-2)3-7-4-9-5-10-7/h4-6,8H,3H2,1-2H3,(H,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Medical Centre
Curated by PDSP Ki Database
| |
Biochem Soc Trans 20: 122-5 (1992)
Article DOI: 10.1042/bst0200122
BindingDB Entry DOI: 10.7270/Q2RX99KH |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM81541

(CAS_195530 | NSC_195530 | S-Beta-Dimethylhistamine...)Show InChI InChI=1S/C7H13N3/c1-5(6(2)8)7-3-9-4-10-7/h3-6H,8H2,1-2H3,(H,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Medical Centre
Curated by PDSP Ki Database
| |
Biochem Soc Trans 20: 122-5 (1992)
Article DOI: 10.1042/bst0200122
BindingDB Entry DOI: 10.7270/Q2RX99KH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583640
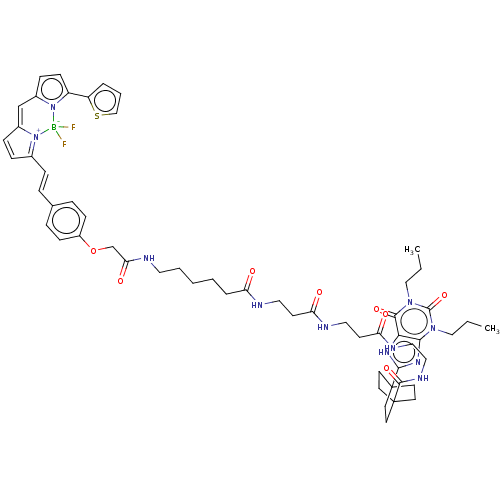
(CHEMBL5075285)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\C2=[N+]3C(C=C2)=Cc2ccc(-c4cccs4)n2[B-]3(F)F)cc1 |c:66,68,t:63| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cells by radioligand competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50094037
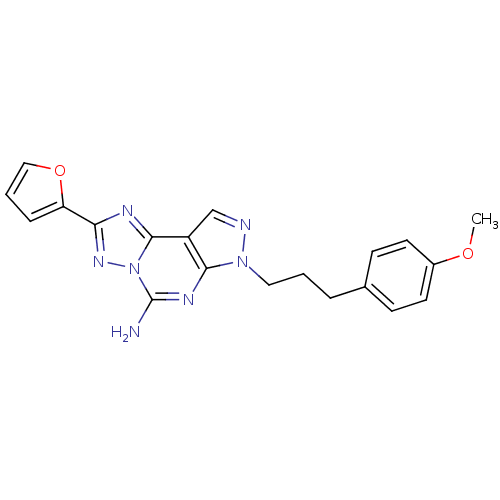
(2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...)Show SMILES COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C20H19N7O2/c1-28-14-8-6-13(7-9-14)4-2-10-26-18-15(12-22-26)19-23-17(16-5-3-11-29-16)25-27(19)20(21)24-18/h3,5-9,11-12H,2,4,10H2,1H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human adenosine A2A receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02169
BindingDB Entry DOI: 10.7270/Q23200R3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22916
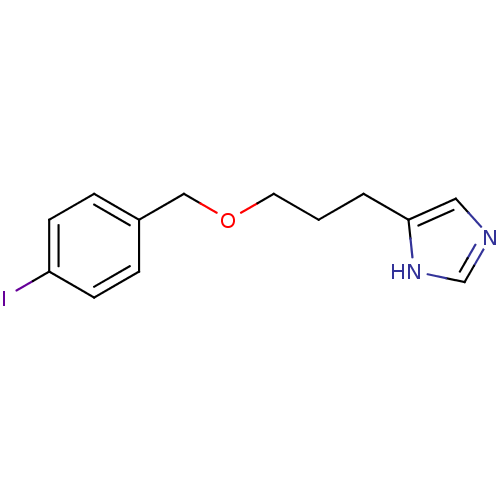
(5-{3-[(4-iodophenyl)methoxy]propyl}-1H-imidazole |...)Show InChI InChI=1S/C13H15IN2O/c14-12-5-3-11(4-6-12)9-17-7-1-2-13-8-15-10-16-13/h3-6,8,10H,1-2,7,9H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Histamine H3 receptor affinity of compound was determined in rat cortical membranes using the H3 selective agonist ligand, [3H]N-alpha-methylhistamin... |
Bioorg Med Chem Lett 7: 3017-3022 (1997)
Article DOI: 10.1016/S0960-894X(97)10137-8
BindingDB Entry DOI: 10.7270/Q2280843 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50074627
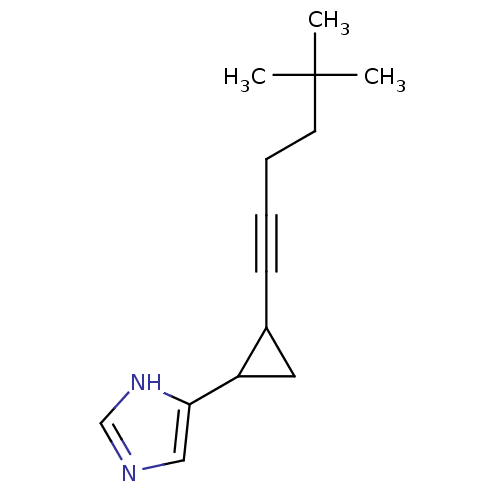
(4-[2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl]-1H-imi...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. |
J Med Chem 42: 903-9 (1999)
Article DOI: 10.1021/jm980310g
BindingDB Entry DOI: 10.7270/Q2ST7P1D |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02169
BindingDB Entry DOI: 10.7270/Q23200R3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604668
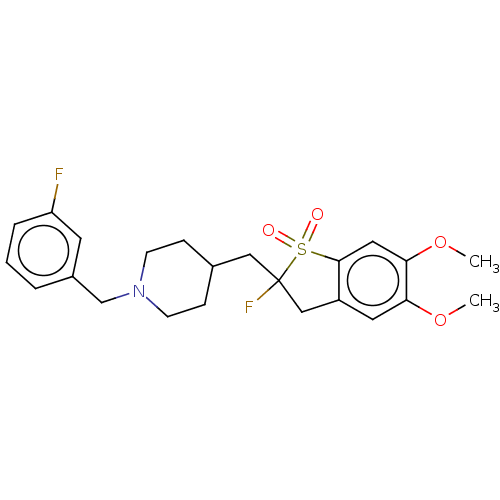
(CHEMBL5180947)Show SMILES COc1cc2CC(F)(CC3CCN(Cc4cccc(F)c4)CC3)S(=O)(=O)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114305
BindingDB Entry DOI: 10.7270/Q26M3BX2 |
More data for this
Ligand-Target Pair | |
Promotilin
(Homo sapiens (Human)) | BDBM86314
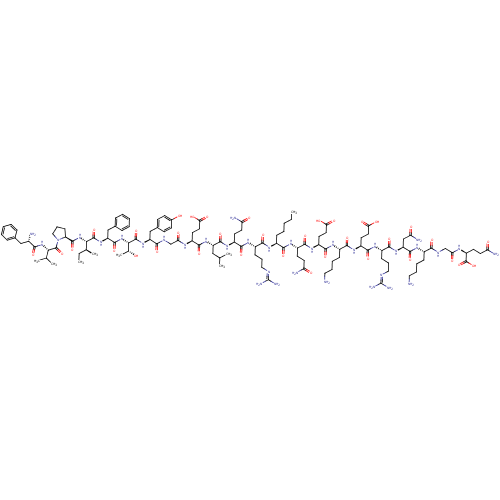
(CAS_0 | NSC_0 | [Nle13]-Motilin)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](-[#6])-[#6])-[#6@@H](-[#6])-[#6]-[#6])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C122H192N34O35/c1-9-11-14-30-74(104(174)145-79(40-46-89(126)159)110(180)147-82(45-51-97(169)170)111(181)142-75(32-20-22-53-124)105(175)146-81(44-50-96(167)168)112(182)144-77(34-24-55-135-122(132)133)107(177)150-87(61-92(129)162)114(184)140-73(31-19-21-52-123)102(172)136-63-94(164)139-83(120(190)191)42-48-91(128)161)141-106(176)76(33-23-54-134-121(130)131)143-109(179)80(41-47-90(127)160)148-113(183)84(57-64(3)4)149-108(178)78(43-49-95(165)166)138-93(163)62-137-103(173)85(60-70-36-38-71(158)39-37-70)151-118(188)100(67(8)157)155-115(185)86(59-69-28-17-13-18-29-69)152-117(187)99(66(7)10-2)154-116(186)88-35-25-56-156(88)119(189)98(65(5)6)153-101(171)72(125)58-68-26-15-12-16-27-68/h12-13,15-18,26-29,36-39,64-67,72-88,98-100,157-158H,9-11,14,19-25,30-35,40-63,123-125H2,1-8H3,(H2,126,159)(H2,127,160)(H2,128,161)(H2,129,162)(H,136,172)(H,137,173)(H,138,163)(H,139,164)(H,140,184)(H,141,176)(H,142,181)(H,143,179)(H,144,182)(H,145,174)(H,146,175)(H,147,180)(H,148,183)(H,149,178)(H,150,177)(H,151,188)(H,152,187)(H,153,171)(H,154,186)(H,155,185)(H,165,166)(H,167,168)(H,169,170)(H,190,191)(H4,130,131,134)(H4,132,133,135)/t66-,67+,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,98-,99-,100-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by PDSP Ki Database
| |
Br J Pharmacol 140: 948-54 (2003)
Article DOI: 10.1038/sj.bjp.0705505
BindingDB Entry DOI: 10.7270/Q2N58JZF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02169
BindingDB Entry DOI: 10.7270/Q23200R3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581955
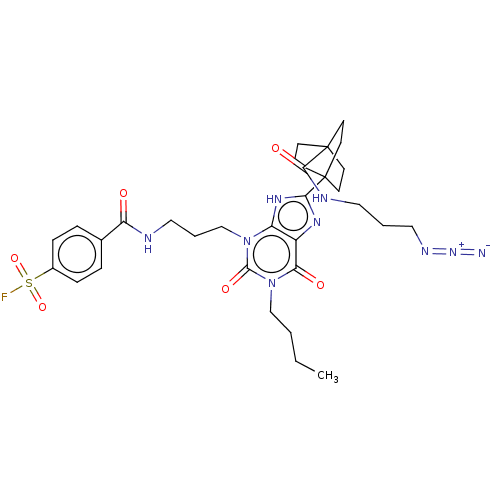
(CHEMBL5073669)Show SMILES CCCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)S(F)(=O)=O)c2[nH]c(nc2c1=O)C12CCC(CC1)(CC2)C(=O)NCCCN=[N+]=[N-] | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02169
BindingDB Entry DOI: 10.7270/Q23200R3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50070217
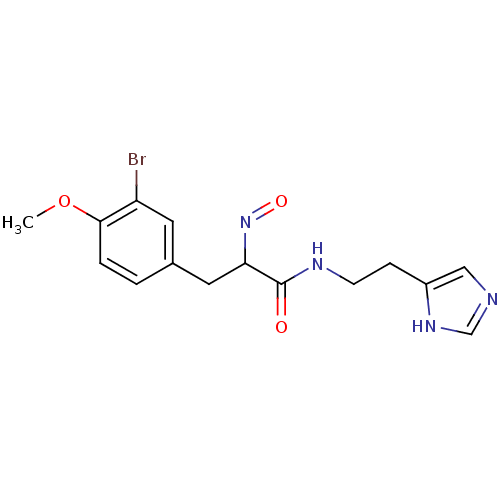
(3-(3-Bromo-4-methoxy-phenyl)-2-[(E)-hydroxyimino]-...)Show InChI InChI=1S/C15H17BrN4O3/c1-23-14-3-2-10(6-12(14)16)7-13(20-22)15(21)18-5-4-11-8-17-9-19-11/h2-3,6,8-9,13H,4-5,7H2,1H3,(H,17,19)(H,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor |
Bioorg Med Chem Lett 8: 1133-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GF0SPX |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Medical Centre
Curated by PDSP Ki Database
| |
Biochem Soc Trans 20: 122-5 (1992)
Article DOI: 10.1042/bst0200122
BindingDB Entry DOI: 10.7270/Q2RX99KH |
More data for this
Ligand-Target Pair | |
Ecdysone receptor/Protein ultraspiracle homolog
(Choristoneura fumiferana) | BDBM50178988
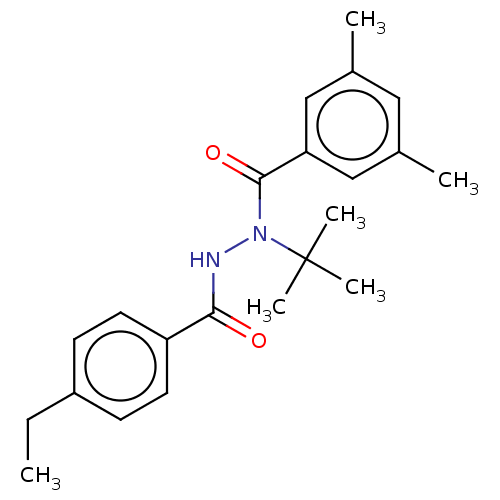
(CHEBI:38452 | Tebufenozide)Show SMILES CCc1ccc(cc1)C(=O)NN(C(=O)c1cc(C)cc(C)c1)C(C)(C)C Show InChI InChI=1S/C22H28N2O2/c1-7-17-8-10-18(11-9-17)20(25)23-24(22(4,5)6)21(26)19-13-15(2)12-16(3)14-19/h8-14H,7H2,1-6H3,(H,23,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Materials Science and Engineering and CSIRO Food and Nutritional Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to Choristoneura fumiferana EcR |
Pest Manag Sci 67: 1457-67 (2011)
Article DOI: 10.1002/ps.2200
BindingDB Entry DOI: 10.7270/Q2B27Z6B |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02169
BindingDB Entry DOI: 10.7270/Q23200R3 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50211457
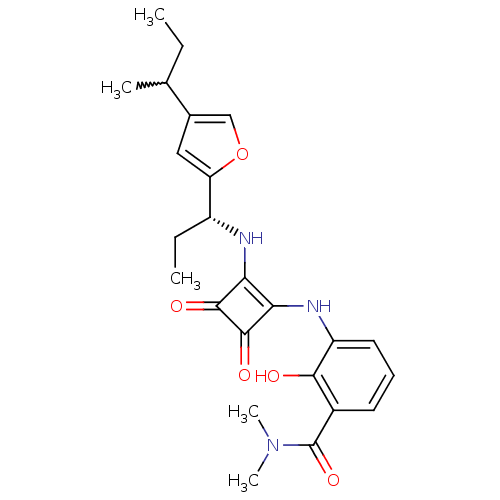
(3-(2-((R)-1-(4-sec-butylfuran-2-yl)propylamino)-3,...)Show SMILES CCC(C)c1coc(c1)[C@@H](CC)Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O |w:2.2| Show InChI InChI=1S/C24H29N3O5/c1-6-13(3)14-11-18(32-12-14)16(7-2)25-19-20(23(30)22(19)29)26-17-10-8-9-15(21(17)28)24(31)27(4)5/h8-13,16,25-26,28H,6-7H2,1-5H3/t13?,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA |
Bioorg Med Chem Lett 17: 3778-83 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.016
BindingDB Entry DOI: 10.7270/Q25D8RH5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581957
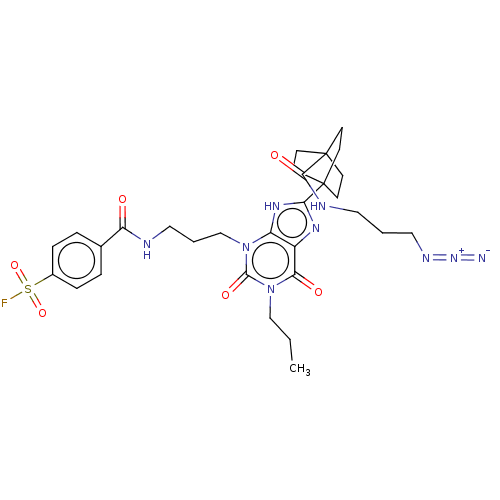
(CHEMBL5080823)Show SMILES CCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)S(F)(=O)=O)c2[nH]c(nc2c1=O)C12CCC(CC1)(CC2)C(=O)NCCCN=[N+]=[N-] | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02169
BindingDB Entry DOI: 10.7270/Q23200R3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02169
BindingDB Entry DOI: 10.7270/Q23200R3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038757
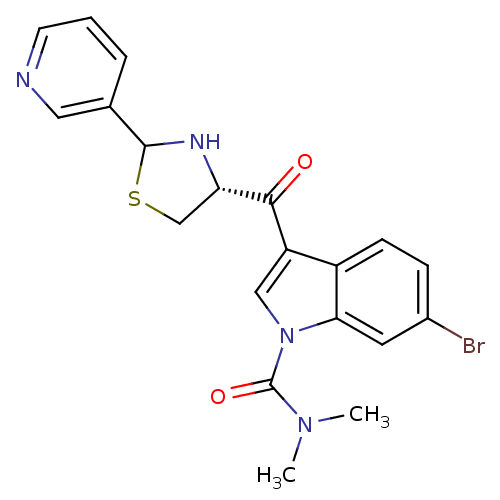
(6-Bromo-3-((R)-2-pyridin-3-yl-thiazolidine-4-carbo...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(Br)cc12 Show InChI InChI=1S/C20H19BrN4O2S/c1-24(2)20(27)25-10-15(14-6-5-13(21)8-17(14)25)18(26)16-11-28-19(23-16)12-4-3-7-22-9-12/h3-10,16,19,23H,11H2,1-2H3/t16-,19?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581964
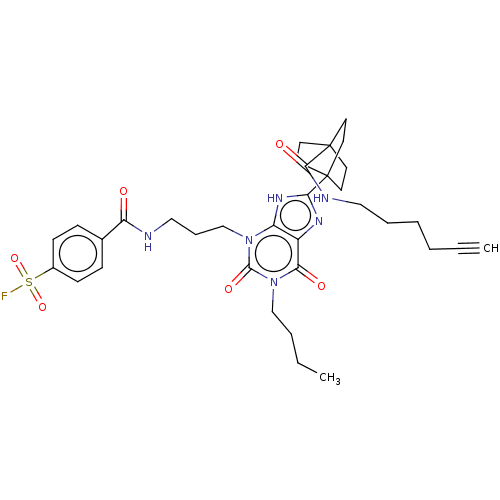
(CHEMBL5092788)Show SMILES CCCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)S(F)(=O)=O)c2[nH]c(nc2c1=O)C12CCC(CC1)(CC2)C(=O)NCCCCC#C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02169
BindingDB Entry DOI: 10.7270/Q23200R3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50583641
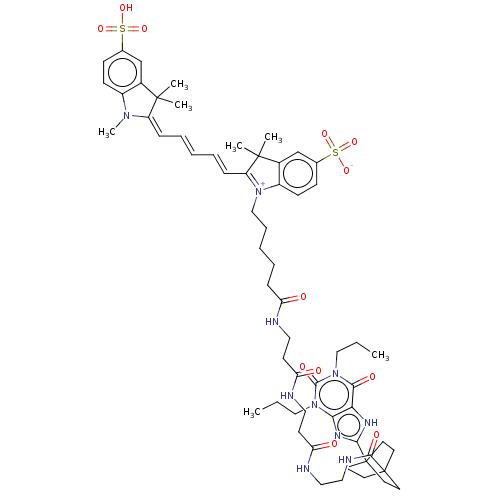
(CHEMBL5086197)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)CCCCC[N+]1=C(\C=C\C=C\C=C2\N(C)c3ccc(cc3C2(C)C)S(O)(=O)=O)C(C)(C)c2cc(ccc12)S([O-])(=O)=O |c:52| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NanoLuc human A1 adenosine receptor expressed in HEK293-A cells in prescence of SLV320 by NanoBRET competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038809
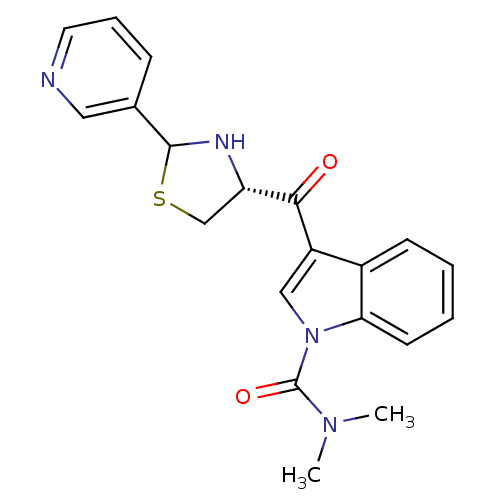
(3-((R)-2-Pyridin-3-yl-thiazolidine-4-carbonyl)-ind...)Show SMILES CN(C)C(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccccc12 Show InChI InChI=1S/C20H20N4O2S/c1-23(2)20(26)24-11-15(14-7-3-4-8-17(14)24)18(25)16-12-27-19(22-16)13-6-5-9-21-10-13/h3-11,16,19,22H,12H2,1-2H3/t16-,19?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50070214
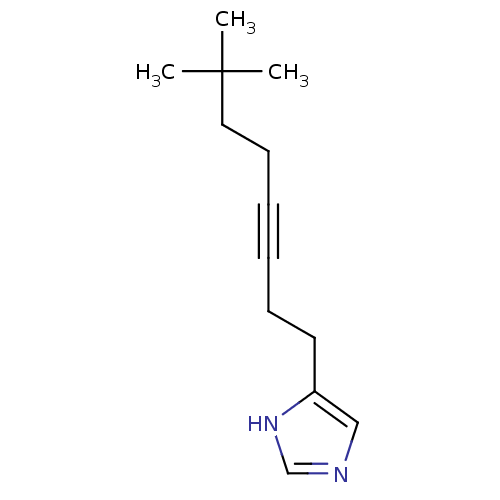
(4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...)Show InChI InChI=1S/C13H20N2/c1-13(2,3)9-7-5-4-6-8-12-10-14-11-15-12/h10-11H,6-9H2,1-3H3,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor |
Bioorg Med Chem Lett 8: 1133-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GF0SPX |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM81542
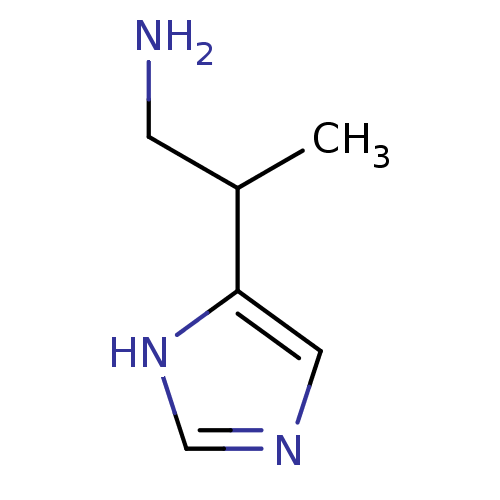
(Beta-Methylhistamine | CAS_565544 | NSC_565544)Show InChI InChI=1S/C6H11N3/c1-5(2-7)6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Medical Centre
Curated by PDSP Ki Database
| |
Biochem Soc Trans 20: 122-5 (1992)
Article DOI: 10.1042/bst0200122
BindingDB Entry DOI: 10.7270/Q2RX99KH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50211449
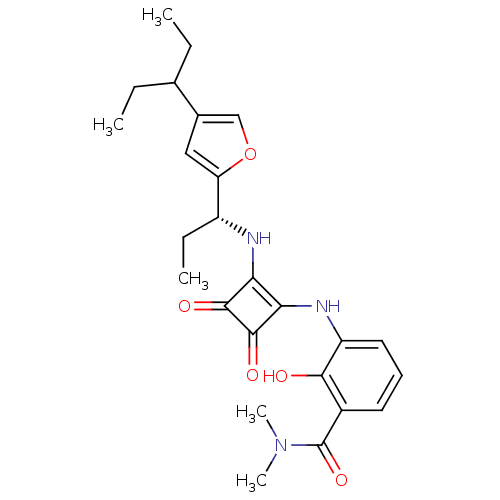
((R)-3-(3,4-dioxo-2-(1-(4-(pentan-3-yl)furan-2-yl)p...)Show SMILES CCC(CC)c1coc(c1)[C@@H](CC)Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O Show InChI InChI=1S/C25H31N3O5/c1-6-14(7-2)15-12-19(33-13-15)17(8-3)26-20-21(24(31)23(20)30)27-18-11-9-10-16(22(18)29)25(32)28(4)5/h9-14,17,26-27,29H,6-8H2,1-5H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA |
Bioorg Med Chem Lett 17: 3778-83 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.016
BindingDB Entry DOI: 10.7270/Q25D8RH5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50070214

(4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...)Show InChI InChI=1S/C13H20N2/c1-13(2,3)9-7-5-4-6-8-12-10-14-11-15-12/h10-11H,6-9H2,1-3H3,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. |
J Med Chem 42: 903-9 (1999)
Article DOI: 10.1021/jm980310g
BindingDB Entry DOI: 10.7270/Q2ST7P1D |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50070214

(4-(7,7-Dimethyl-oct-3-ynyl)-1H-imidazole | CHEMBL2...)Show InChI InChI=1S/C13H20N2/c1-13(2,3)9-7-5-4-6-8-12-10-14-11-15-12/h10-11H,6-9H2,1-3H3,(H,14,15) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM86314
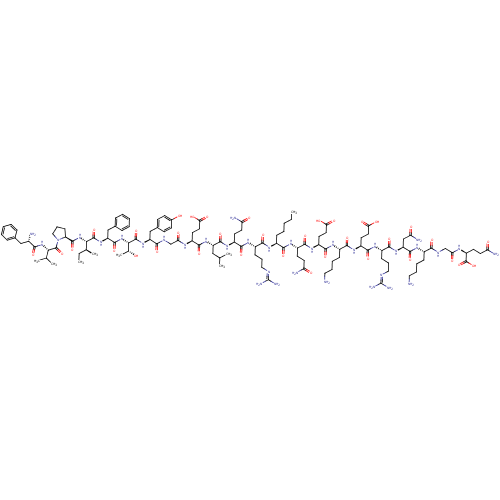
(CAS_0 | NSC_0 | [Nle13]-Motilin)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](-[#6])-[#6])-[#6@@H](-[#6])-[#6]-[#6])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C122H192N34O35/c1-9-11-14-30-74(104(174)145-79(40-46-89(126)159)110(180)147-82(45-51-97(169)170)111(181)142-75(32-20-22-53-124)105(175)146-81(44-50-96(167)168)112(182)144-77(34-24-55-135-122(132)133)107(177)150-87(61-92(129)162)114(184)140-73(31-19-21-52-123)102(172)136-63-94(164)139-83(120(190)191)42-48-91(128)161)141-106(176)76(33-23-54-134-121(130)131)143-109(179)80(41-47-90(127)160)148-113(183)84(57-64(3)4)149-108(178)78(43-49-95(165)166)138-93(163)62-137-103(173)85(60-70-36-38-71(158)39-37-70)151-118(188)100(67(8)157)155-115(185)86(59-69-28-17-13-18-29-69)152-117(187)99(66(7)10-2)154-116(186)88-35-25-56-156(88)119(189)98(65(5)6)153-101(171)72(125)58-68-26-15-12-16-27-68/h12-13,15-18,26-29,36-39,64-67,72-88,98-100,157-158H,9-11,14,19-25,30-35,40-63,123-125H2,1-8H3,(H2,126,159)(H2,127,160)(H2,128,161)(H2,129,162)(H,136,172)(H,137,173)(H,138,163)(H,139,164)(H,140,184)(H,141,176)(H,142,181)(H,143,179)(H,144,182)(H,145,174)(H,146,175)(H,147,180)(H,148,183)(H,149,178)(H,150,177)(H,151,188)(H,152,187)(H,153,171)(H,154,186)(H,155,185)(H,165,166)(H,167,168)(H,169,170)(H,190,191)(H4,130,131,134)(H4,132,133,135)/t66-,67+,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,98-,99-,100-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by PDSP Ki Database
| |
Br J Pharmacol 140: 948-54 (2003)
Article DOI: 10.1038/sj.bjp.0705505
BindingDB Entry DOI: 10.7270/Q2N58JZF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50070220
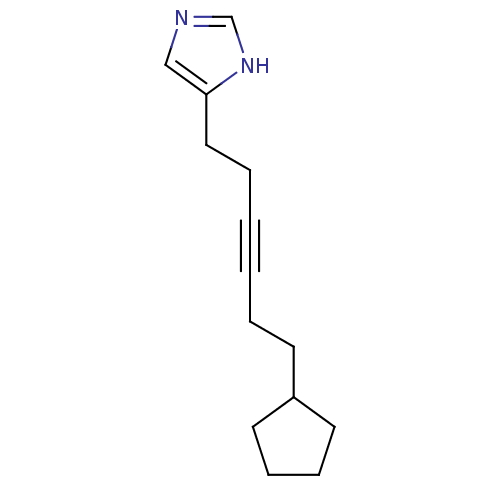
(4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...)Show InChI InChI=1S/C14H20N2/c1(3-7-13-8-5-6-9-13)2-4-10-14-11-15-12-16-14/h11-13H,3-10H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor in rat cortical membranes by [3H]-Nalpha-methylhistamine displacement. |
J Med Chem 42: 903-9 (1999)
Article DOI: 10.1021/jm980310g
BindingDB Entry DOI: 10.7270/Q2ST7P1D |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM81542

(Beta-Methylhistamine | CAS_565544 | NSC_565544)Show InChI InChI=1S/C6H11N3/c1-5(2-7)6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Medical Centre
Curated by PDSP Ki Database
| |
Biochem Soc Trans 20: 122-5 (1992)
Article DOI: 10.1042/bst0200122
BindingDB Entry DOI: 10.7270/Q2RX99KH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50070220

(4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...)Show InChI InChI=1S/C14H20N2/c1(3-7-13-8-5-6-9-13)2-4-10-14-11-15-12-16-14/h11-13H,3-10H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor |
Bioorg Med Chem Lett 8: 1133-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GF0SPX |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50070220

(4-(6-Cyclopentyl-hex-3-ynyl)-1H-imidazole | CHEMBL...)Show InChI InChI=1S/C14H20N2/c1(3-7-13-8-5-6-9-13)2-4-10-14-11-15-12-16-14/h11-13H,3-10H2,(H,15,16) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Promotilin
(RABBIT) | BDBM50143037

(CHEMBL411576 | MOTILIN)Show SMILES CCC(C)C(NC(=O)C1CCCN1C(=O)C(NC(=O)C(N)Cc1ccccc1)C(C)C)C(=O)NC(Cc1ccccc1)C(=O)NC(C(C)O)C(=O)NC(Cc1ccc(O)cc1)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(N)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CCSC)C(=O)NC(CCC(N)=O)C(=O)NC(CCC(O)=O)C(=O)NC(CCCCN)C(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CC(N)=O)C(=O)NC(CCCCN)C(=O)NCC(=O)NC(CCC(N)=O)C(O)=O Show InChI InChI=1S/C120H188N34O35S/c1-9-64(6)97(152-114(184)86-31-22-53-154(86)117(187)96(63(4)5)151-99(169)70(123)56-66-23-12-10-13-24-66)115(185)150-84(57-67-25-14-11-15-26-67)113(183)153-98(65(7)155)116(186)149-83(58-68-32-34-69(156)35-33-68)101(171)135-60-91(161)136-75(39-45-93(163)164)105(175)147-82(55-62(2)3)111(181)145-77(37-43-88(125)158)106(176)140-73(29-20-51-132-119(128)129)103(173)146-80(48-54-190-8)110(180)142-76(36-42-87(124)157)107(177)144-79(41-47-95(167)168)108(178)139-72(28-17-19-50-122)102(172)143-78(40-46-94(165)166)109(179)141-74(30-21-52-133-120(130)131)104(174)148-85(59-90(127)160)112(182)138-71(27-16-18-49-121)100(170)134-61-92(162)137-81(118(188)189)38-44-89(126)159/h10-15,23-26,32-35,62-65,70-86,96-98,155-156H,9,16-22,27-31,36-61,121-123H2,1-8H3,(H2,124,157)(H2,125,158)(H2,126,159)(H2,127,160)(H,134,170)(H,135,171)(H,136,161)(H,137,162)(H,138,182)(H,139,178)(H,140,176)(H,141,179)(H,142,180)(H,143,172)(H,144,177)(H,145,181)(H,146,173)(H,147,175)(H,148,174)(H,149,186)(H,150,185)(H,151,169)(H,152,184)(H,153,183)(H,163,164)(H,165,166)(H,167,168)(H,188,189)(H4,128,129,132)(H4,130,131,133) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by PDSP Ki Database
| |
Br J Pharmacol 140: 948-54 (2003)
Article DOI: 10.1038/sj.bjp.0705505
BindingDB Entry DOI: 10.7270/Q2N58JZF |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM85407
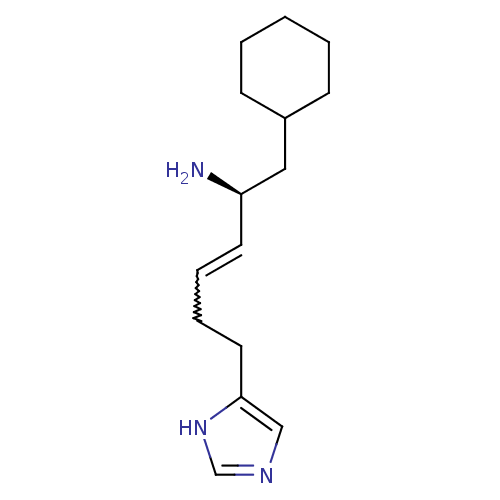
(GT 2231)Show SMILES N[C@@H](CC1CCCCC1)C=CCCc1cnc[nH]1 |r,w:10.11| Show InChI InChI=1S/C15H25N3/c16-14(10-13-6-2-1-3-7-13)8-4-5-9-15-11-17-12-18-15/h4,8,11-14H,1-3,5-7,9-10,16H2,(H,17,18)/b8-4+/t14-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 1151-9 (1999)
BindingDB Entry DOI: 10.7270/Q2BZ64KX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Oryctolagus cuniculus (Rabbit)) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 122-8 (1997)
BindingDB Entry DOI: 10.7270/Q2J67FFH |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's Medical Centre
Curated by PDSP Ki Database
| |
Biochem Soc Trans 20: 122-5 (1992)
Article DOI: 10.1042/bst0200122
BindingDB Entry DOI: 10.7270/Q2RX99KH |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50211458
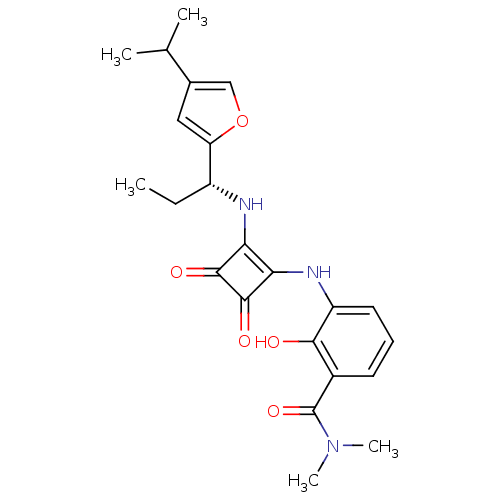
((R)-2-hydroxy-3-(2-(1-(4-isopropylfuran-2-yl)propy...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cc(co1)C(C)C Show InChI InChI=1S/C23H27N3O5/c1-6-15(17-10-13(11-31-17)12(2)3)24-18-19(22(29)21(18)28)25-16-9-7-8-14(20(16)27)23(30)26(4)5/h7-12,15,24-25,27H,6H2,1-5H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA |
Bioorg Med Chem Lett 17: 3778-83 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.016
BindingDB Entry DOI: 10.7270/Q25D8RH5 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50211454
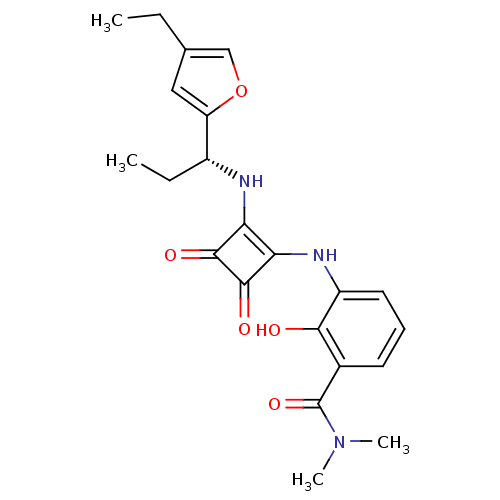
((R)-3-(2-(1-(4-ethylfuran-2-yl)propylamino)-3,4-di...)Show SMILES CC[C@@H](Nc1c(Nc2cccc(C(=O)N(C)C)c2O)c(=O)c1=O)c1cc(CC)co1 Show InChI InChI=1S/C22H25N3O5/c1-5-12-10-16(30-11-12)14(6-2)23-17-18(21(28)20(17)27)24-15-9-7-8-13(19(15)26)22(29)25(3)4/h7-11,14,23-24,26H,5-6H2,1-4H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from human CXCR2 expressed in CHO cells by SPA |
Bioorg Med Chem Lett 17: 3778-83 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.016
BindingDB Entry DOI: 10.7270/Q25D8RH5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM7967

(1-methyl-5-(beta-aminoethyl)-imidazole | 2-(1-meth...)Show InChI InChI=1S/C6H11N3/c1-9-5-8-4-6(9)2-3-7/h4-5H,2-3,7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gliatech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- N-alpha-methylhistamine from histamine H3 receptor |
Bioorg Med Chem Lett 8: 1133-8 (1999)
BindingDB Entry DOI: 10.7270/Q2GF0SPX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50581950

(CHEMBL4204703)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(nc2[nH]ccc12)-c1ccccc1 |r,wU:4.7,wD:1.0,(5.09,-4.45,;6.42,-5.22,;6.43,-6.76,;7.77,-7.53,;9.09,-6.74,;9.09,-5.21,;7.76,-4.45,;10.43,-7.5,;10.44,-9.04,;9.12,-9.82,;9.13,-11.35,;10.47,-12.12,;11.8,-11.34,;13.26,-11.81,;14.16,-10.57,;13.25,-9.33,;11.79,-9.81,;7.8,-12.13,;6.47,-11.36,;5.14,-12.13,;5.14,-13.68,;6.47,-14.45,;7.81,-13.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Oryctolagus cuniculus (Rabbit)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 280: 122-8 (1997)
BindingDB Entry DOI: 10.7270/Q2J67FFH |
More data for this
Ligand-Target Pair | |
Platelet-activating factor receptor
(Cavia porcellus) | BDBM50038792

(6-Benzyloxy-3-((R)-2-pyridin-3-yl-thiazolidine-4-c...)Show SMILES CC(C)(C)OC(=O)n1cc(C(=O)[C@@H]2CSC(N2)c2cccnc2)c2ccc(OCc3ccccc3)cc12 Show InChI InChI=1S/C29H29N3O4S/c1-29(2,3)36-28(34)32-16-23(26(33)24-18-37-27(31-24)20-10-7-13-30-15-20)22-12-11-21(14-25(22)32)35-17-19-8-5-4-6-9-19/h4-16,24,27,31H,17-18H2,1-3H3/t24-,27?/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligand |
J Med Chem 37: 2011-32 (1994)
BindingDB Entry DOI: 10.7270/Q2GH9JM7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data