

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50004774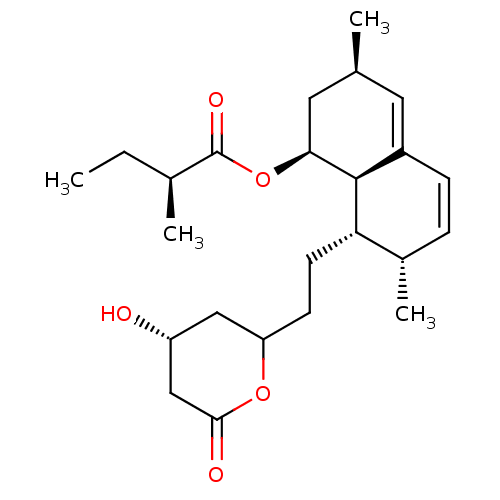 ((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Tested for inhibition of rat liver microsomal HMG-CoA reductase | J Med Chem 37: 3240-6 (1994) BindingDB Entry DOI: 10.7270/Q25H7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142068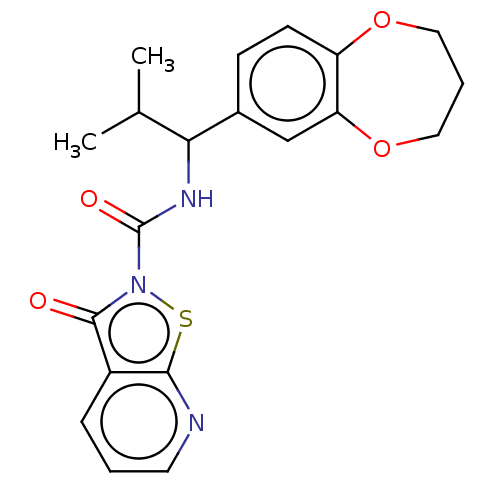 (US8933024, 46) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142060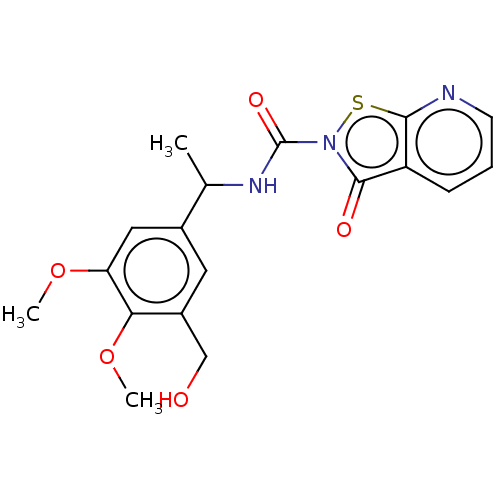 (US8933024, 4) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142062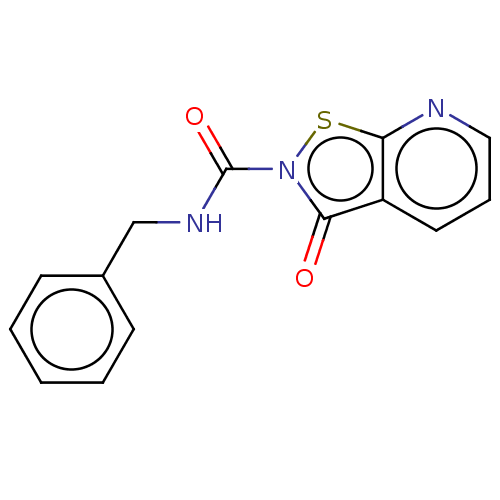 (US8933024, 18) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142080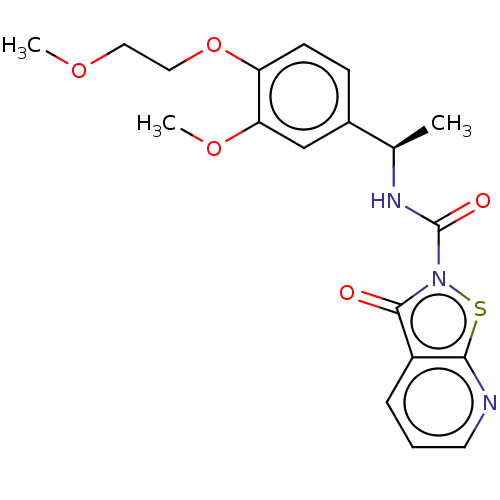 (US8933024, 161) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142076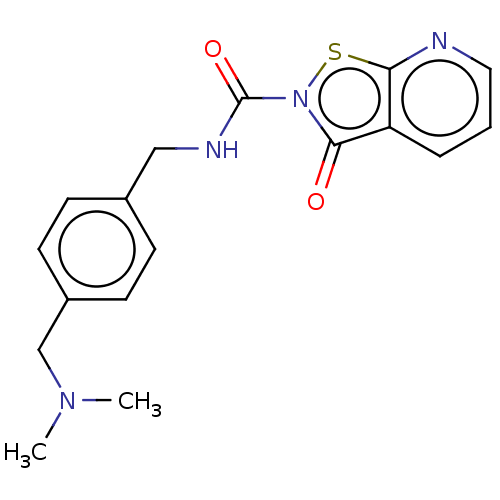 (US8933024, 130) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142063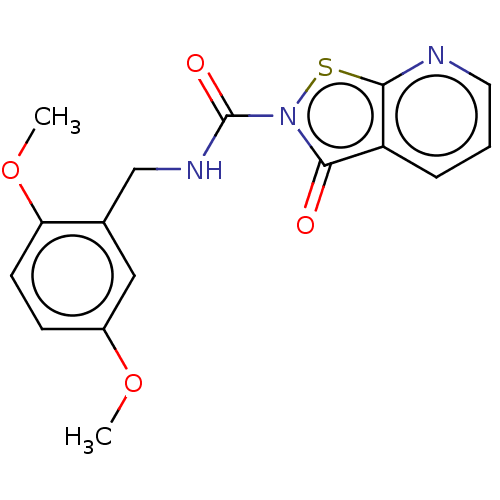 (US8933024, 28) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142070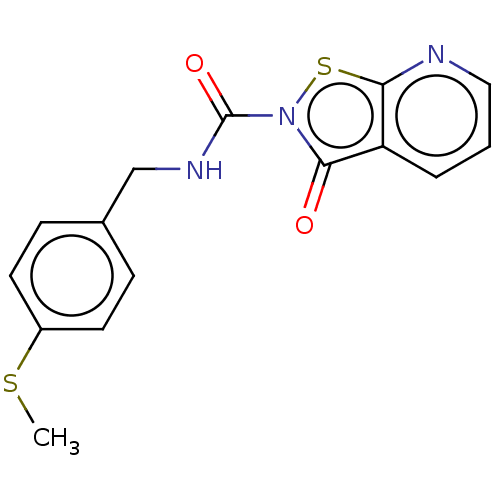 (US8933024, 55) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142067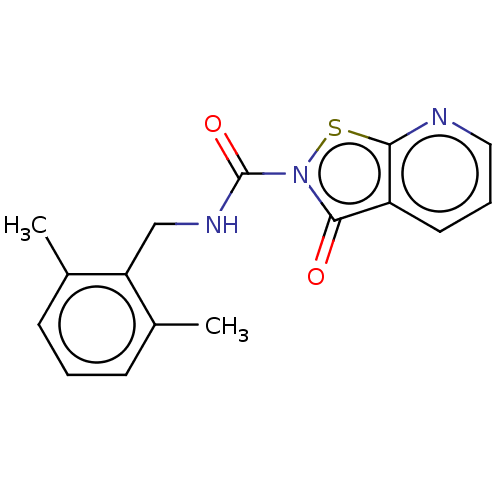 (US8933024, 39) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142069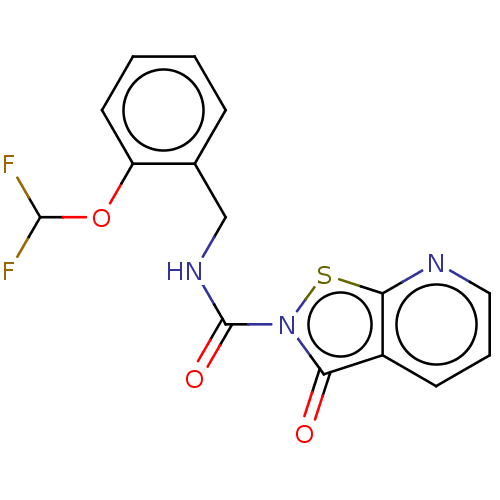 (US8933024, 50) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142065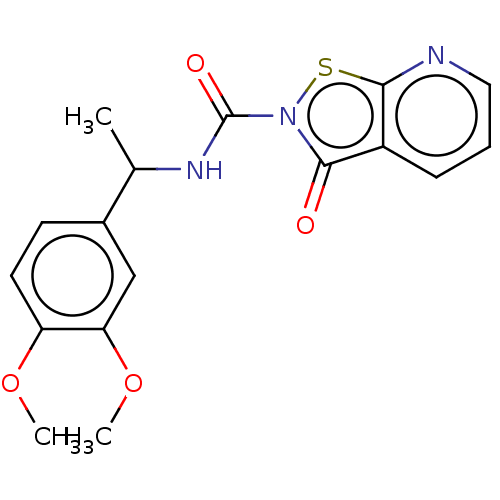 (US8933024, 37) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142066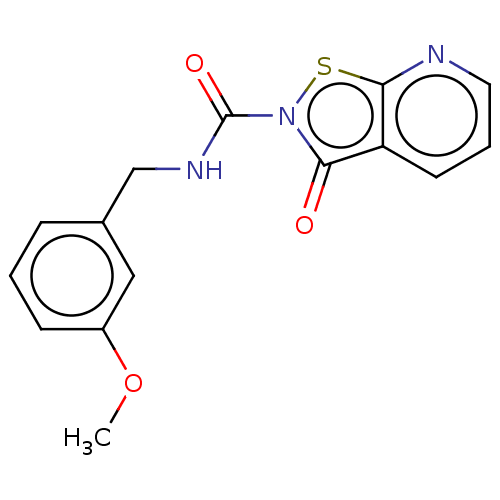 (US8933024, 38) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142061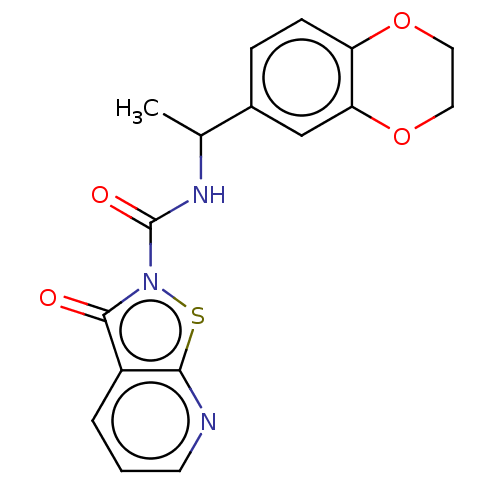 (US8933024, 5) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50037255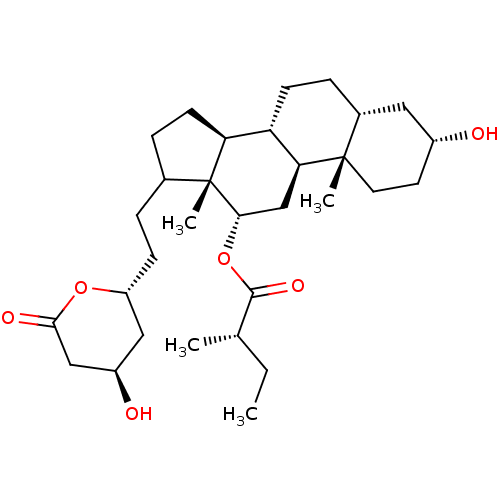 ((S)-2-Methyl-butyric acid (3R,5R,8R,9S,10S,12S,13R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Tested for inhibition of rat liver microsomal HMG-CoA reductase | J Med Chem 37: 3240-6 (1994) BindingDB Entry DOI: 10.7270/Q25H7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142072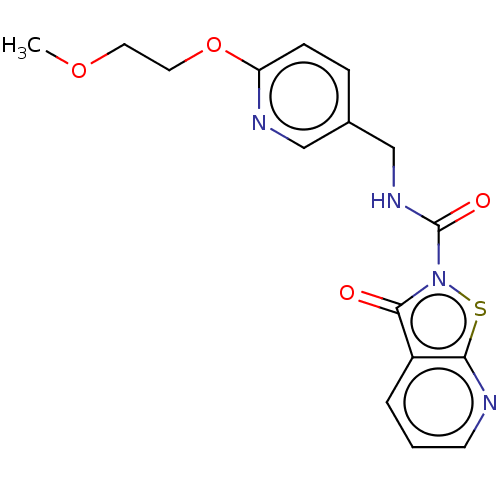 (US8933024, 114) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142075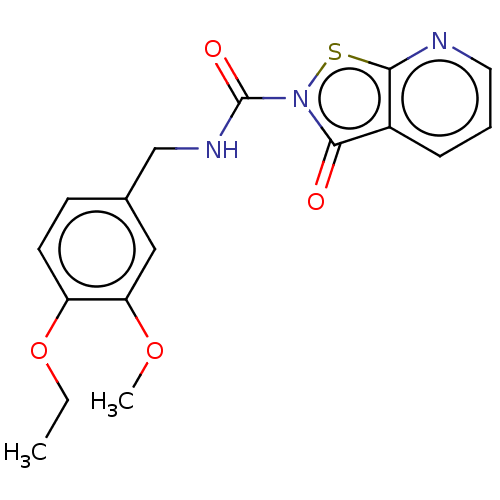 (US8933024, 129) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142071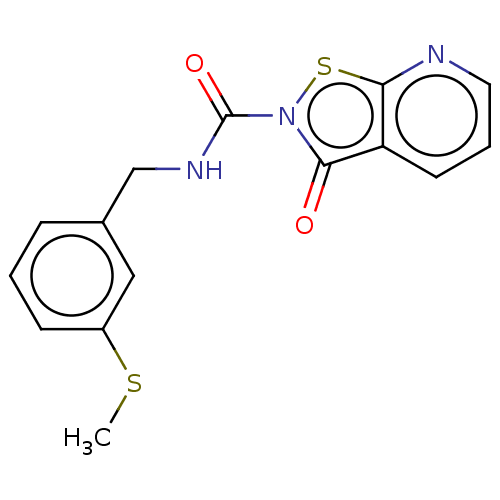 (US8933024, 59) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 264 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142077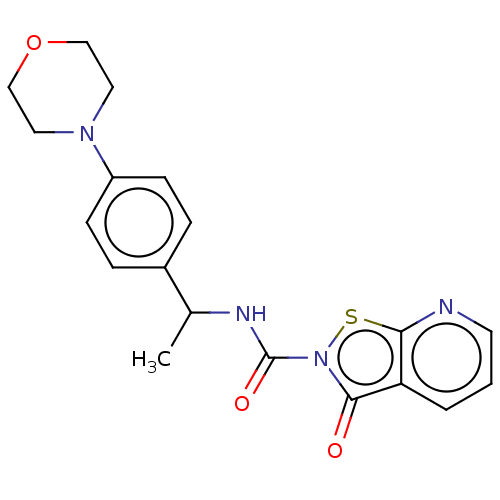 (US8933024, 134) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 391 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142078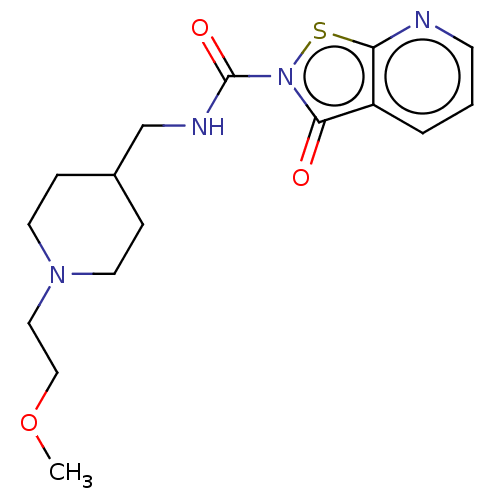 (US8933024, 142) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50037257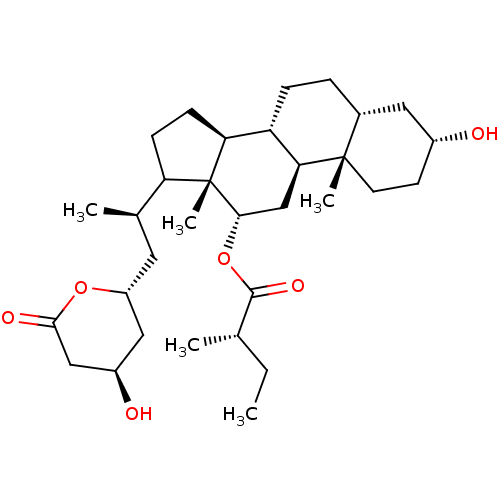 ((S)-2-Methyl-butyric acid (3R,5R,8R,9S,10S,12S,13R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Tested for inhibition of rat liver microsomal HMG-CoA reductase | J Med Chem 37: 3240-6 (1994) BindingDB Entry DOI: 10.7270/Q25H7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142059 (US8933024, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 926 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50037254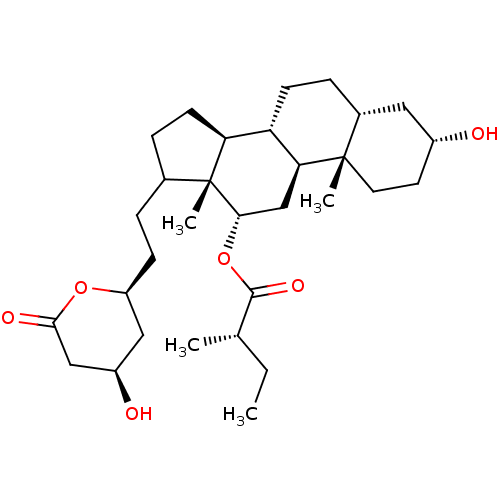 ((S)-2-Methyl-butyric acid (3R,5R,8R,9S,10S,12S,13R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Tested for inhibition of rat liver microsomal HMG-CoA reductase | J Med Chem 37: 3240-6 (1994) BindingDB Entry DOI: 10.7270/Q25H7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142073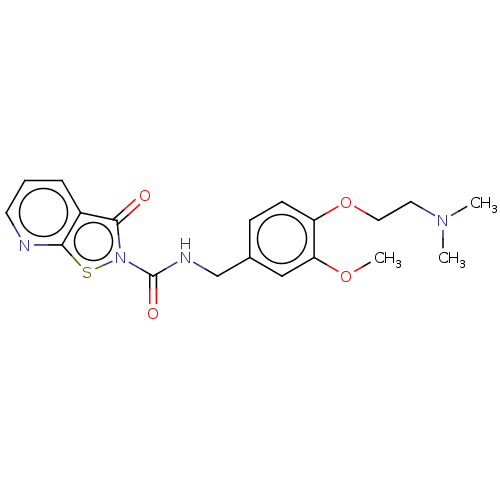 (US8933024, 115) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142079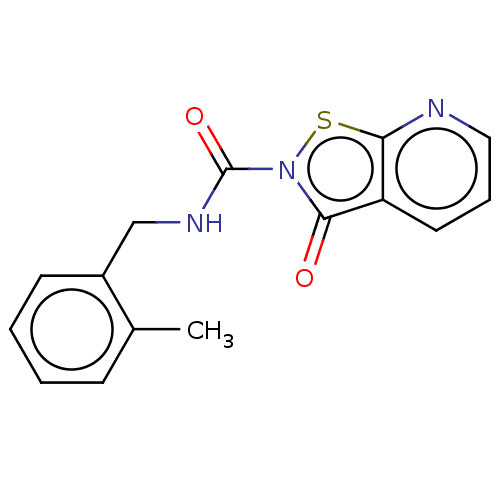 (US8933024, 156) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142074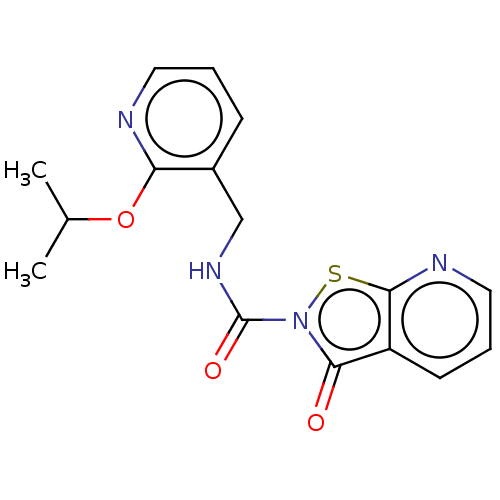 (US8933024, 116) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM142064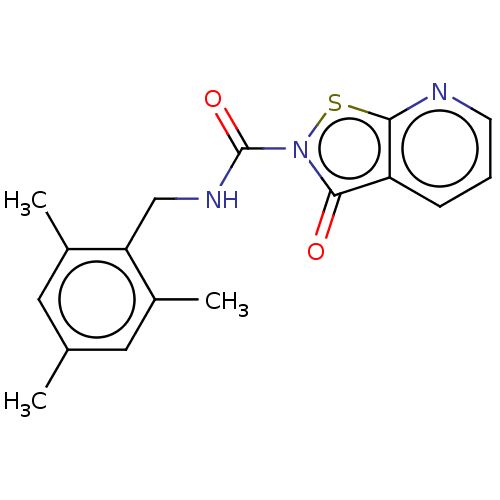 (US8933024, 29) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description For characterization of the enzymatic activity of endothelial lipase and the effect of inhibitors, the phospholipase-specific substrate 1,2-bis(4,4-d... | US Patent US8933024 (2015) BindingDB Entry DOI: 10.7270/Q228069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50037256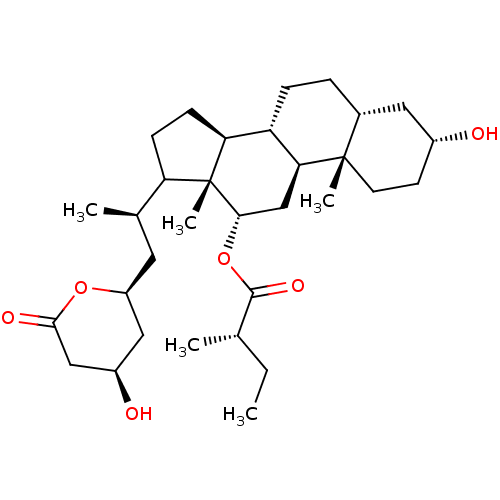 ((S)-2-Methyl-butyric acid (3R,5R,8R,9S,10S,12S,13R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst AG Curated by ChEMBL | Assay Description Tested for inhibition of rat liver microsomal HMG-CoA reductase | J Med Chem 37: 3240-6 (1994) BindingDB Entry DOI: 10.7270/Q25H7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||