

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.800 | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591671 (CHEMBL5191603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591673 (CHEMBL5198400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591672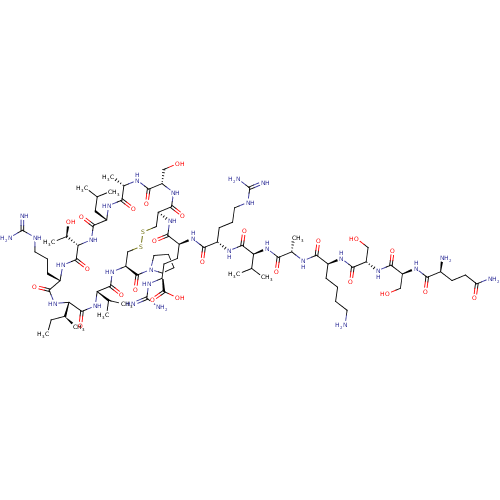 (CHEMBL5184798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591674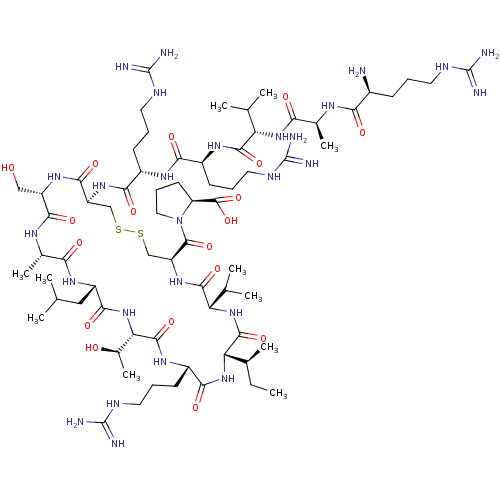 (CHEMBL5173140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178102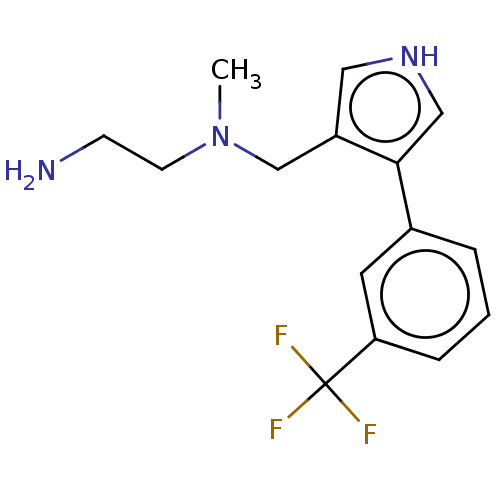 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082611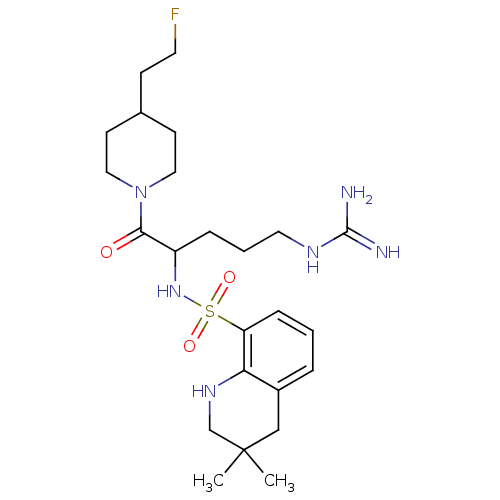 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082575 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090249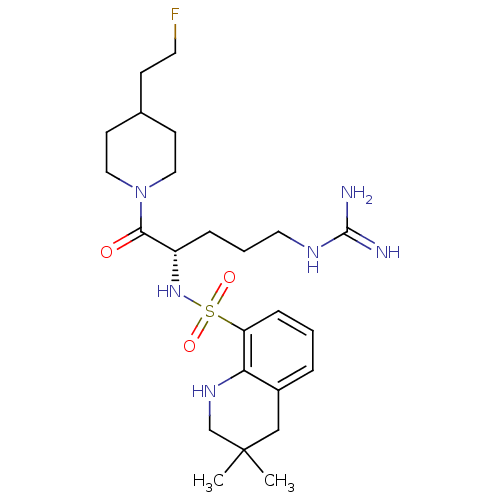 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082578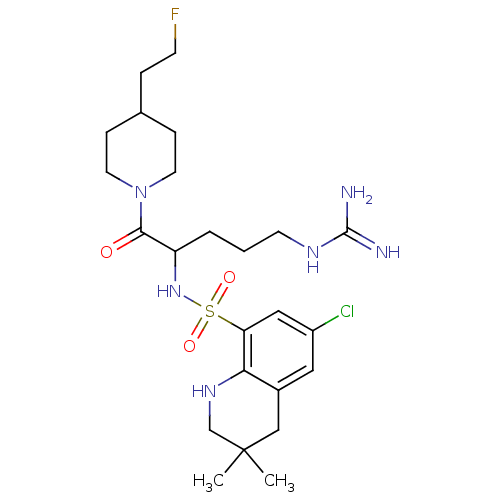 (6-Chloro-3,3-dimethyl-1,2,3,4-tetrahydro-quinoline...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591676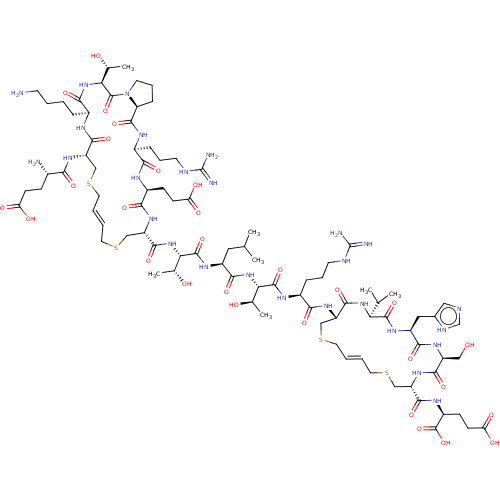 (CHEMBL5202369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 11 | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50082589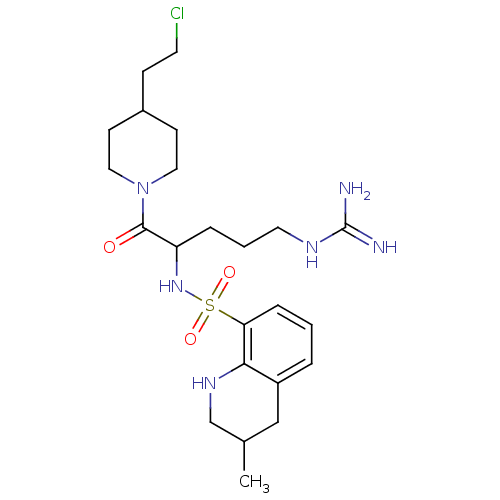 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description The compound was evaluated for the inhibitory activity against human thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082579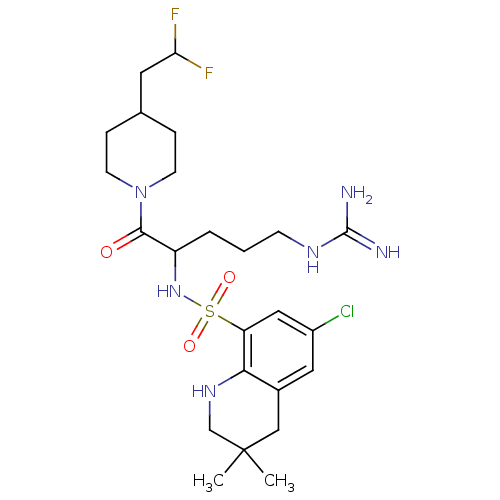 (6-Chloro-3,3-dimethyl-1,2,3,4-tetrahydro-quinoline...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082583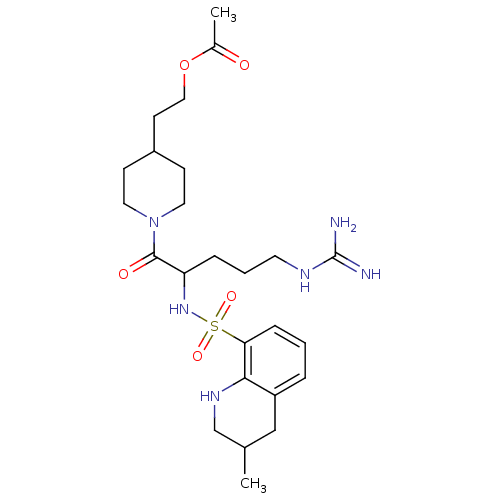 (Acetic acid 2-{1-[5-guanidino-2-(3-methyl-1,2,3,4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073283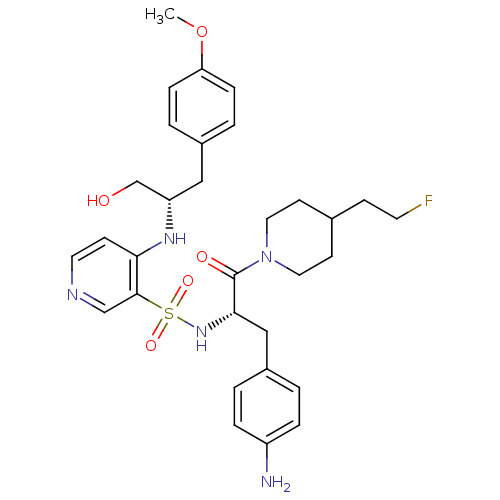 (4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082589 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073297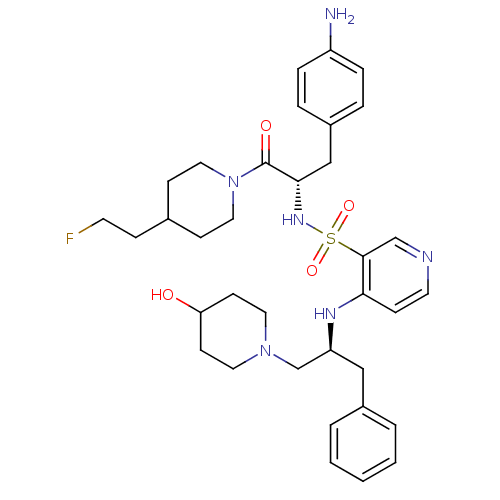 (4-[(S)-1-(4-Hydroxy-piperidin-1-ylmethyl)-2-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073283 (4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073298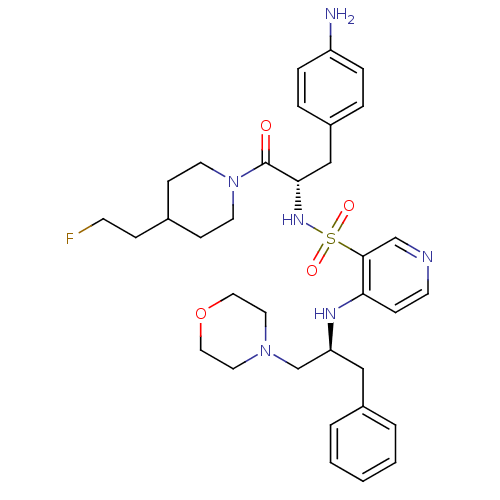 (4-((S)-1-Benzyl-2-morpholin-4-yl-ethylamino)-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073298 (4-((S)-1-Benzyl-2-morpholin-4-yl-ethylamino)-pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 23 | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082598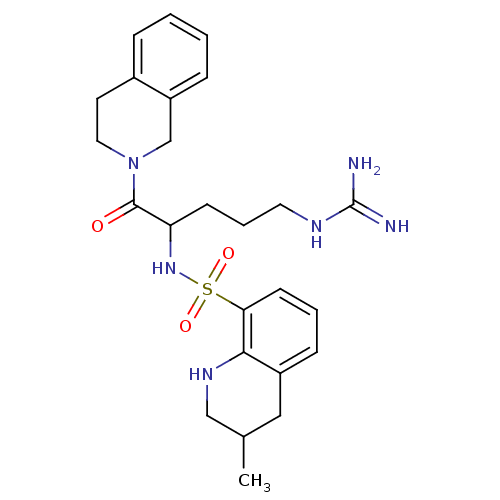 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082577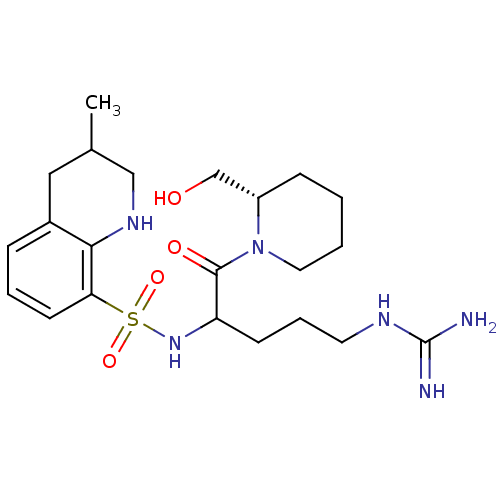 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073294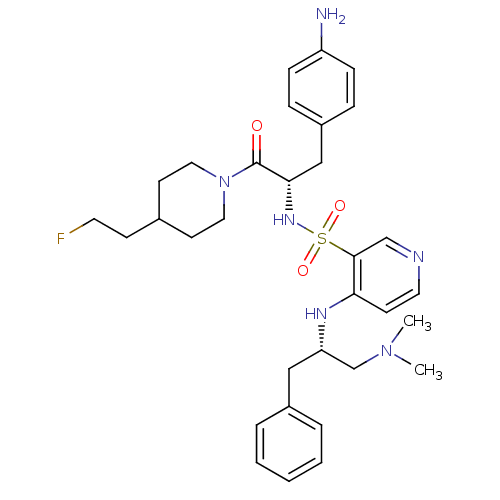 (4-((S)-1-Benzyl-2-dimethylamino-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50077058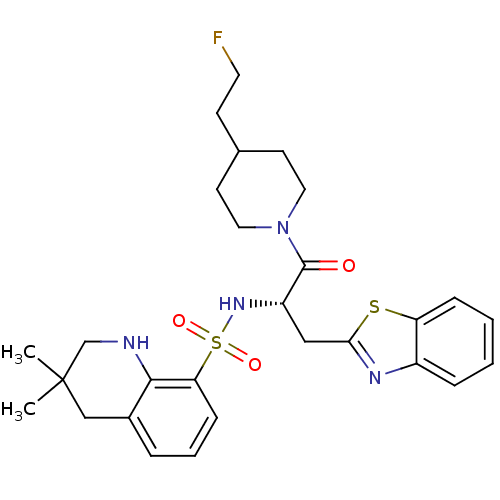 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082612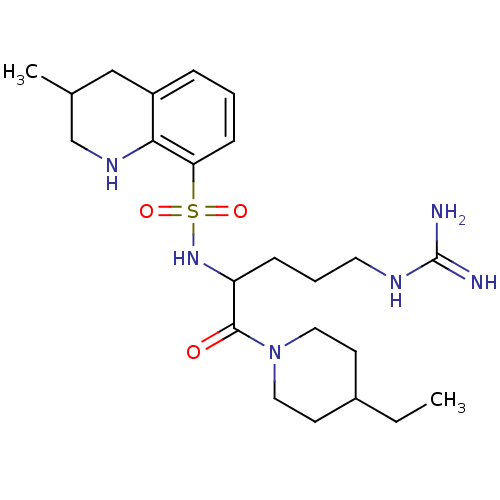 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090244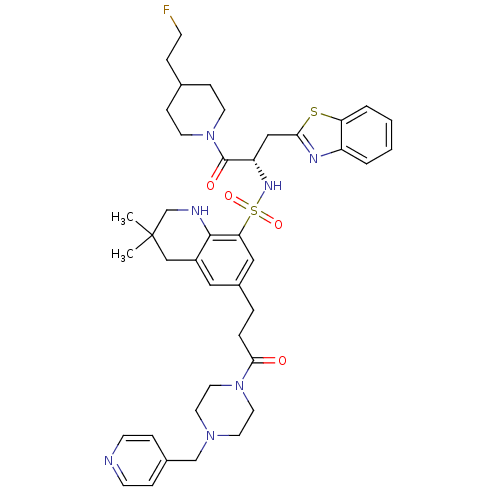 (3,3-Dimethyl-6-[3-oxo-3-(4-pyridin-4-ylmethyl-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075870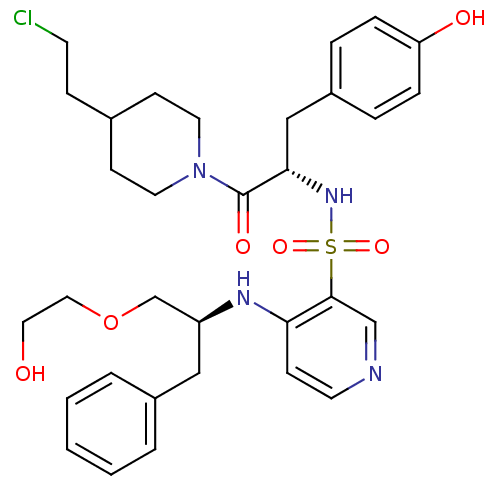 (4-[(S)-1-(2-Hydroxy-ethoxymethyl)-2-phenyl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50591678 (CHEMBL5174448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00056 BindingDB Entry DOI: 10.7270/Q26977J9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082580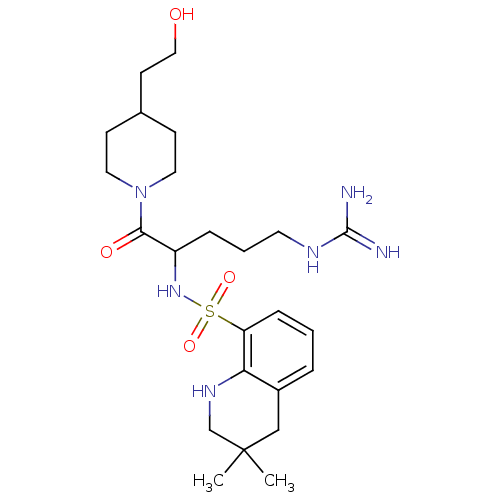 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description The compound was evaluated for the inhibitory activity against human thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090250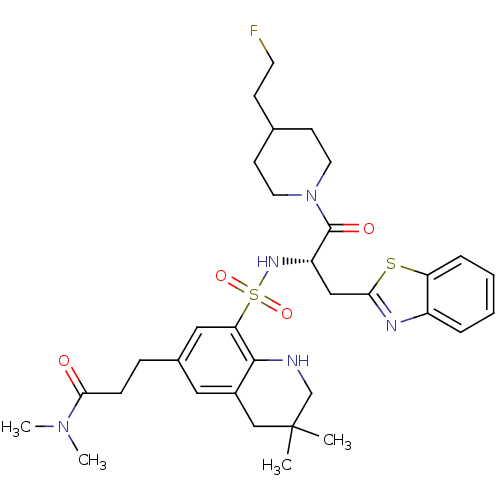 (3-(8-{(S)-1-Benzothiazol-2-ylmethyl-2-[4-(2-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090247 (6-[3-(4-Acetyl-piperazin-1-yl)-3-oxo-propyl]-3,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082608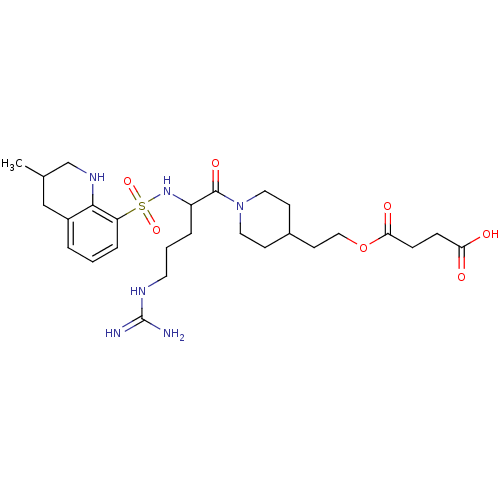 (CHEMBL139718 | Succinic acid mono-(2-{1-[5-guanidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090243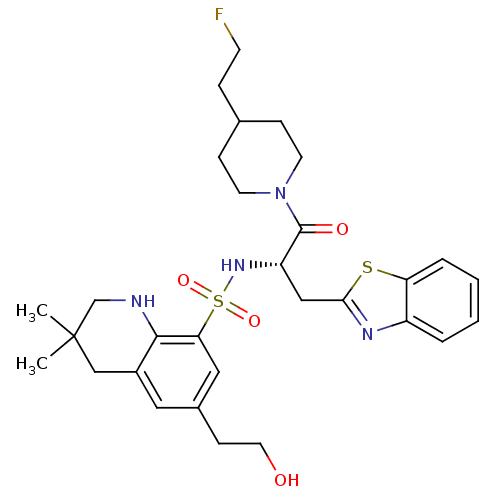 (6-(2-Hydroxy-ethyl)-3,3-dimethyl-1,2,3,4-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073295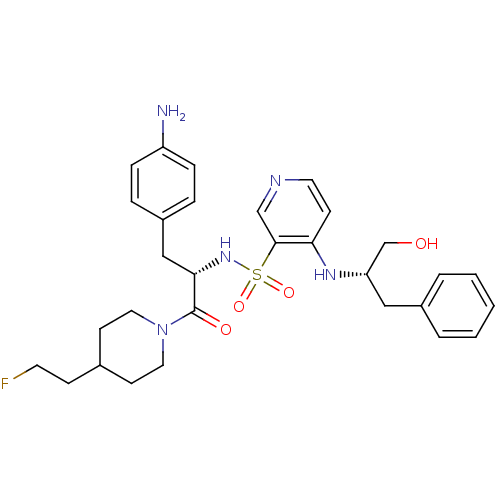 (4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073295 (4-((S)-1-Hydroxymethyl-2-phenyl-ethylamino)-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075873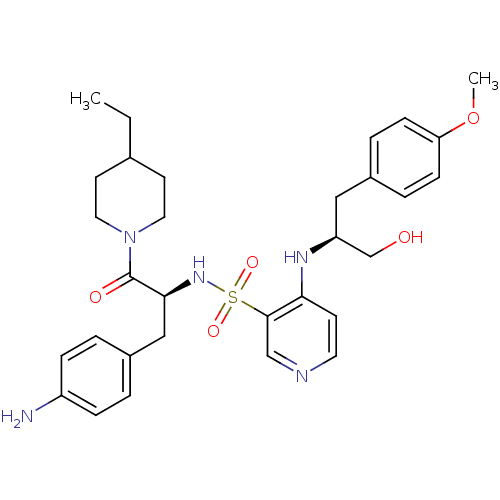 (4-[(S)-1-Hydroxymethyl-2-(4-methoxy-phenyl)-ethyla...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082581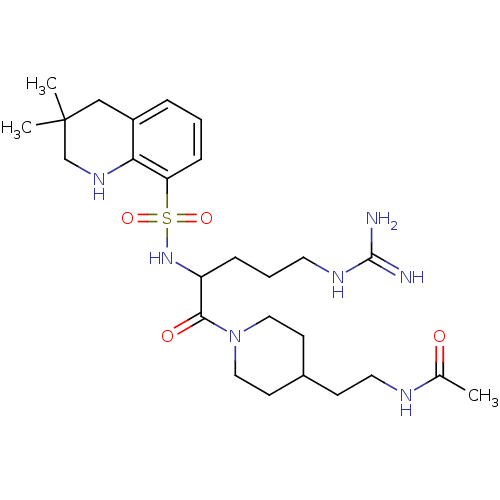 (CHEMBL143203 | N-(2-{1-[2-(3,3-Dimethyl-1,2,3,4-te...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082573 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description The compound was evaluated for the inhibitory activity against human thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082591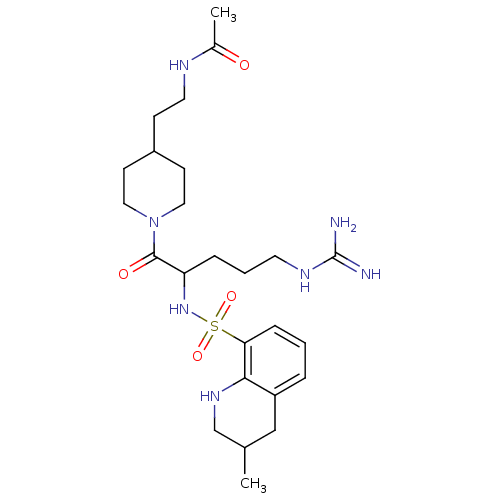 (CHEMBL139606 | N-(2-{1-[5-Guanidino-2-(3-methyl-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082603 ((R)-3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090246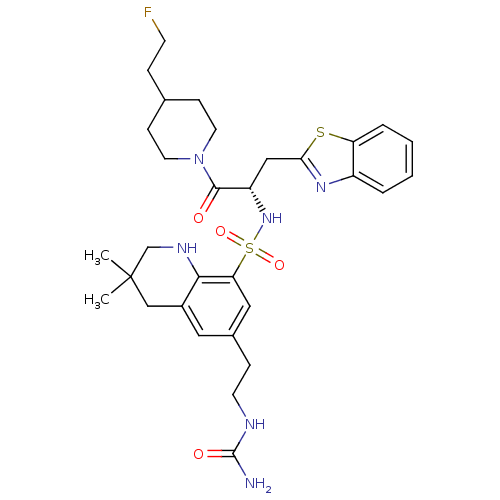 (3,3-Dimethyl-6-(2-ureido-ethyl)-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Evaluated for the binding affinity against thrombin | Bioorg Med Chem Lett 10: 1567-70 (2000) BindingDB Entry DOI: 10.7270/Q2RX9BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082580 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073292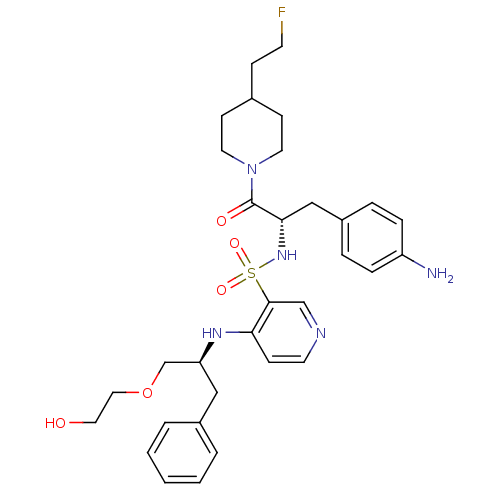 (4-[(S)-1-(2-Hydroxy-ethoxymethyl)-2-phenyl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073292 (4-[(S)-1-(2-Hydroxy-ethoxymethyl)-2-phenyl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center Curated by ChEMBL | Assay Description In vitro inhibtion of human thrombin. | Bioorg Med Chem Lett 9: 737-42 (1999) BindingDB Entry DOI: 10.7270/Q2BG2N6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082594 (CHEMBL356067 | N-[1-(3,4-Dihydro-1H-isoquinoline-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50082573 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Inhibitory activity against bovine thrombin | J Med Chem 42: 4584-603 (1999) BindingDB Entry DOI: 10.7270/Q28G8MDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 481 total ) | Next | Last >> |