

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361235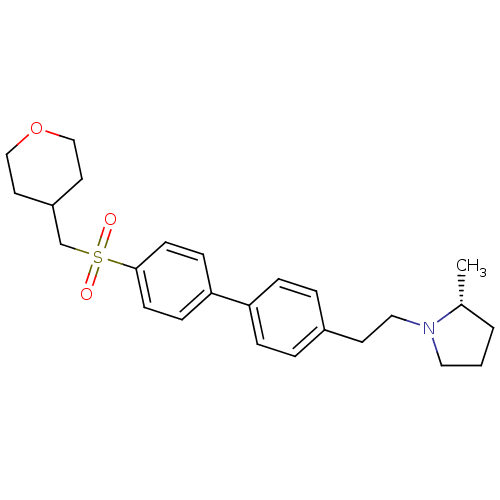 (CHEMBL1934525) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352357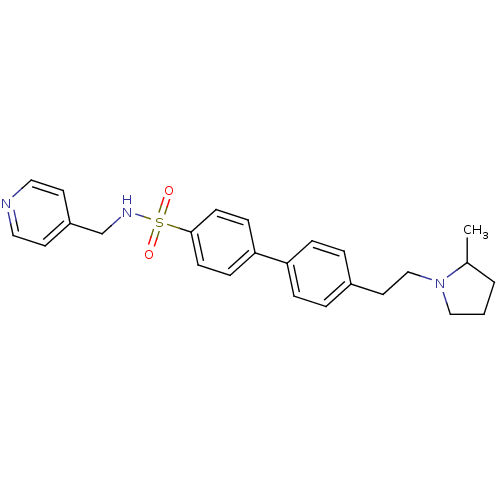 (CHEMBL558655) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361237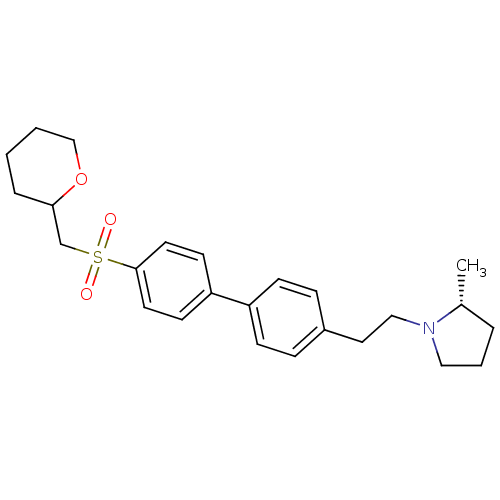 (CHEMBL1934527) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160935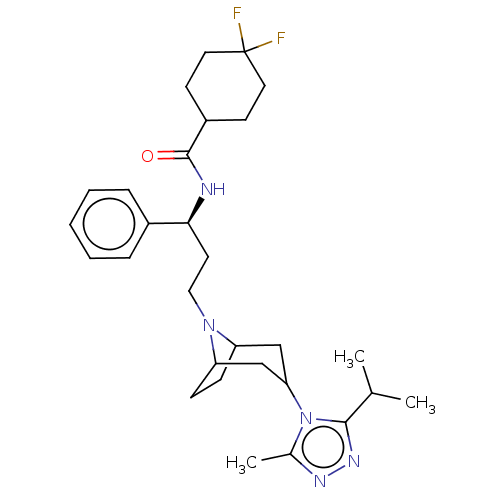 (US10167299, Maraviroc | US9107954, maraviroc) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description Afterwards, the pharmacological profile of bivalent ligand 1 at the chemokine receptor CCR5 was characterized similarly. The competitive radioligand ... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50334986 (4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1alpha from CCR5 in rhesus monkey Chem-1 cell membranes after 120 mins by liquid scintillation counting analysis | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50464147 (CHEMBL256907) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]MIP-1alpha from CCR5 receptor in rhesus monkey membrane incubated for 120 mins by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352358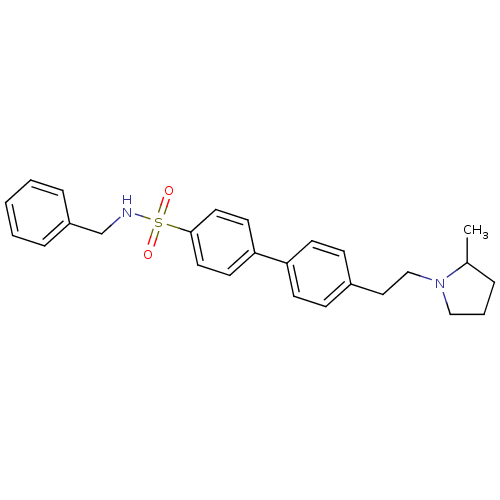 (CHEMBL558456) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-NLX from mouse mu opioid receptor expressed in CHO cells measured after 1.5 hrs by competitive-binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50324540 (CHEMBL1215661 | Pruvanserin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells after 1 hr by scintillation counting | J Med Chem 53: 5696-706 (2010) Article DOI: 10.1021/jm100479q BindingDB Entry DOI: 10.7270/Q2CV4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50414743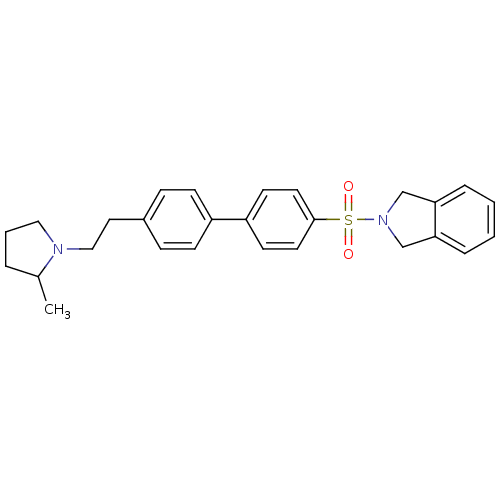 (CHEMBL564803) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50324541 (1-[3-(4-Bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells after 1 hr by scintillation counting | J Med Chem 53: 5696-706 (2010) Article DOI: 10.1021/jm100479q BindingDB Entry DOI: 10.7270/Q2CV4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361228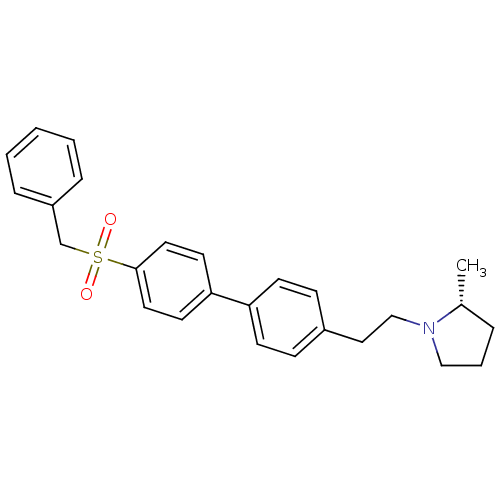 (CHEMBL1934358) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361226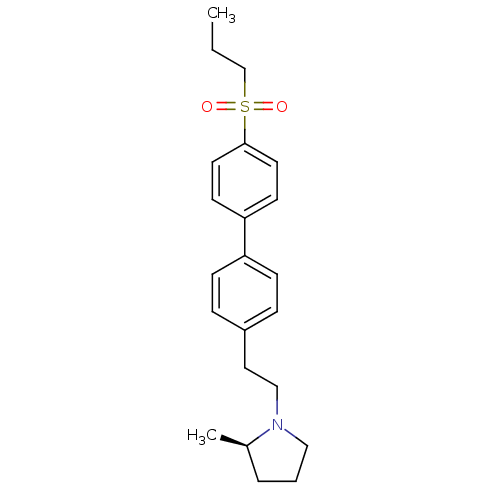 (CHEMBL1934356) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352354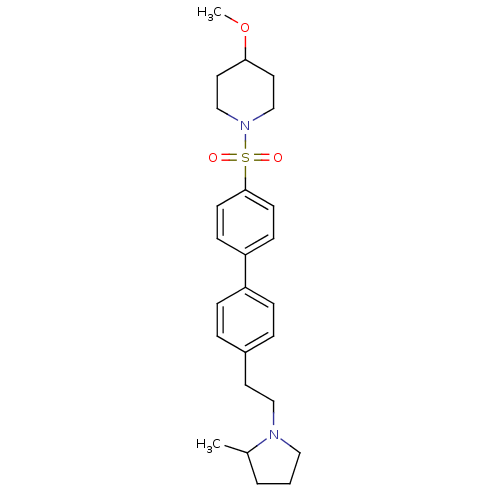 (CHEMBL554506) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361233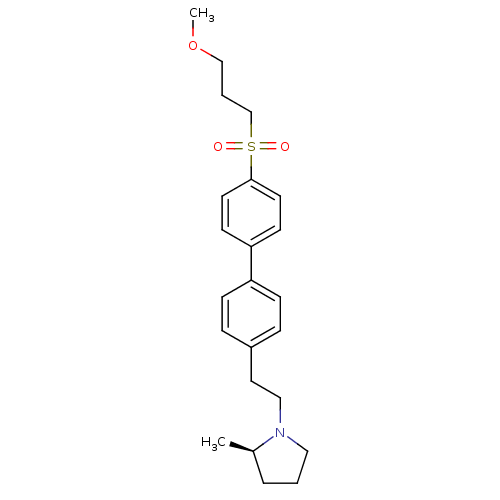 (CHEMBL1934523) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-naloxone from mouse MOR expressed in CHO cells incubated for 1.5 hrs by competitive radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University US Patent | Assay Description A bivalent ligand 1 (FIG. 14) that combines the pharmacophores of naltrexone (a MOR antagonist) and maraviroc (a CCR5 antagonist) into one molecule w... | US Patent US9107954 (2015) BindingDB Entry DOI: 10.7270/Q27943D1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from human mu opioid receptor expressed in CHO cells after 1 hr | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50392801 (CHEMBL2151247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361234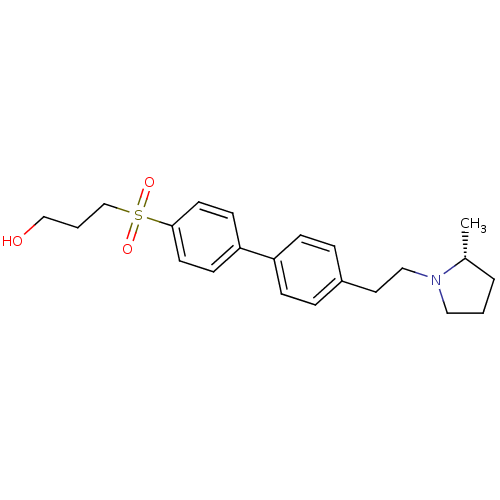 (CHEMBL1934524) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361225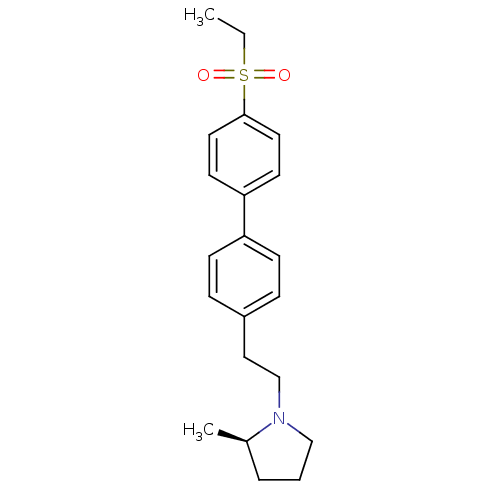 (CHEMBL1934355) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352355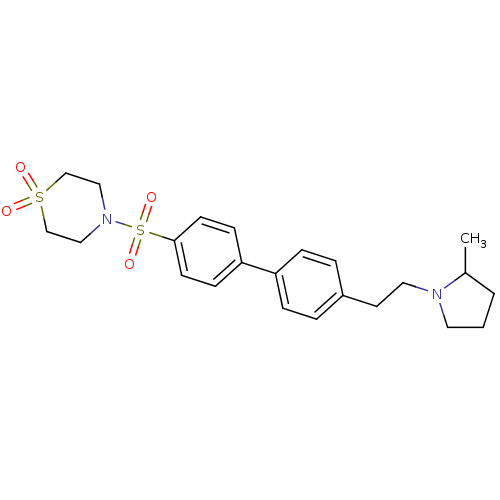 (CHEMBL560140) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361232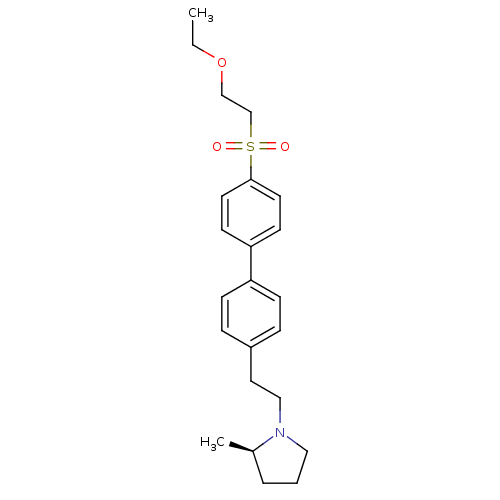 (CHEMBL1934522) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361229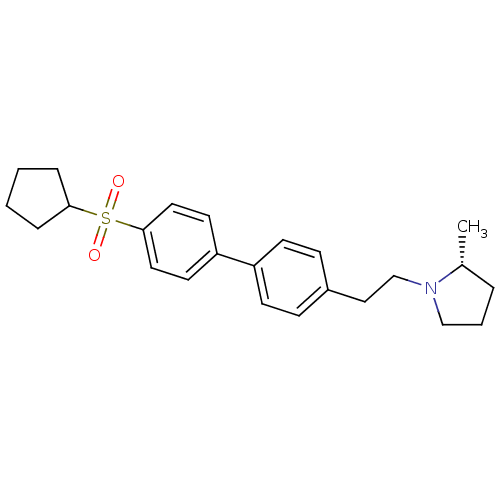 (CHEMBL1934519) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50297368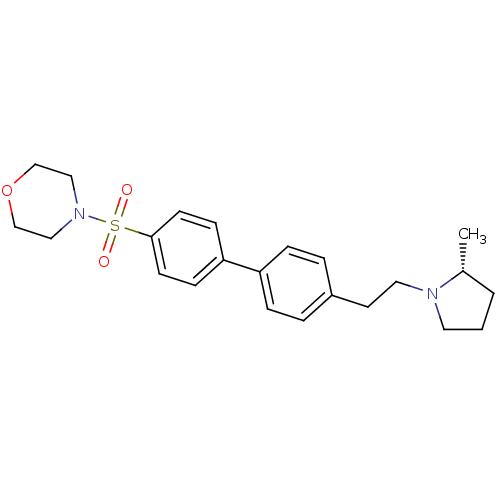 (4-{4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity against human histamine H3 receptor by [35S]GTPgamma binding assay | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50297367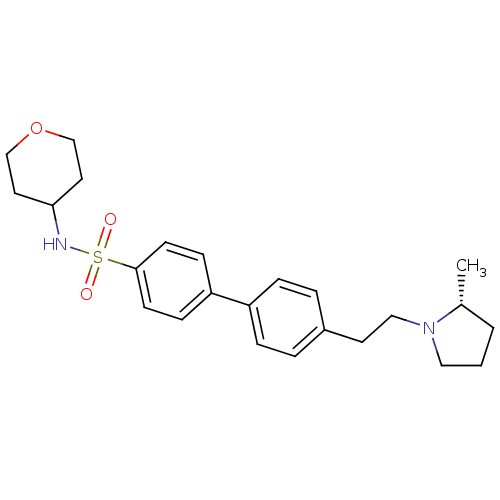 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity against human histamine H3 receptor by [35S]GTPgamma binding assay | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50297366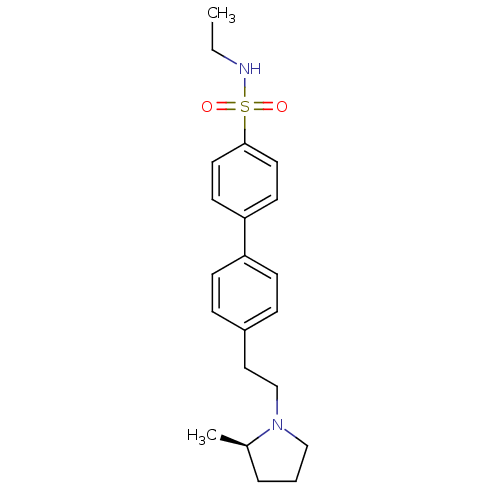 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Inverse agonist activity against human histamine H3 receptor by [35S]GTPgamma binding assay | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361236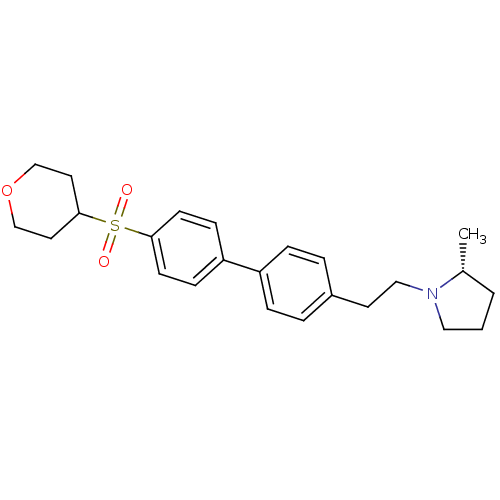 (CHEMBL1934526) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50324551 ((4-Bromo-1-methyl-1H-pyrazol-3-yl)-{4-[2-(2,4-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells after 1 hr by scintillation counting | J Med Chem 53: 5696-706 (2010) Article DOI: 10.1021/jm100479q BindingDB Entry DOI: 10.7270/Q2CV4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50558602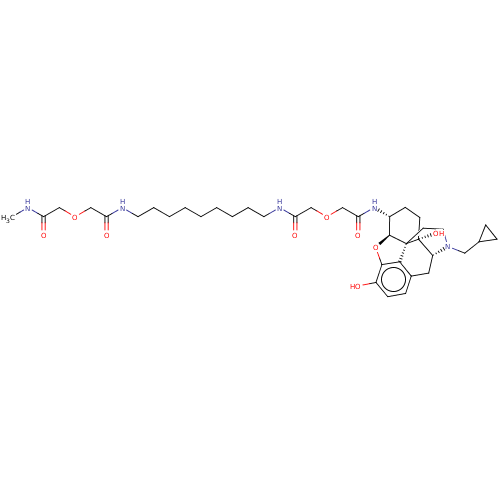 (CHEMBL4741368) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361231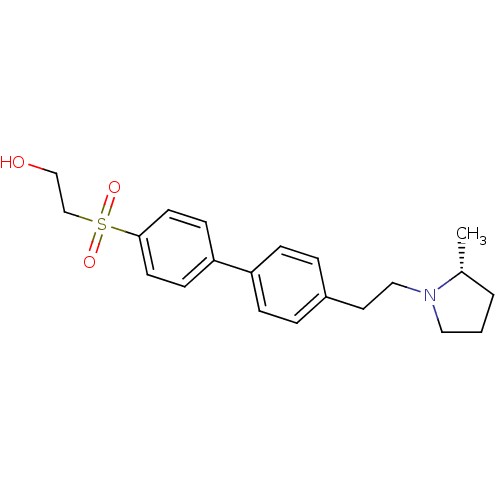 (CHEMBL1934521) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361224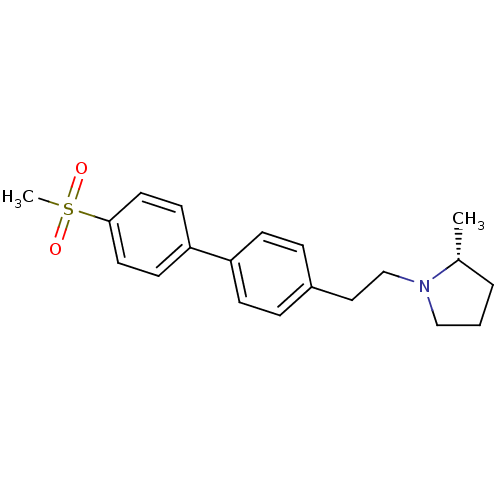 (CHEMBL1934354) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361230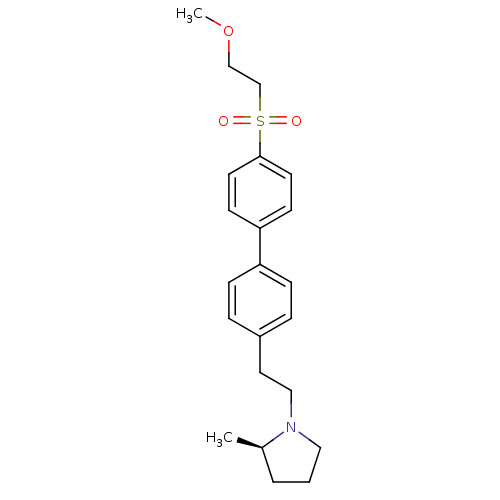 (CHEMBL1934520) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50414742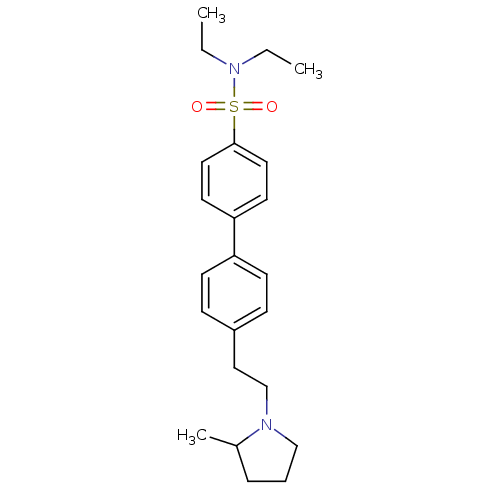 (CHEMBL560799) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50297368 (4-{4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352364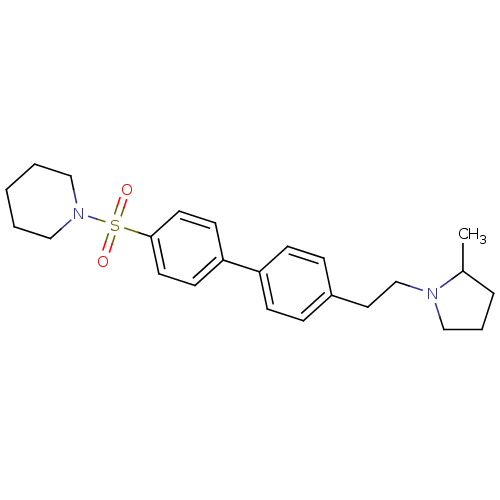 (CHEMBL562629) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50297367 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352359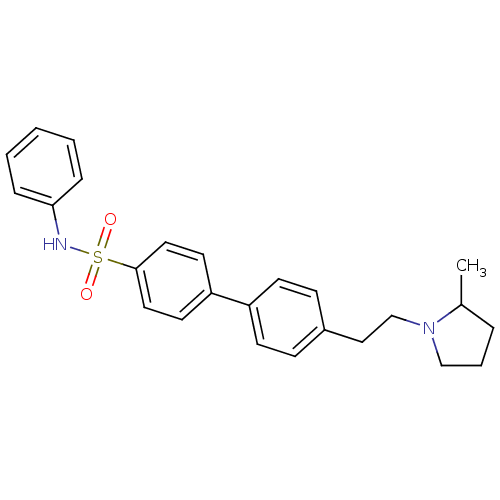 (CHEMBL552279) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352360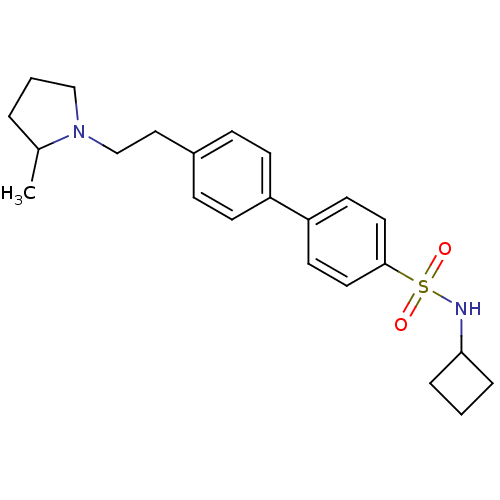 (CHEMBL563118) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50583787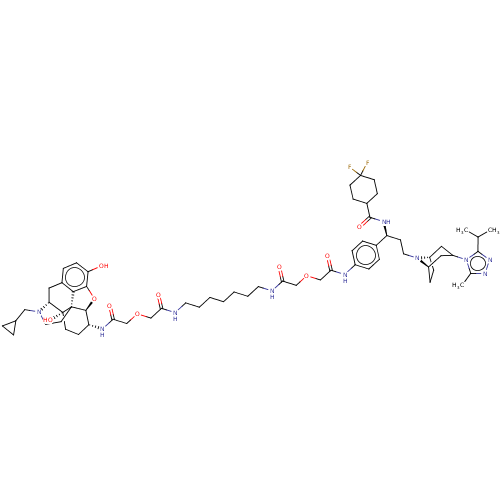 (CHEMBL5071060) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]1-alpha from recombinant rhesus macaque CCR5 expressed in Chem-1 cells incubated for 90 mins by competitive radioligand binding... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00408 BindingDB Entry DOI: 10.7270/Q2R49VPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50324542 (2-[4-(4-Chloro-1-methyl-1H-pyrazole-3-carbonyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals. Curated by ChEMBL | Assay Description Binding affinity to rat 5HT2A receptor | J Med Chem 53: 5696-706 (2010) Article DOI: 10.1021/jm100479q BindingDB Entry DOI: 10.7270/Q2CV4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361238 (CHEMBL1934528) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352353 (CHEMBL1823053) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50352361 (CHEMBL558260) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50324567 (2-[4-(4-Chloro-1-ethyl-1H-pyrazole-3-carbonyl)pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells after 1 hr by scintillation counting | J Med Chem 53: 5696-706 (2010) Article DOI: 10.1021/jm100479q BindingDB Entry DOI: 10.7270/Q2CV4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50361227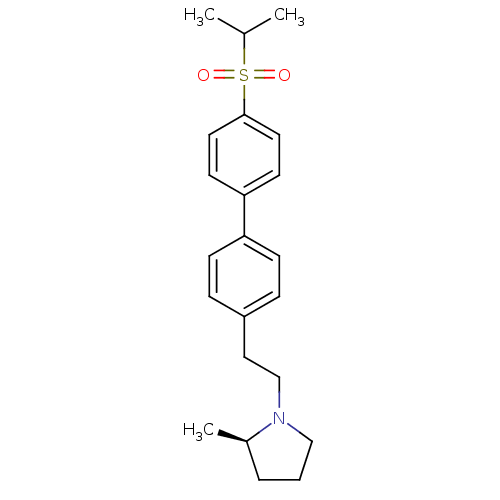 (CHEMBL1934357) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes | Bioorg Med Chem Lett 22: 71-5 (2011) Article DOI: 10.1016/j.bmcl.2011.11.075 BindingDB Entry DOI: 10.7270/Q27M08C7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Canis familiaris) | BDBM50324542 (2-[4-(4-Chloro-1-methyl-1H-pyrazole-3-carbonyl)pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals. Curated by ChEMBL | Assay Description Binding affinity to dog 5HT2A receptor | J Med Chem 53: 5696-706 (2010) Article DOI: 10.1021/jm100479q BindingDB Entry DOI: 10.7270/Q2CV4HZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50561916 (CHEMBL4779258) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-NLX from mouse mu opioid receptor expressed in CHO cells measured after 1.5 hrs by competitive-binding assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00444 BindingDB Entry DOI: 10.7270/Q2M04947 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50297369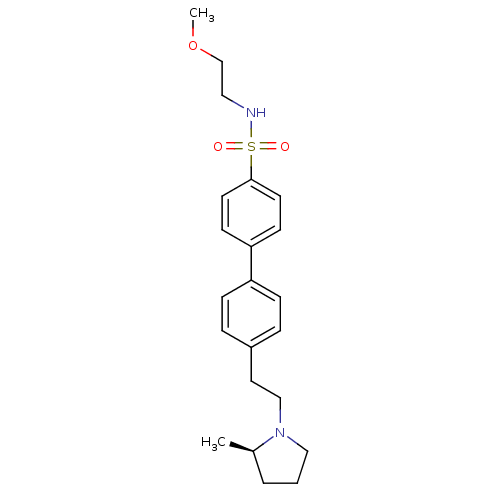 (4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane | J Med Chem 52: 5603-11 (2009) Article DOI: 10.1021/jm900857n BindingDB Entry DOI: 10.7270/Q2KW5G2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 442 total ) | Next | Last >> |