 Found 14926 hits with Last Name = 'chen' and Initial = 'k'
Found 14926 hits with Last Name = 'chen' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50147818
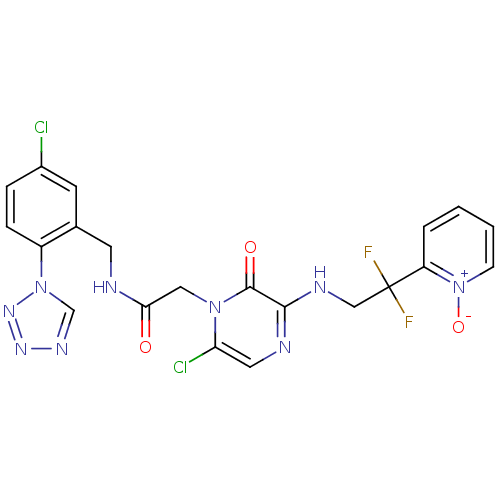
((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147818

((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147824
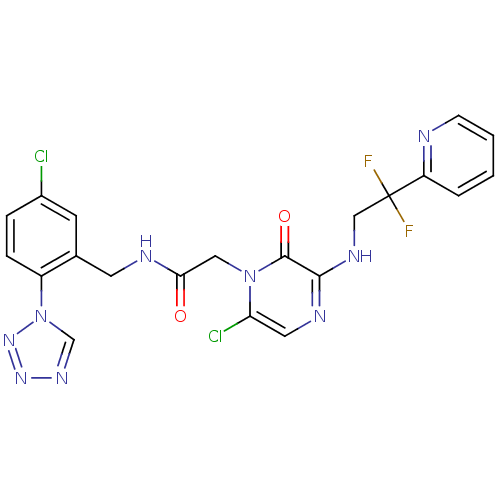
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147824

(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457933

(CHEMBL327265)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O Show InChI InChI=1S/C22H18Cl2F2N8O3/c23-15-4-5-16(33-13-27-12-31-33)14(7-15)8-28-19(35)10-32-18(24)9-29-20(21(32)36)30-11-22(25,26)17-3-1-2-6-34(17)37/h1-7,9,12-13H,8,10-11H2,(H,28,35)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457929
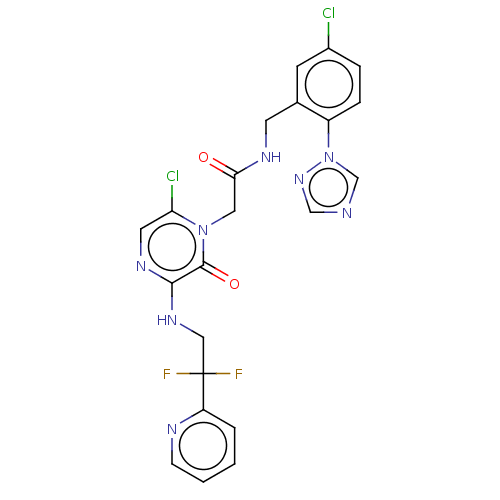
(CHEMBL104951)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O)c1ccccn1 Show InChI InChI=1S/C22H18Cl2F2N8O2/c23-15-4-5-16(34-13-27-12-32-34)14(7-15)8-29-19(35)10-33-18(24)9-30-20(21(33)36)31-11-22(25,26)17-3-1-2-6-28-17/h1-7,9,12-13H,8,10-11H2,(H,29,35)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457933

(CHEMBL327265)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O Show InChI InChI=1S/C22H18Cl2F2N8O3/c23-15-4-5-16(33-13-27-12-31-33)14(7-15)8-28-19(35)10-32-18(24)9-29-20(21(32)36)30-11-22(25,26)17-3-1-2-6-34(17)37/h1-7,9,12-13H,8,10-11H2,(H,28,35)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50457929

(CHEMBL104951)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cncn2)c1=O)c1ccccn1 Show InChI InChI=1S/C22H18Cl2F2N8O2/c23-15-4-5-16(34-13-27-12-32-34)14(7-15)8-29-19(35)10-33-18(24)9-30-20(21(33)36)31-11-22(25,26)17-3-1-2-6-28-17/h1-7,9,12-13H,8,10-11H2,(H,29,35)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM85357
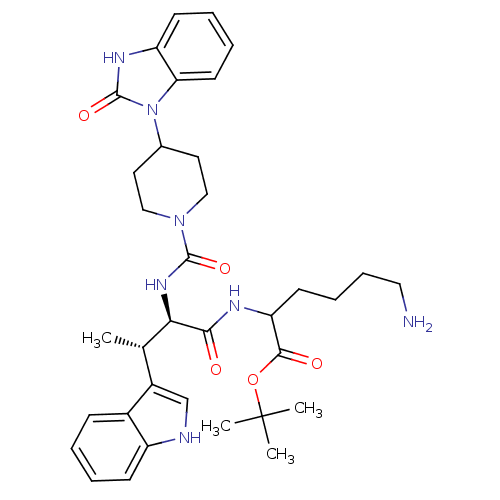
(2-[[(2R,3S)-2-[[4-[(2-Oxo-2,3-dihydro-1H-benzimida...)Show SMILES C[C@H]([C@@H](NC(=O)N1CCC(CC1)n1c2ccccc2[nH]c1=O)C(=O)NC(CCCCN)C(=O)OC(C)(C)C)c1c[nH]c2ccccc12 Show InChI InChI=1S/C35H47N7O5/c1-22(25-21-37-26-12-6-5-11-24(25)26)30(31(43)38-28(14-9-10-18-36)32(44)47-35(2,3)4)40-33(45)41-19-16-23(17-20-41)42-29-15-8-7-13-27(29)39-34(42)46/h5-8,11-13,15,21-23,28,30,37H,9-10,14,16-20,36H2,1-4H3,(H,38,43)(H,39,46)(H,40,45)/t22-,28?,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using S-2765 as substrate after 30 mins by spectrophotometric method |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50375695

(CHEMBL270984)Show SMILES Cc1cc(O)cc(O)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C43H49N5O9/c1-27-20-32(49)23-38(50)33(27)24-34(44)41(53)48-25-31-17-9-8-16-30(31)22-37(48)40(52)47-36(21-28-12-4-2-5-13-28)39(51)46-35(42(54)55)18-10-11-19-45-43(56)57-26-29-14-6-3-7-15-29/h2-9,12-17,20,23,34-37,49-50H,10-11,18-19,21-22,24-26,44H2,1H3,(H,45,56)(H,46,51)(H,47,52)(H,54,55)/t34-,35-,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane |
J Med Chem 51: 1817-23 (2008)
Article DOI: 10.1021/jm7014765
BindingDB Entry DOI: 10.7270/Q24T6K7K |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50258507

(CHEMBL4078345)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C30H32N2O4/c1-23(33)17-19-27(20-18-24-11-5-2-6-12-24)31-29(34)28(21-25-13-7-3-8-14-25)32-30(35)36-22-26-15-9-4-10-16-26/h2-17,19,27-28H,18,20-22H2,1H3,(H,31,34)(H,32,35)/b19-17+/t27-,28+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... |
J Med Chem 60: 6911-6923 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00405
BindingDB Entry DOI: 10.7270/Q2FJ2K73 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50064772
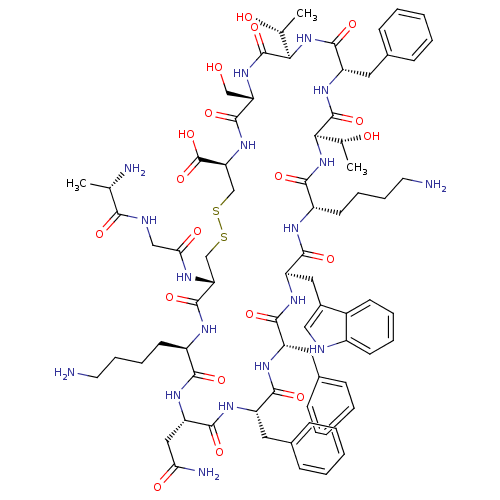
(Ala-Gly-cyclo[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52-,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human sst2 receptor expressed in CHO-K1 cells |
J Med Chem 41: 2175-9 (1998)
Article DOI: 10.1021/jm980194h
BindingDB Entry DOI: 10.7270/Q2XW4KGS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
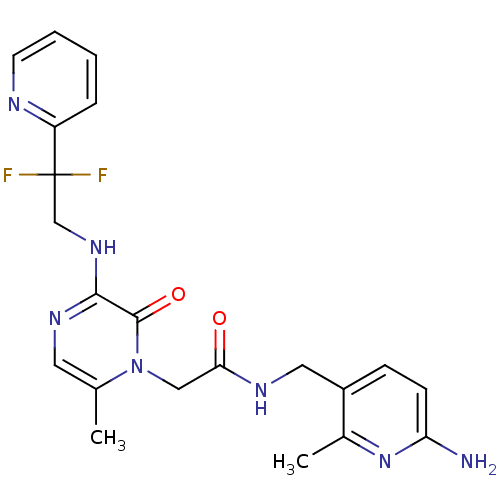
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hefei University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
Eur J Med Chem 146: 299-317 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.067
BindingDB Entry DOI: 10.7270/Q2251MTJ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81766
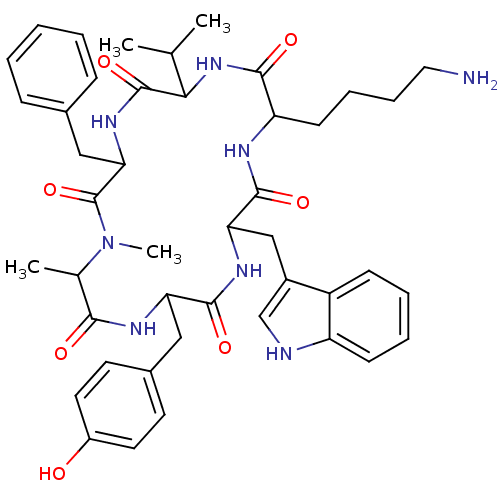
(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099273
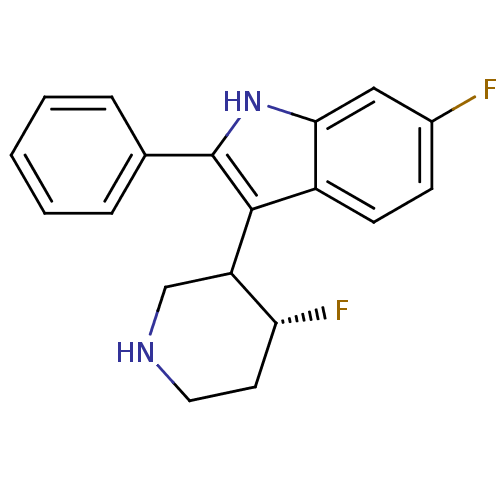
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370582
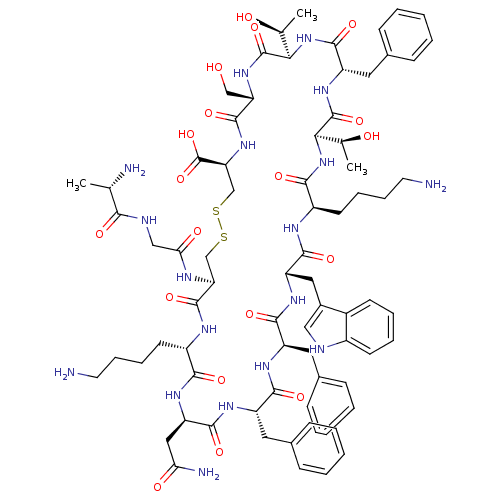
(CHEMBL1791306)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42-,43-,50-,51+,52-,53+,54-,55-,56+,57-,58-,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50258514
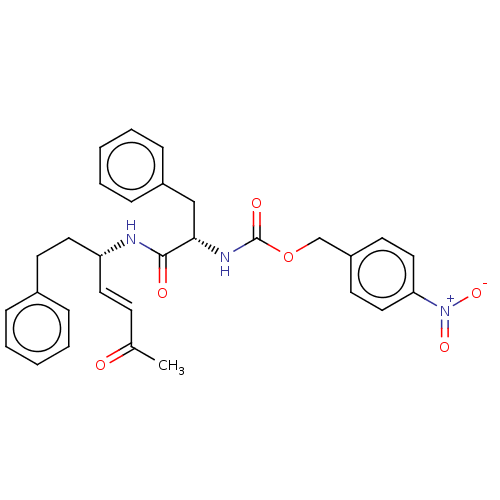
(CHEMBL4062015)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C30H31N3O6/c1-22(34)12-16-26(17-13-23-8-4-2-5-9-23)31-29(35)28(20-24-10-6-3-7-11-24)32-30(36)39-21-25-14-18-27(19-15-25)33(37)38/h2-12,14-16,18-19,26,28H,13,17,20-21H2,1H3,(H,31,35)(H,32,36)/b16-12+/t26-,28+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris using Cbz-Phe-Arg-AMC as substrate after 30 min by fl... |
J Med Chem 60: 6911-6923 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00405
BindingDB Entry DOI: 10.7270/Q2FJ2K73 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328391
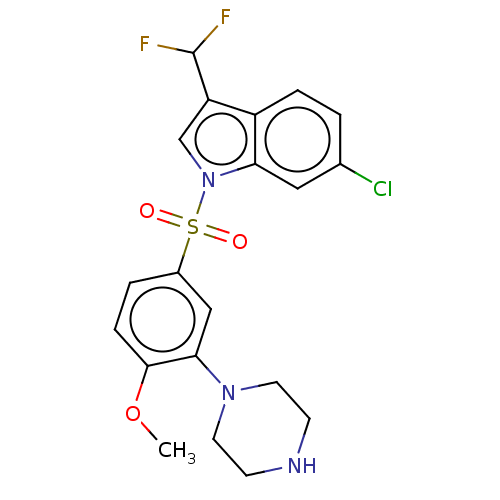
(6-chloro-3-(difluoromethyl)-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(Cl)cc12 Show InChI InChI=1S/C20H20ClF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50095027
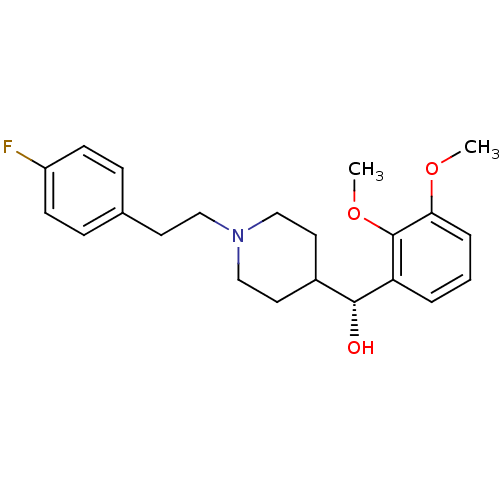
((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...)Show SMILES COc1cccc([C@H](O)C2CCN(CCc3ccc(F)cc3)CC2)c1OC |r| Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50375694
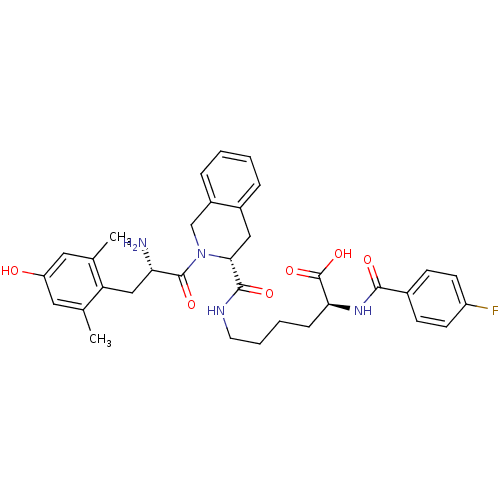
(CHEMBL244265)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(O)=O Show InChI InChI=1S/C34H39FN4O6/c1-20-15-26(40)16-21(2)27(20)18-28(36)33(43)39-19-24-8-4-3-7-23(24)17-30(39)32(42)37-14-6-5-9-29(34(44)45)38-31(41)22-10-12-25(35)13-11-22/h3-4,7-8,10-13,15-16,28-30,40H,5-6,9,14,17-19,36H2,1-2H3,(H,37,42)(H,38,41)(H,44,45)/t28-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane |
J Med Chem 51: 1817-23 (2008)
Article DOI: 10.1021/jm7014765
BindingDB Entry DOI: 10.7270/Q24T6K7K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592784
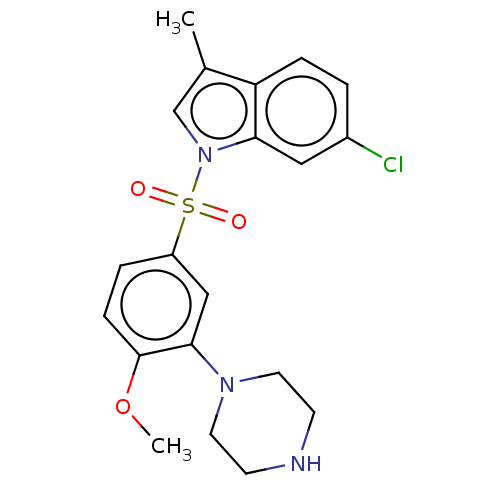
(CHEMBL5177311)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Cl)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108690
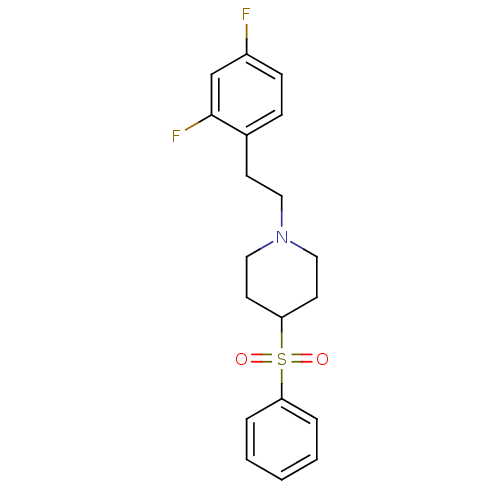
(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50217956

((1R,5S)-(+)-5-(3-hydroxyphenyl)-9-methylene-2-phen...)Show SMILES Oc1cccc(c1)[C@]12CCC[C@@H](N(CCc3ccccc3)CC1)C2=C |TLB:24:23:8.9.10:12.21.22| Show InChI InChI=1S/C23H27NO/c1-18-22-11-6-13-23(18,20-9-5-10-21(25)17-20)14-16-24(22)15-12-19-7-3-2-4-8-19/h2-5,7-10,17,22,25H,1,6,11-16H2/t22-,23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells |
J Med Chem 50: 3765-76 (2007)
Article DOI: 10.1021/jm061325e
BindingDB Entry DOI: 10.7270/Q2D21XB2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50217952
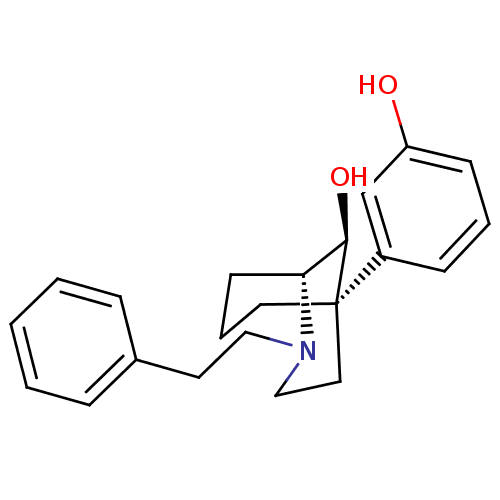
((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...)Show SMILES O[C@@H]1[C@H]2CCC[C@@]1(CCN2CCc1ccccc1)c1cccc(O)c1 |TLB:10:9:5.4.3:1| Show InChI InChI=1S/C22H27NO2/c24-19-9-4-8-18(16-19)22-12-5-10-20(21(22)25)23(15-13-22)14-11-17-6-2-1-3-7-17/h1-4,6-9,16,20-21,24-25H,5,10-15H2/t20-,21-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells |
J Med Chem 50: 3765-76 (2007)
Article DOI: 10.1021/jm061325e
BindingDB Entry DOI: 10.7270/Q2D21XB2 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19968
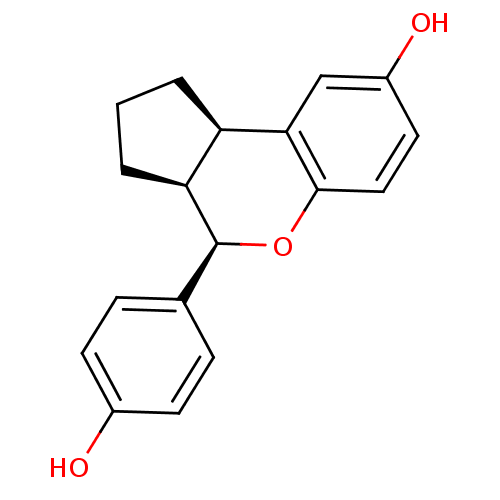
((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...)Show SMILES Oc1ccc(cc1)[C@@H]1Oc2ccc(O)cc2[C@@H]2CCC[C@H]12 |r| Show InChI InChI=1S/C18H18O3/c19-12-6-4-11(5-7-12)18-15-3-1-2-14(15)16-10-13(20)8-9-17(16)21-18/h4-10,14-15,18-20H,1-3H2/t14-,15+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.190 | -54.9 | n/a | n/a | 0.660 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories
| Assay Description
The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... |
J Med Chem 49: 6155-7 (2006)
Article DOI: 10.1021/jm060491j
BindingDB Entry DOI: 10.7270/Q2N014SV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50546246
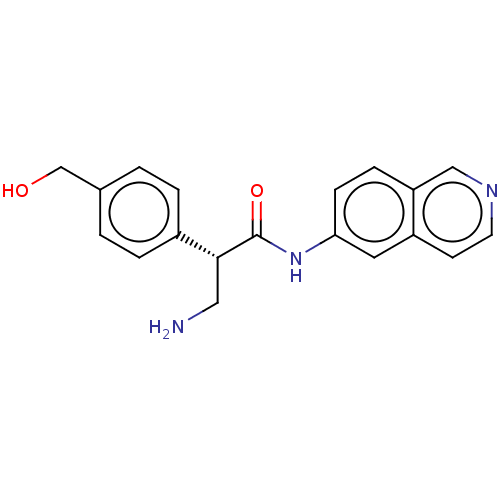
(CHEMBL4753043 | US11608319, Compound AR-13503)Show SMILES NC[C@@H](C(=O)Nc1ccc2cnccc2c1)c1ccc(CO)cc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ROCK2 expressed in baculovirus expression system by Kinase-Glo luminescent Assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b01033
BindingDB Entry DOI: 10.7270/Q2ZK5M83 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50546246

(CHEMBL4753043 | US11608319, Compound AR-13503)Show SMILES NC[C@@H](C(=O)Nc1ccc2cnccc2c1)c1ccc(CO)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ROCK1 expressed in baculovirus expression system by Kinase-Glo luminescent Assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b01033
BindingDB Entry DOI: 10.7270/Q2ZK5M83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
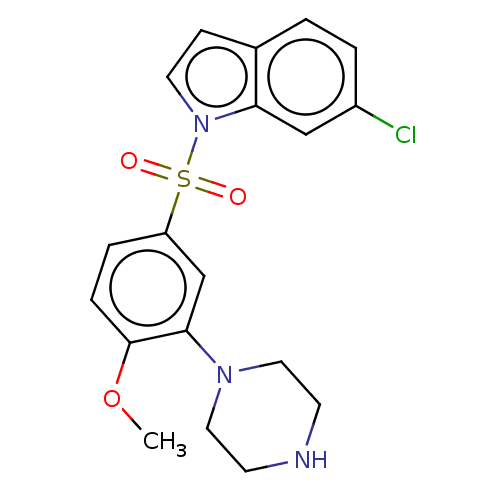
(Homo sapiens (Human)) | BDBM328379
(6-chloro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(Cl)cc12 Show InChI InChI=1S/C19H20ClN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
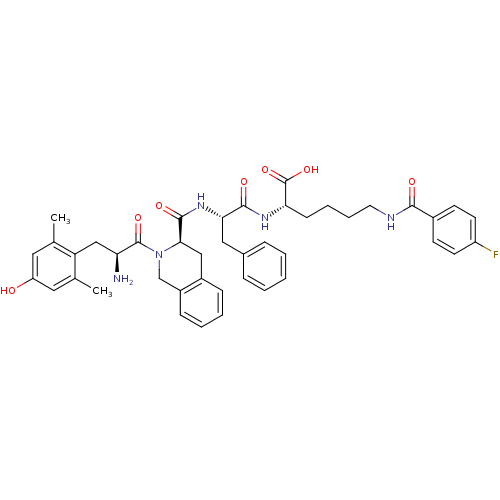
(Rattus norvegicus (rat)) | BDBM50375696
(CHEMBL269842)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCNC(=O)c1ccc(F)cc1)C(O)=O Show InChI InChI=1S/C43H48FN5O7/c1-26-20-33(50)21-27(2)34(26)24-35(45)42(54)49-25-31-13-7-6-12-30(31)23-38(49)41(53)48-37(22-28-10-4-3-5-11-28)40(52)47-36(43(55)56)14-8-9-19-46-39(51)29-15-17-32(44)18-16-29/h3-7,10-13,15-18,20-21,35-38,50H,8-9,14,19,22-25,45H2,1-2H3,(H,46,51)(H,47,52)(H,48,53)(H,55,56)/t35-,36-,37-,38+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane |
J Med Chem 51: 1817-23 (2008)
Article DOI: 10.1021/jm7014765
BindingDB Entry DOI: 10.7270/Q24T6K7K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108689
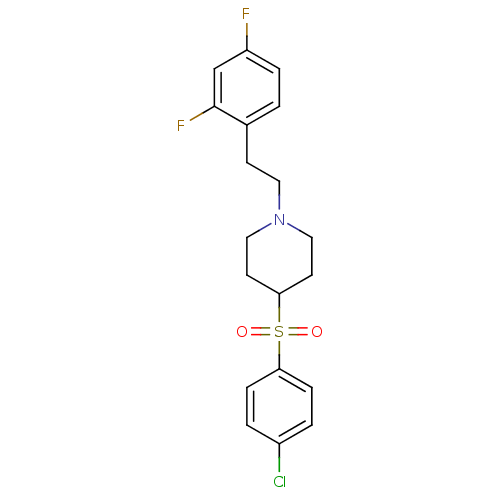
(4-(4-Chloro-benzenesulfonyl)-1-[2-(2,4-difluoro-ph...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C19H20ClF2NO2S/c20-15-2-5-17(6-3-15)26(24,25)18-8-11-23(12-9-18)10-7-14-1-4-16(21)13-19(14)22/h1-6,13,18H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130285
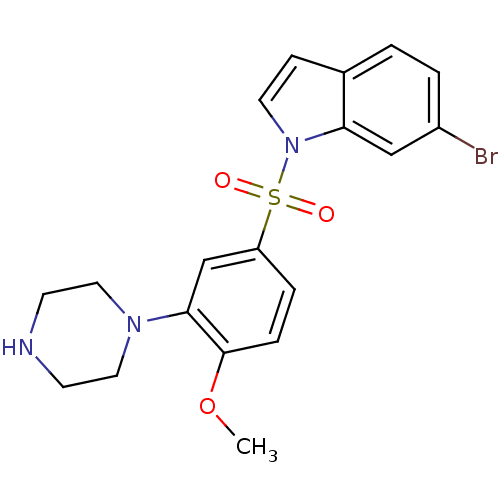
(6-Bromo-1-(4-methoxy-3-piperazin-1-yl-benzenesulfo...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1ccc2ccc(Br)cc12 Show InChI InChI=1S/C19H20BrN3O3S/c1-26-19-5-4-16(13-18(19)22-10-7-21-8-11-22)27(24,25)23-9-6-14-2-3-15(20)12-17(14)23/h2-6,9,12-13,21H,7-8,10-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328389

(3-(difluoromethyl)-6-fluoro-1-((4-methoxy-3-(piper...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(F)cc12 Show InChI InChI=1S/C20H20F3N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50592785
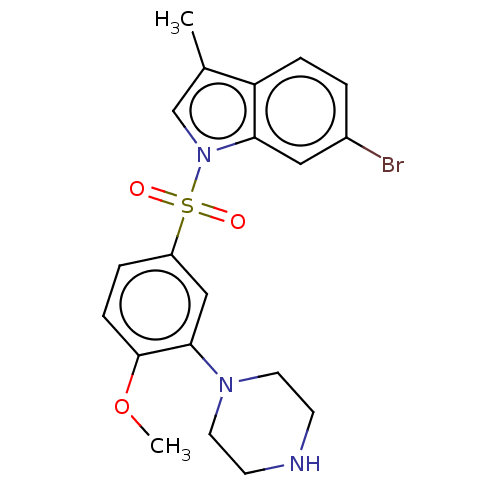
(CHEMBL5206617)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C)c2ccc(Br)cc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50059090
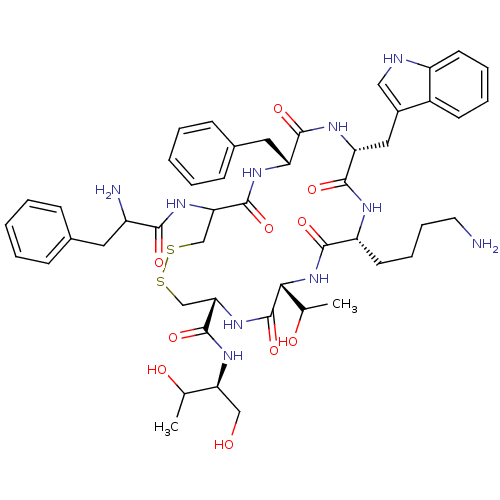
(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES CC(O)[C@H](CO)NC(=O)[C@@H]1CSSCC(NC(=O)C(N)Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C(=O)N[C@H](C(C)O)C(=O)N1 Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28?,29?,34?,36-,37-,38-,39+,40?,41+,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50258880
(8-(dio-tolylmethyl)-3-phenyl-8-azabicyclo[3.2.1]oc...)Show SMILES Cc1ccccc1C(N1C2CCC1CC(O)(C2)c1ccccc1)c1ccccc1C |THB:15:14:8:10.11| Show InChI InChI=1S/C28H31NO/c1-20-10-6-8-14-25(20)27(26-15-9-7-11-21(26)2)29-23-16-17-24(29)19-28(30,18-23)22-12-4-3-5-13-22/h3-15,23-24,27,30H,16-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human cloned NOP receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2519-23 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.031
BindingDB Entry DOI: 10.7270/Q20V8CPS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50095027

((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...)Show SMILES COc1cccc([C@H](O)C2CCN(CCc3ccc(F)cc3)CC2)c1OC |r| Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM328393

(3-(difluoromethyl)-6-bromo-1-((4-methoxy-3-(pipera...)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)n1cc(C(F)F)c2ccc(Br)cc12 Show InChI InChI=1S/C20H20BrF2N3O3S/c1-29-19-5-3-14(11-18(19)25-8-6-24-7-9-25)30(27,28)26-12-16(20(22)23)15-4-2-13(21)10-17(15)26/h2-5,10-12,20,24H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116917
BindingDB Entry DOI: 10.7270/Q21C21V3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152234

(CHEMBL184061 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES Nc1nc(NCCN2CCN(CC2)c2ccc(F)cc2F)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C20H21F2N9O/c21-13-3-4-15(14(22)12-13)30-9-7-29(8-10-30)6-5-24-19-26-18(23)31-20(27-19)25-17(28-31)16-2-1-11-32-16/h1-4,11-12H,5-10H2,(H3,23,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50152234

(CHEMBL184061 | N*5*-{2-[4-(2,4-Difluoro-phenyl)-pi...)Show SMILES Nc1nc(NCCN2CCN(CC2)c2ccc(F)cc2F)nc2nc(nn12)-c1ccco1 Show InChI InChI=1S/C20H21F2N9O/c21-13-3-4-15(14(22)12-13)30-9-7-29(8-10-30)6-5-24-19-26-18(23)31-20(27-19)25-17(28-31)16-2-1-11-32-16/h1-4,11-12H,5-10H2,(H3,23,24,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108690

(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50108688
(1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...)Show SMILES COc1ccc2OC3(CCN(CCCC(=O)c4ccc(F)cc4)CC3)CCc2c1 Show InChI InChI=1S/C24H28FNO3/c1-28-21-8-9-23-19(17-21)10-11-24(29-23)12-15-26(16-13-24)14-2-3-22(27)18-4-6-20(25)7-5-18/h4-9,17H,2-3,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 95: 10836-41 (1998)
Article DOI: 10.1073/pnas.95.18.10836
BindingDB Entry DOI: 10.7270/Q2XW4HCM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108688

(1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...)Show SMILES COc1ccc2OC3(CCN(CCCC(=O)c4ccc(F)cc4)CC3)CCc2c1 Show InChI InChI=1S/C24H28FNO3/c1-28-21-8-9-23-19(17-21)10-11-24(29-23)12-15-26(16-13-24)14-2-3-22(27)18-4-6-20(25)7-5-18/h4-9,17H,2-3,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1
(Homo sapiens (Human)) | BDBM50064772

(Ala-Gly-cyclo[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-...)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50+,51-,52-,53-,54-,55+,56-,57-,58+,59-,62+,63+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards human sst1 receptor expressed in CHO-K1 cells |
J Med Chem 41: 2175-9 (1998)
Article DOI: 10.1021/jm980194h
BindingDB Entry DOI: 10.7270/Q2XW4KGS |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50258507

(CHEMBL4078345)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C30H32N2O4/c1-23(33)17-19-27(20-18-24-11-5-2-6-12-24)31-29(34)28(21-25-13-7-3-8-14-25)32-30(35)36-22-26-15-9-4-10-16-26/h2-17,19,27-28H,18,20-22H2,1H3,(H,31,34)(H,32,35)/b19-17+/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay |
J Med Chem 60: 6911-6923 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00405
BindingDB Entry DOI: 10.7270/Q2FJ2K73 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from wild type human adenosine receptor A2a expressed in HEK293 cell membranes after 240 mins by scintillation counting |
J Med Chem 59: 6470-9 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00653
BindingDB Entry DOI: 10.7270/Q29S1VHS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50036756
(1'-phenethylspiro[1,2,3,4-tetrahydronaphthalene-2,...)Show InChI InChI=1S/C22H27NO/c24-21-20-9-5-4-8-19(20)10-12-22(21)13-16-23(17-14-22)15-11-18-6-2-1-3-7-18/h1-9,21,24H,10-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data