

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50378656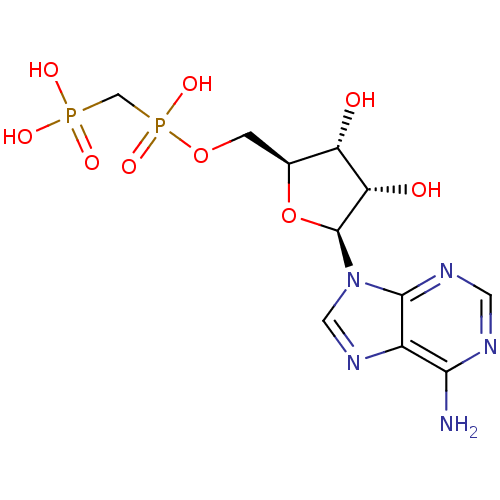 (CHEMBL598619) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437933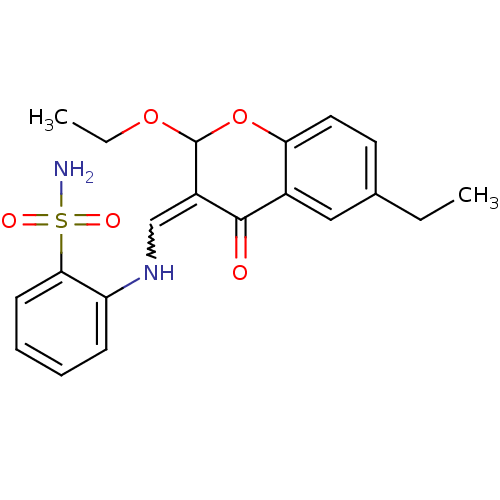 (CHEMBL2408703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437932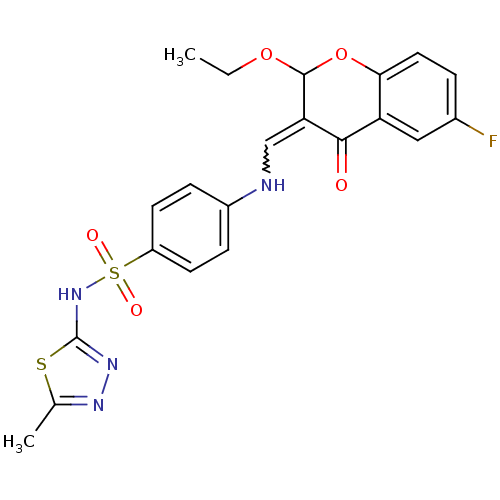 (CHEMBL2408704) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165144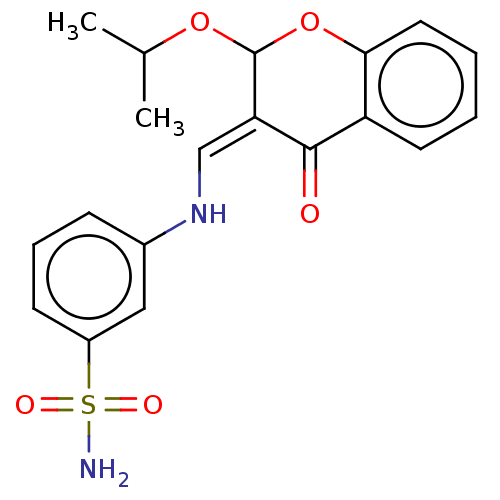 (CHEMBL3798753) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165146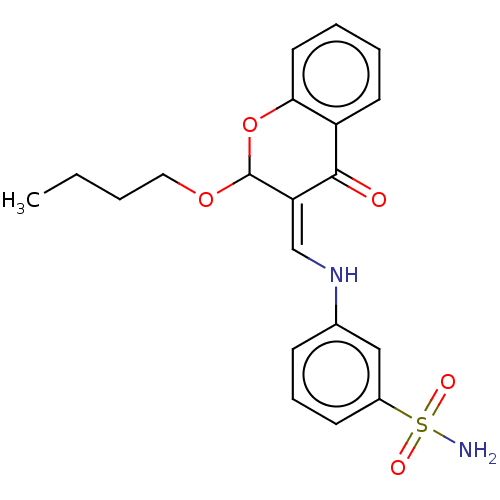 (CHEMBL3798795) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165147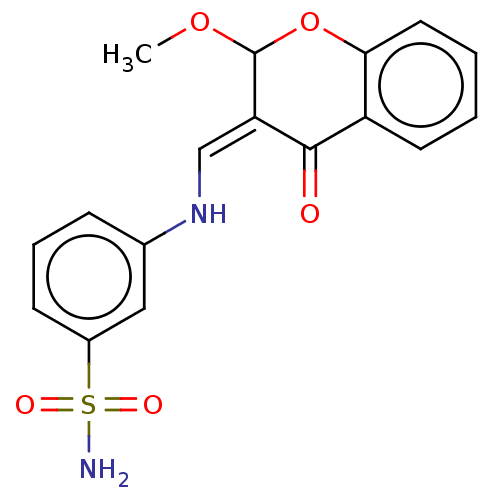 (CHEMBL3797302) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50300873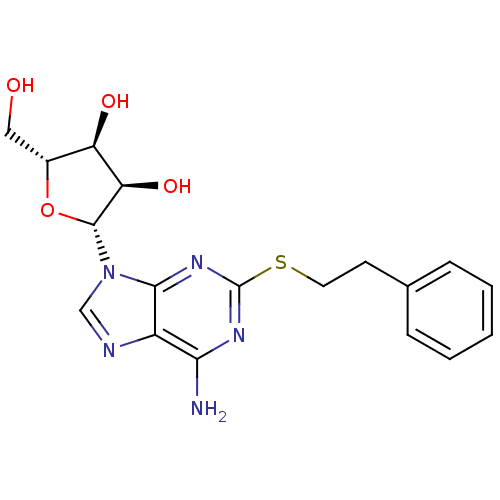 ((2R,3R,4S,5R)-2-(6-amino-2-(phenethylthio)-9H-puri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from adenosine A2A receptor in rat brain striatal membrane after 60 mins | J Med Chem 52: 7669-77 (2009) Article DOI: 10.1021/jm900538v BindingDB Entry DOI: 10.7270/Q2J10379 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437935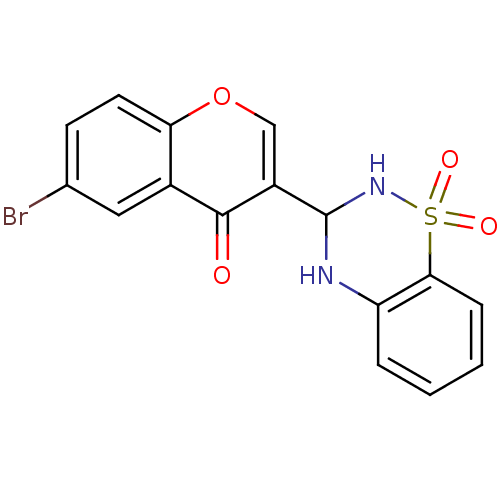 (CHEMBL2408701) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165143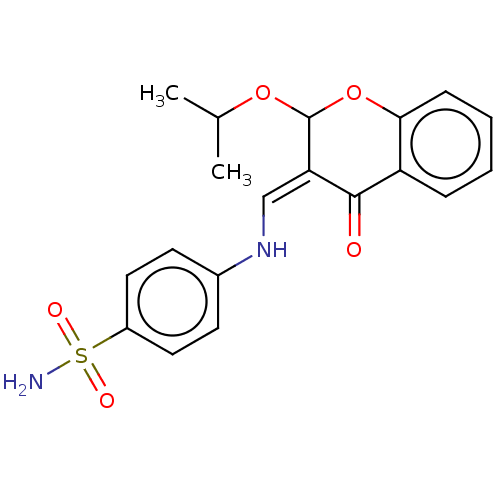 (CHEMBL3798555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165149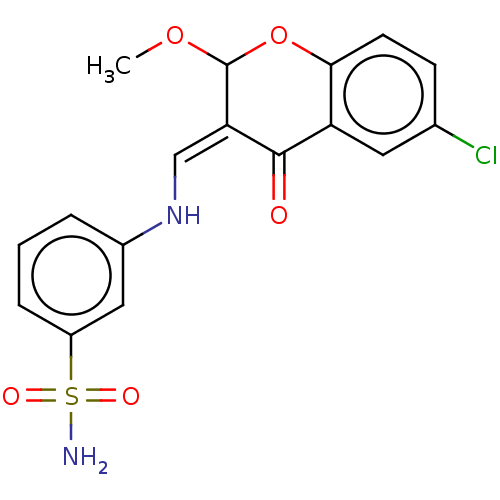 (CHEMBL3799691) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437939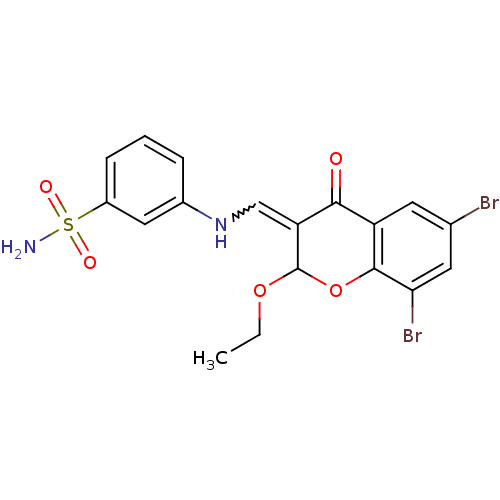 (CHEMBL1814399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92554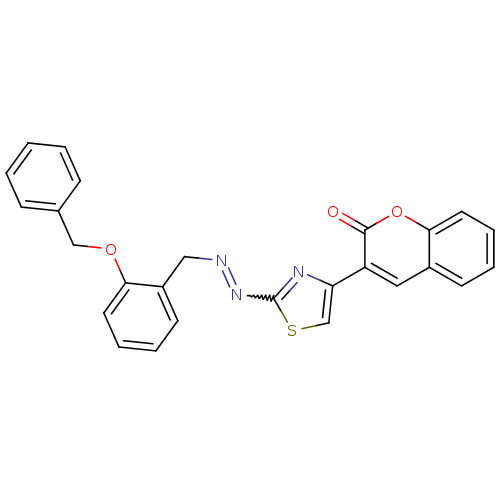 (Coumarin analogue, 3l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92543 (Coumarin analogue, 3a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165137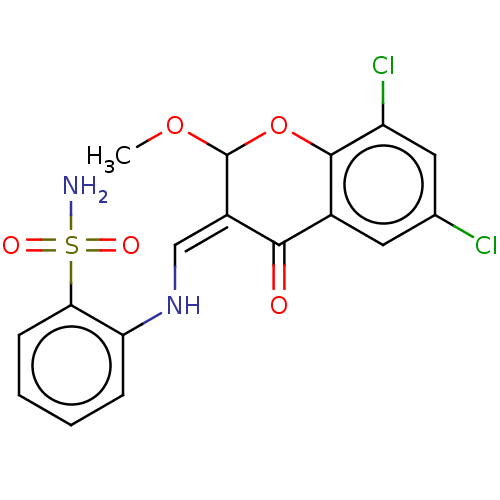 (CHEMBL3800324) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 45.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165136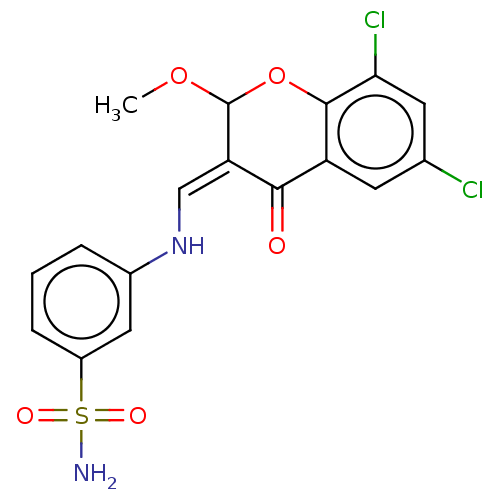 (CHEMBL3800429) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437941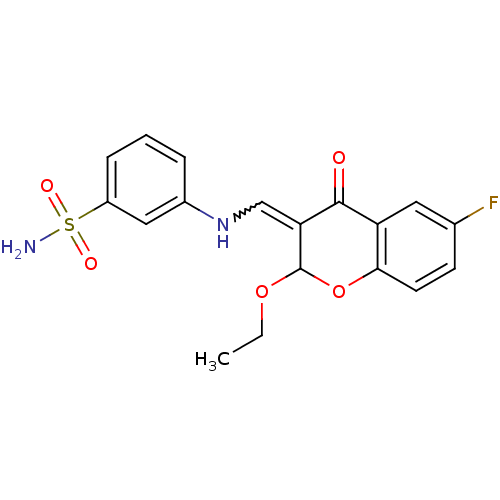 (CHEMBL1814397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437937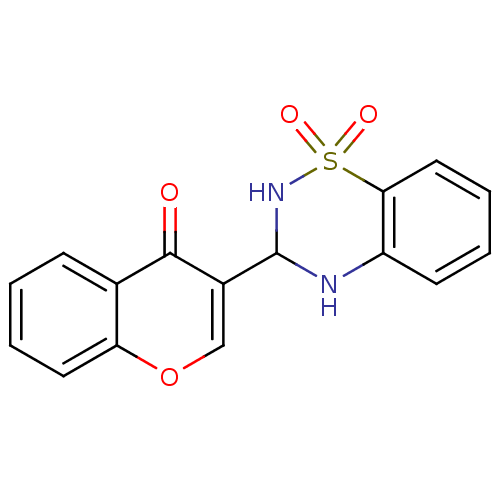 (CHEMBL2408699) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068225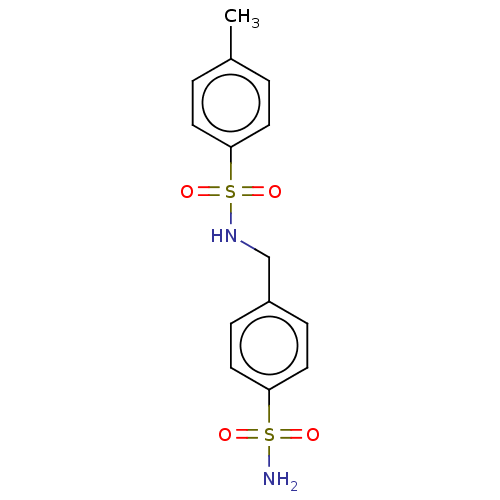 (CHEMBL3403324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165126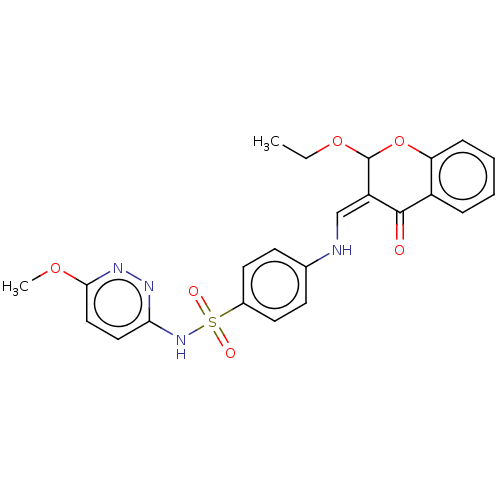 (CHEMBL3797319) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165143 (CHEMBL3798555) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068229 (CHEMBL3403327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92545 (Coumarin analogue, 3c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165150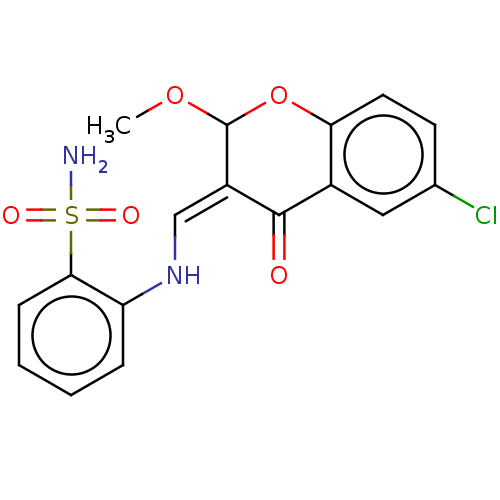 (CHEMBL3799027) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165145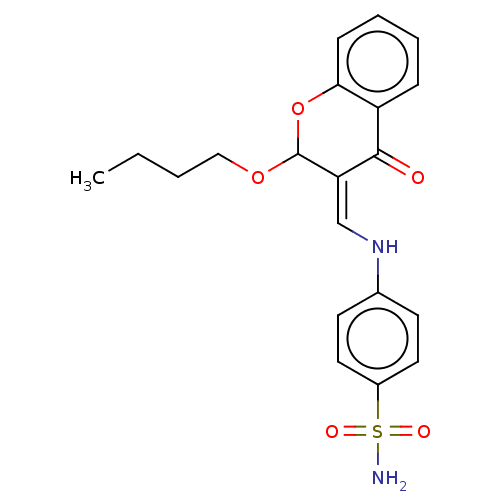 (CHEMBL3800455) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165150 (CHEMBL3799027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92544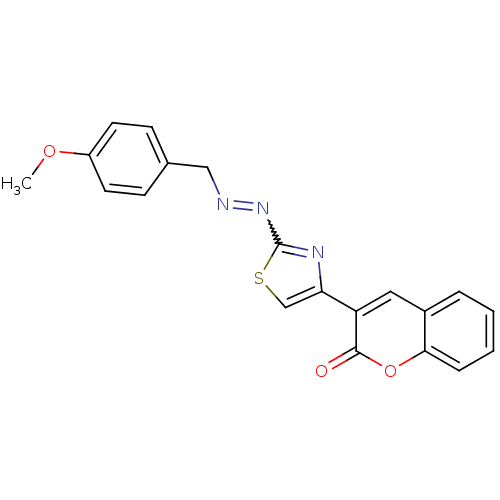 (Coumarin analogue, 3b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50428400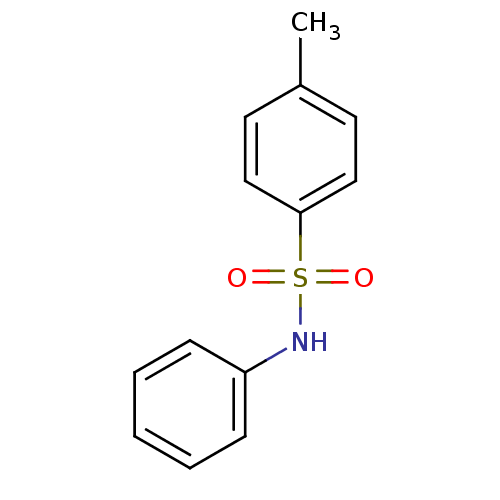 (CA inhibitor, 2 | CHEMBL182659 | [(4-Methylphenyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92548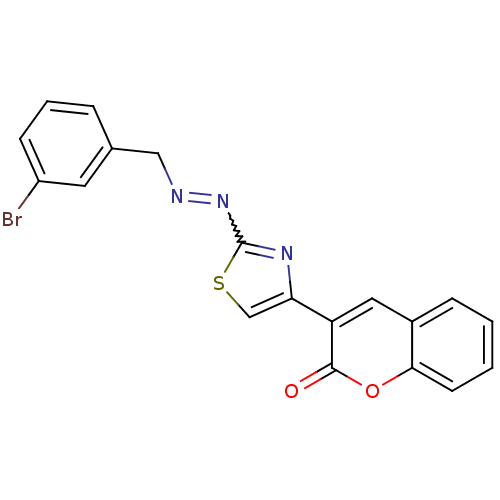 (Coumarin analogue, 3f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92553 (Coumarin analogue, 3k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92551 (Coumarin analogue, 3i) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92549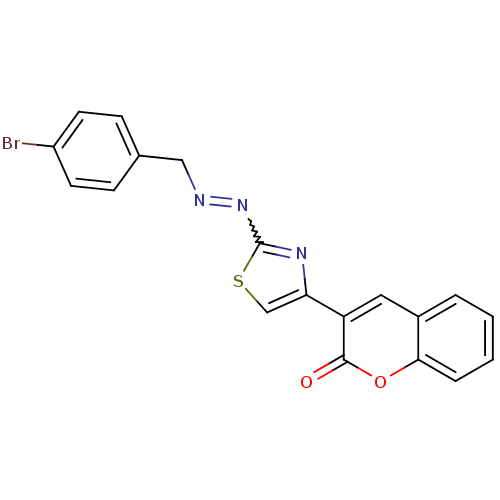 (Coumarin analogue, 3g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50165140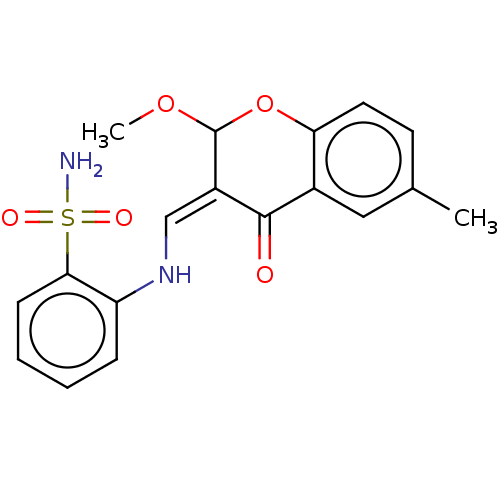 (CHEMBL3797297) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine tissue non-specific alkaline phosphatase preincubated for 3 to 5 mins followed by CDP-star substrate addition measured after 15 ... | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165148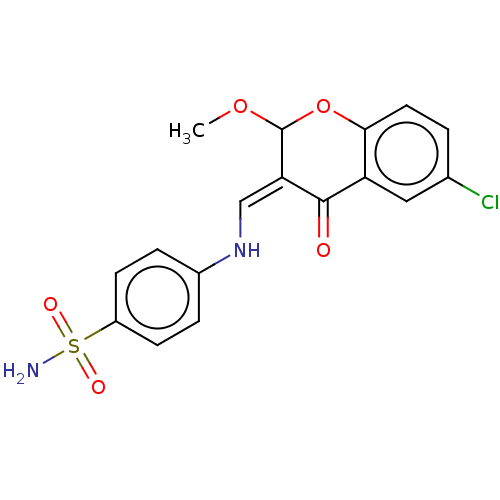 (CHEMBL3797640) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92550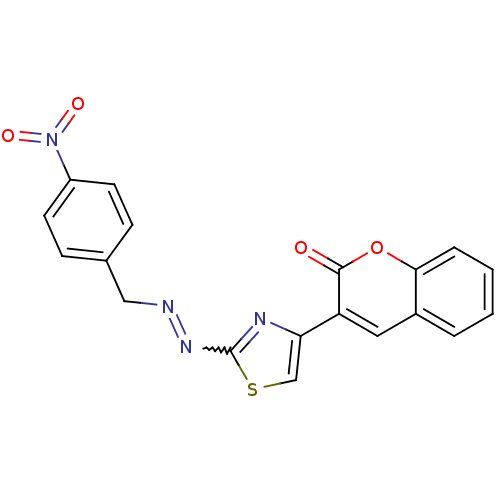 (Coumarin analogue, 3h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165148 (CHEMBL3797640) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50437931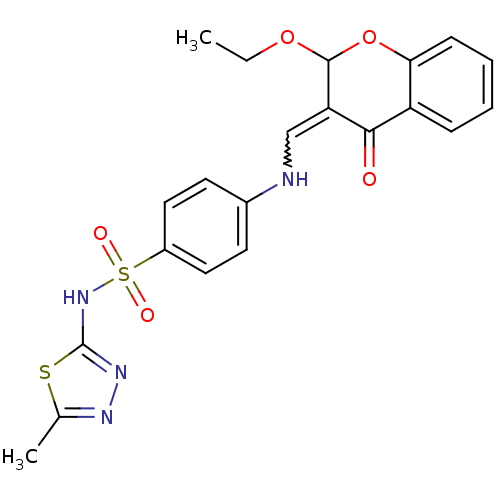 (CHEMBL2408705) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine intestinal alkaline phosphatase using p-NPP as substrate treated 10 mins before substrate addition measured after 30 mins by spe... | Eur J Med Chem 66: 438-49 (2013) Article DOI: 10.1016/j.ejmech.2013.06.015 BindingDB Entry DOI: 10.7270/Q20K29ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165137 (CHEMBL3800324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356190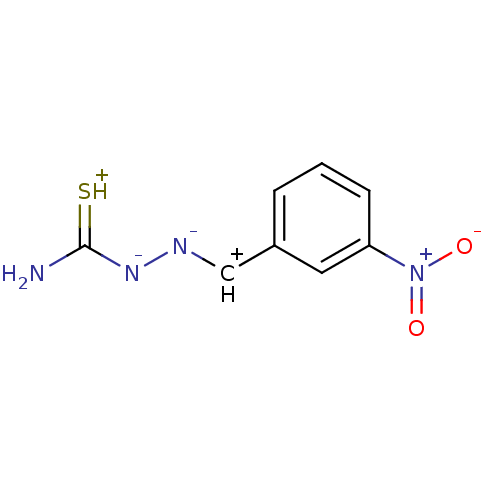 (CHEMBL193474) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50165129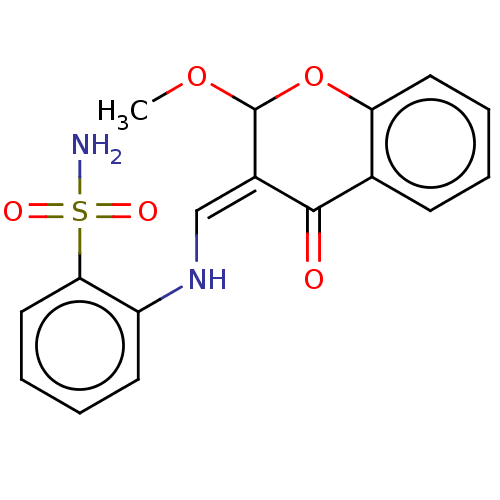 (CHEMBL3799469) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of human Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50437946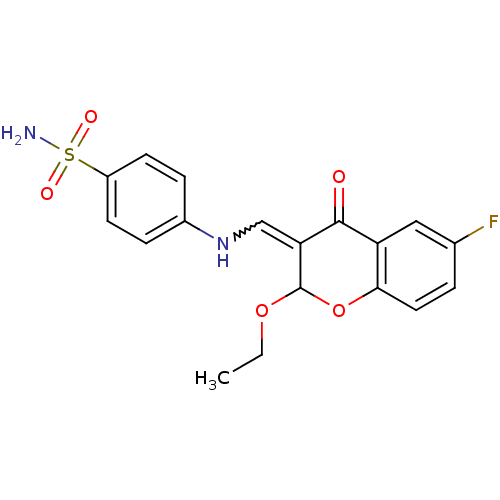 (CHEMBL1814392) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165149 (CHEMBL3799691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Rattus norvegicus (Rat)) | BDBM50165129 (CHEMBL3799469) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of rat Ecto-5'-nucleotidase transfected in COS7 cells preincubated for 10 mins followed by AMP addition measured after 10 mins | Eur J Med Chem 115: 484-94 (2016) Article DOI: 10.1016/j.ejmech.2016.02.073 BindingDB Entry DOI: 10.7270/Q2K939D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50300874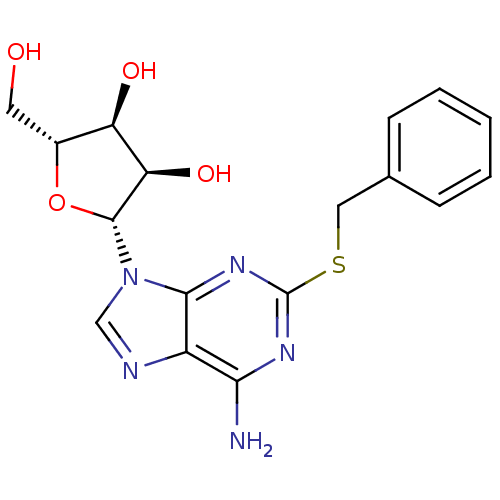 ((2R,3R,4S,5R)-2-(6-amino-2-(benzylthio)-9H-purin-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-11 from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins | J Med Chem 52: 7669-77 (2009) Article DOI: 10.1021/jm900538v BindingDB Entry DOI: 10.7270/Q2J10379 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356195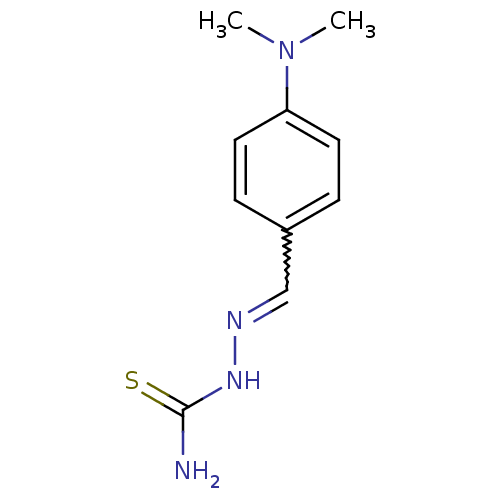 (CHEMBL241902) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM53627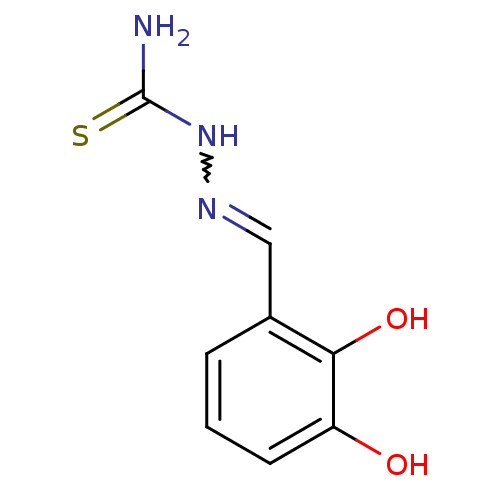 (1-[[(E)-(5-oxidanyl-6-oxidanylidene-cyclohexa-2,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356192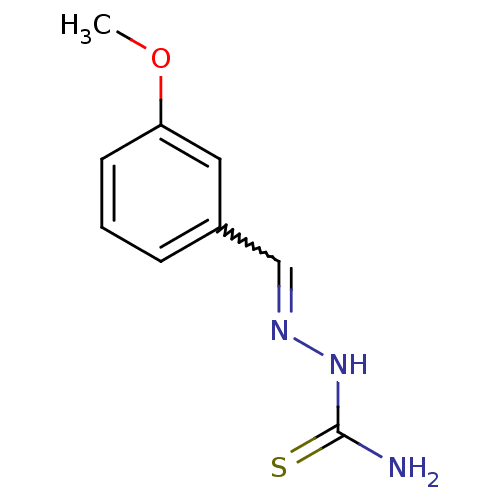 (CHEMBL241894) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Bos taurus (Cattle)) | BDBM50068230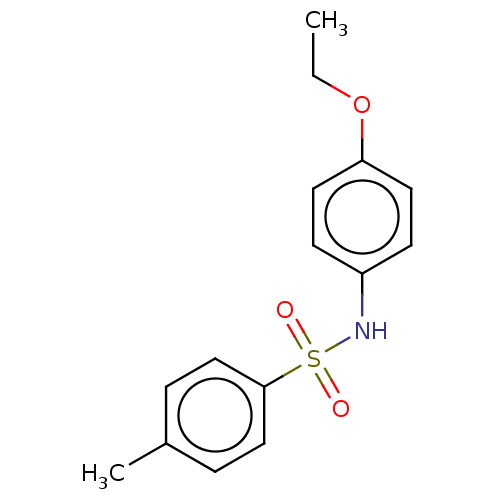 (CHEMBL3403329) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Forman Christian College (A Chartered University) Curated by ChEMBL | Assay Description Inhibition of bovine kidney non-specific alkaline phosphatase using CDP-star chemiluminescent substrate assessed as change in luminescence by spectro... | Bioorg Med Chem 23: 2435-44 (2015) Article DOI: 10.1016/j.bmc.2015.03.054 BindingDB Entry DOI: 10.7270/Q2WW7KC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1837 total ) | Next | Last >> |