 Found 1586 hits with Last Name = 'tran' and Initial = 'l'
Found 1586 hits with Last Name = 'tran' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1B) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM81490
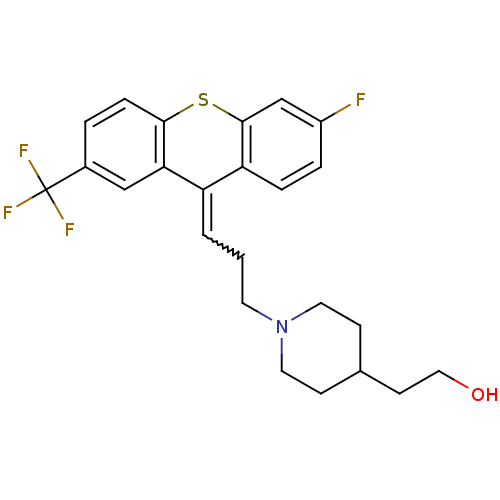
(CAS_60197-32-2 | CIS PIFLUTIXOL | NSC_68714 | Pifl...)Show SMILES OCCC1CCN(CCC=C2c3ccc(F)cc3Sc3ccc(cc23)C(F)(F)F)CC1 |w:9.8| Show InChI InChI=1S/C24H25F4NOS/c25-18-4-5-20-19(2-1-10-29-11-7-16(8-12-29)9-13-30)21-14-17(24(26,27)28)3-6-22(21)31-23(20)15-18/h2-6,14-16,30H,1,7-13H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM81490

(CAS_60197-32-2 | CIS PIFLUTIXOL | NSC_68714 | Pifl...)Show SMILES OCCC1CCN(CCC=C2c3ccc(F)cc3Sc3ccc(cc23)C(F)(F)F)CC1 |w:9.8| Show InChI InChI=1S/C24H25F4NOS/c25-18-4-5-20-19(2-1-10-29-11-7-16(8-12-29)9-13-30)21-14-17(24(26,27)28)3-6-22(21)31-23(20)15-18/h2-6,14-16,30H,1,7-13H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50026957
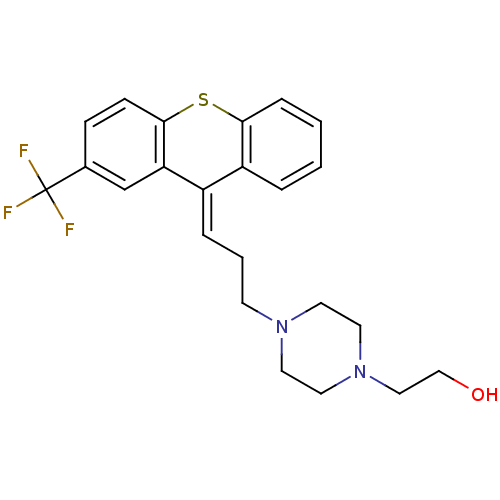
((cis) 2-{4-[3-(2-Trifluoromethyl-thioxanthen-9-yli...)Show SMILES OCCN1CCN(CC\C=C2/c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C23H25F3N2OS/c24-23(25,26)17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)30-22)5-3-9-27-10-12-28(13-11-27)14-15-29/h1-2,4-8,16,29H,3,9-15H2/b18-5+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM50026957

((cis) 2-{4-[3-(2-Trifluoromethyl-thioxanthen-9-yli...)Show SMILES OCCN1CCN(CC\C=C2/c3ccccc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C23H25F3N2OS/c24-23(25,26)17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)30-22)5-3-9-27-10-12-28(13-11-27)14-15-29/h1-2,4-8,16,29H,3,9-15H2/b18-5+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385299
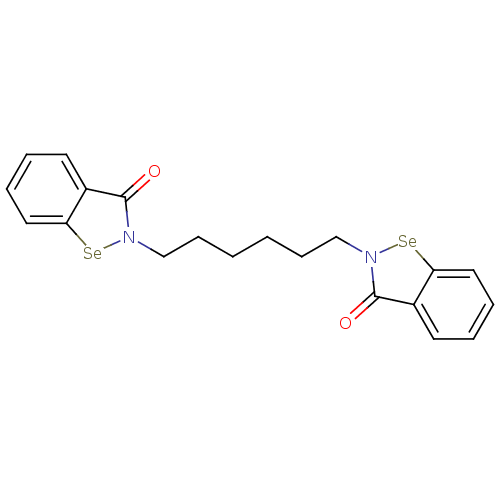
(CHEMBL2035464 | US8592468, EbSe16)Show SMILES O=c1n(CCCCCCn2[se]c3ccccc3c2=O)[se]c2ccccc12 Show InChI InChI=1S/C20H20N2O2Se2/c23-19-15-9-3-5-11-17(15)25-21(19)13-7-1-2-8-14-22-20(24)16-10-4-6-12-18(16)26-22/h3-6,9-12H,1-2,7-8,13-14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 10 | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM60917
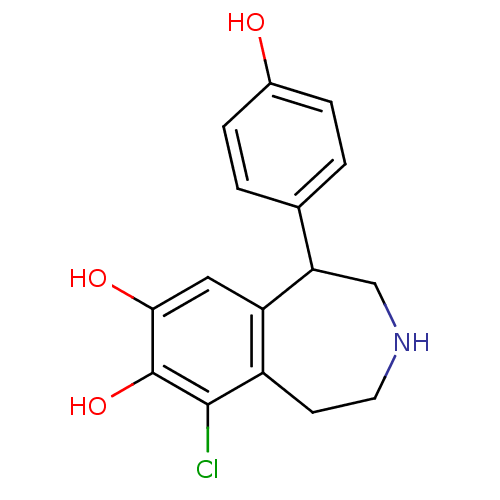
(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM81485
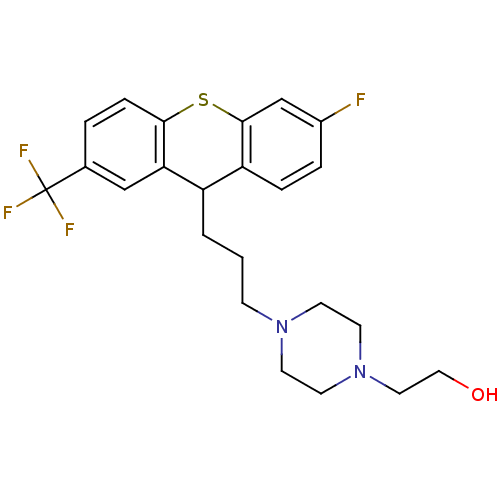
(CAS_68751 | CIS TEFLUTIXOL | NSC_68751 | Teflutixo...)Show SMILES OCCN1CCN(CCCC2c3ccc(F)cc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C23H26F4N2OS/c24-17-4-5-19-18(2-1-7-28-8-10-29(11-9-28)12-13-30)20-14-16(23(25,26)27)3-6-21(20)31-22(19)15-17/h3-6,14-15,18,30H,1-2,7-13H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM81485

(CAS_68751 | CIS TEFLUTIXOL | NSC_68751 | Teflutixo...)Show SMILES OCCN1CCN(CCCC2c3ccc(F)cc3Sc3ccc(cc23)C(F)(F)F)CC1 Show InChI InChI=1S/C23H26F4N2OS/c24-17-4-5-19-18(2-1-7-28-8-10-29(11-9-28)12-13-30)20-14-16(23(25,26)27)3-6-21(20)31-22(19)15-17/h3-6,14-15,18,30H,1-2,7-13H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385302
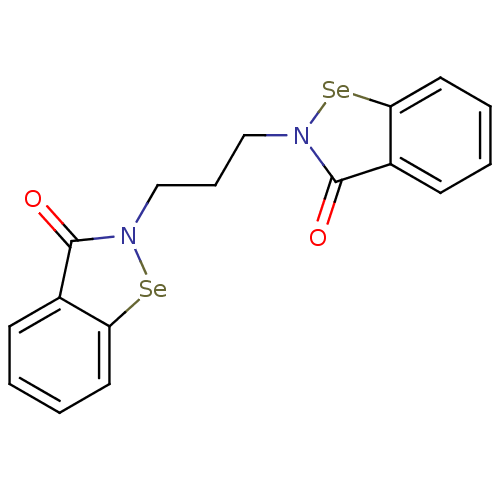
(CHEMBL2035461 | US8592468, EbSe15)Show InChI InChI=1S/C17H14N2O2Se2/c20-16-12-6-1-3-8-14(12)22-18(16)10-5-11-19-17(21)13-7-2-4-9-15(13)23-19/h1-4,6-9H,5,10-11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 40 | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM50513992

(CHEMBL3919119)Show SMILES NCc1ccc(C[C@H](NC(=O)c2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)B(O)O)cc1 |r| Show InChI InChI=1S/C22H31BN6O4/c24-14-16-10-8-15(9-11-16)13-18(28-20(30)17-5-2-1-3-6-17)21(31)29-19(23(32)33)7-4-12-27-22(25)26/h1-3,5-6,8-11,18-19,32-33H,4,7,12-14,24H2,(H,28,30)(H,29,31)(H4,25,26,27)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged Zika virus NS2B (49 to 95 residues) - NS3 (1 to 170 residues) protease domain expressed in Escherichia coli BL21... |
J Med Chem 63: 470-489 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00775
BindingDB Entry DOI: 10.7270/Q2RX9GFS |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM50385303
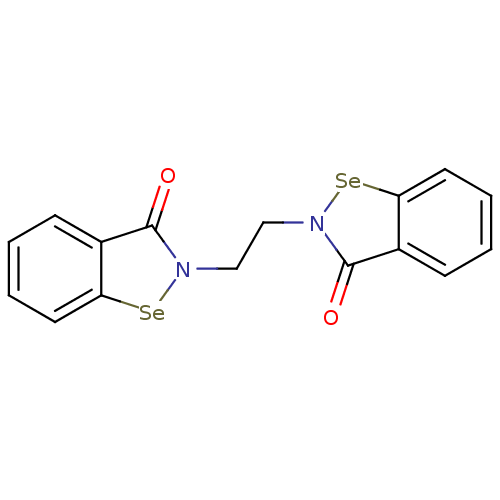
(CHEMBL2035460 | US8592468, EbSe14)Show InChI InChI=1S/C16H12N2O2Se2/c19-15-11-5-1-3-7-13(11)21-17(15)9-10-18-16(20)12-6-2-4-8-14(12)22-18/h1-8H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| 50 | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50040241
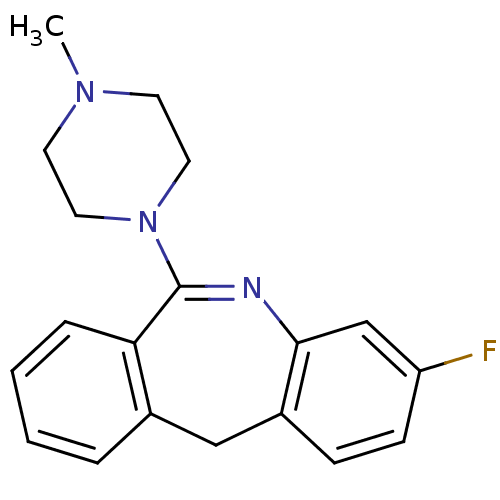
(3-Fluoro-6-(4-methyl-piperazin-1-yl)-11H-dibenzo[b...)Show InChI InChI=1S/C19H20FN3/c1-22-8-10-23(11-9-22)19-17-5-3-2-4-14(17)12-15-6-7-16(20)13-18(15)21-19/h2-7,13H,8-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM50004822
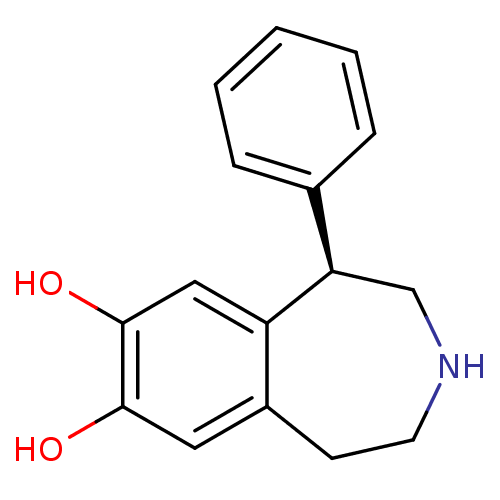
((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004822

((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106948

(US8592468, EbSe12)Show InChI InChI=1S/C12H7N3O3Se/c16-12-8-4-1-2-6-10(8)19-14(12)11-9(15(17)18)5-3-7-13-11/h1-7H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 250 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106941
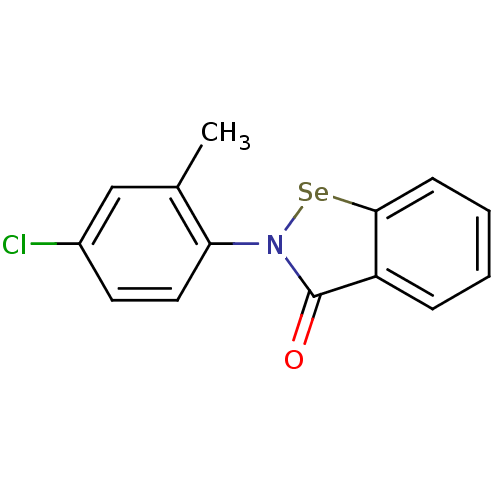
(US8592468, EbSe8)Show InChI InChI=1S/C14H10ClNOSe/c1-9-8-10(15)6-7-12(9)16-14(17)11-4-2-3-5-13(11)18-16/h2-8H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 250 | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 300 | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM50040241

(3-Fluoro-6-(4-methyl-piperazin-1-yl)-11H-dibenzo[b...)Show InChI InChI=1S/C19H20FN3/c1-22-8-10-23(11-9-22)19-17-5-3-2-4-14(17)12-15-6-7-16(20)13-18(15)21-19/h2-7,13H,8-12H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106940
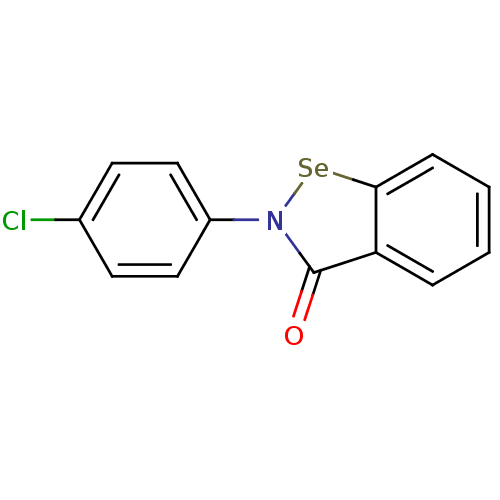
(US8592468, EbSe7)Show InChI InChI=1S/C13H8ClNOSe/c14-9-5-7-10(8-6-9)15-13(16)11-3-1-2-4-12(11)17-15/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 550 | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Nonstructural protein 3
(Zika virus) | BDBM15236

(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...)Show SMILES Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O Show InChI InChI=1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibition of Zika virus Asian/8375 NS2B (48 to 100 residues)-NS3 (14 to 185 residues) expressed in Escherichia coli BL21 (DE3) Star cells preincubat... |
J Med Chem 63: 470-489 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00775
BindingDB Entry DOI: 10.7270/Q2RX9GFS |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106944
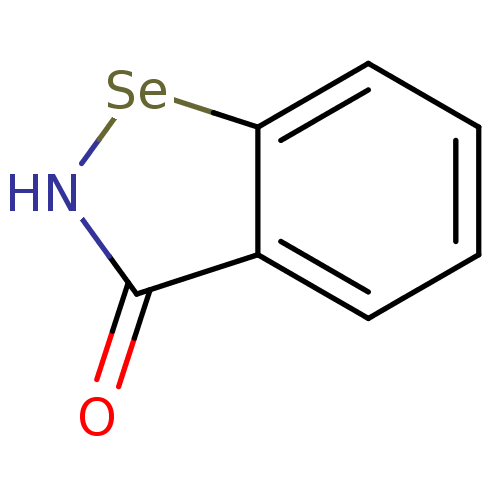
(US8592468, EbSe2)Show InChI InChI=1S/C7H5NOSe/c9-7-5-3-1-2-4-6(5)10-8-7/h1-4H,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.00E+3 | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM81777
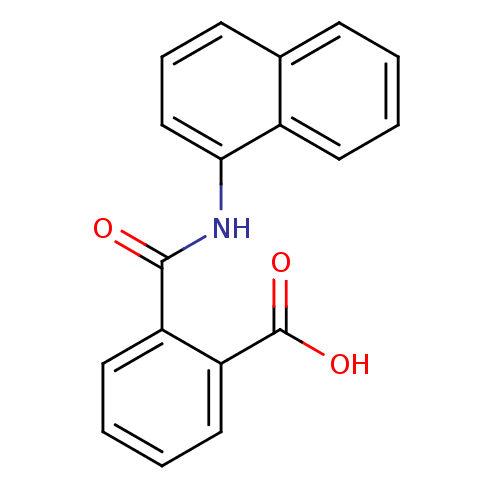
(CAS_132-66-1 | NPA | NPA,(+) | NPA,(-) | NSC_8594)Show InChI InChI=1S/C18H13NO3/c20-17(14-9-3-4-10-15(14)18(21)22)19-16-11-5-7-12-6-1-2-8-13(12)16/h1-11H,(H,19,20)(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106942
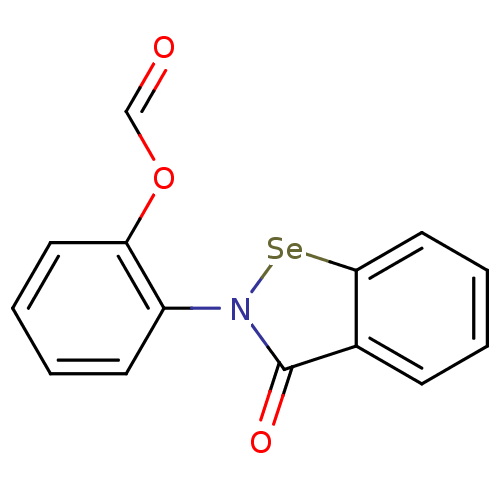
(US8592468, EbSe9)Show InChI InChI=1S/C14H9NO3Se/c16-9-18-12-7-3-2-6-11(12)15-14(17)10-5-1-4-8-13(10)19-15/h1-9H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.20E+3 | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase
(Escherichia coli (strain K12)) | BDBM106949
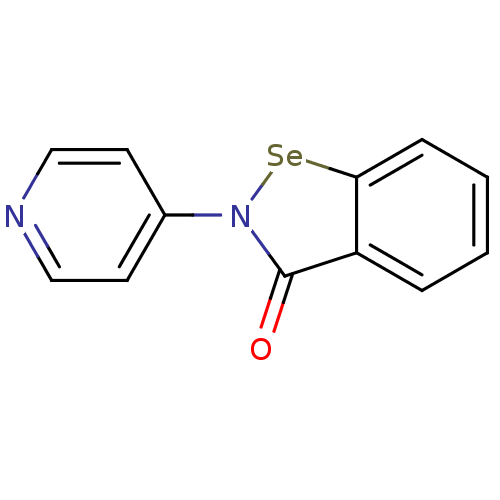
(US8592468, EbSe13)Show InChI InChI=1S/C12H8N2OSe/c15-12-10-3-1-2-4-11(10)16-14(12)9-5-7-13-8-6-9/h1-8H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.50E+3 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Thioredoxin Systems AB
US Patent
| Assay Description
All the benzisoselenazol-3(2H)-one and bisbenzisoselenazol-3(2H)-one
derivatives were tested as potential E. coli TrxR inhibitors by standard
DTNB ... |
US Patent US8592468 (2013)
BindingDB Entry DOI: 10.7270/Q29P3081 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM81777

(CAS_132-66-1 | NPA | NPA,(+) | NPA,(-) | NSC_8594)Show InChI InChI=1S/C18H13NO3/c20-17(14-9-3-4-10-15(14)18(21)22)19-16-11-5-7-12-6-1-2-8-13(12)16/h1-11H,(H,19,20)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(RAT) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371616

((R)-4-(2-(6-Fluoropyridin-3-yl)-4-(trifluoromethyl...)Show SMILES Fc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1ccncn1)C(F)(F)F Show InChI InChI=1S/C25H18F4N4O3S/c26-23-6-1-15(13-31-23)21-11-16(25(27,28)29)2-4-18(21)19-8-10-36-22-12-17(3-5-20(19)22)37(34,35)33-24-7-9-30-14-32-24/h1-7,9,11-14,19H,8,10H2,(H,30,32,33)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
| Assay Description
HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30000 cells/well and incubated at 37° C./5% CO2 f... |
J Med Chem 51: 545-52 (2008)
BindingDB Entry DOI: 10.7270/Q2NG4SZX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371616

((R)-4-(2-(6-Fluoropyridin-3-yl)-4-(trifluoromethyl...)Show SMILES Fc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1ccncn1)C(F)(F)F Show InChI InChI=1S/C25H18F4N4O3S/c26-23-6-1-15(13-31-23)21-11-16(25(27,28)29)2-4-18(21)19-8-10-36-22-12-17(3-5-20(19)22)37(34,35)33-24-7-9-30-14-32-24/h1-7,9,11-14,19H,8,10H2,(H,30,32,33)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371648
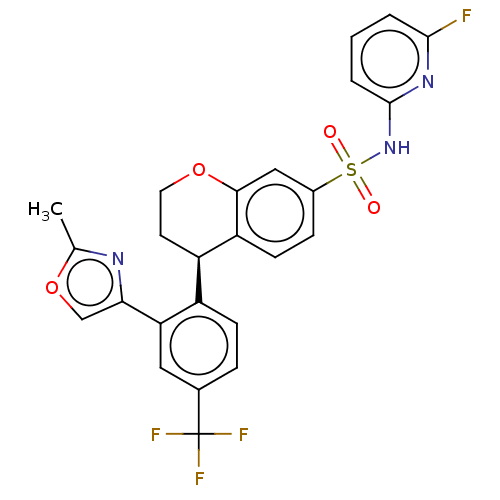
((R)-N-(6-Fluoropyridin-2-yl)-4-(2-(2-methyloxazol-...)Show SMILES Cc1nc(co1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1cccc(F)n1)C(F)(F)F Show InChI InChI=1S/C25H19F4N3O4S/c1-14-30-21(13-36-14)20-11-15(25(27,28)29)5-7-17(20)18-9-10-35-22-12-16(6-8-19(18)22)37(33,34)32-24-4-2-3-23(26)31-24/h2-8,11-13,18H,9-10H2,1H3,(H,31,32)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
| Assay Description
HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30000 cells/well and incubated at 37° C./5% CO2 f... |
J Med Chem 51: 545-52 (2008)
BindingDB Entry DOI: 10.7270/Q2NG4SZX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371618
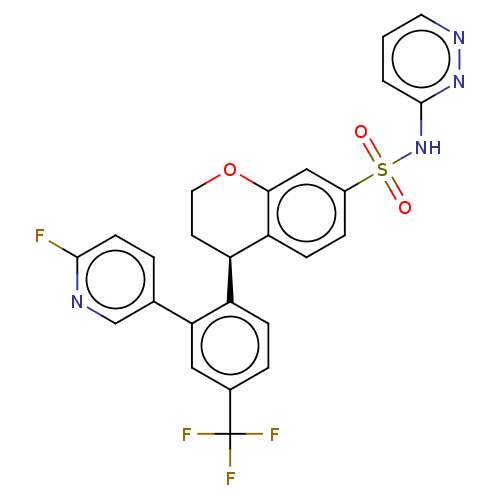
((R)-4-(2-(6-Fluoropyridin-3-yl)-4-(trifluoromethyl...)Show SMILES Fc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1cccnn1)C(F)(F)F Show InChI InChI=1S/C25H18F4N4O3S/c26-23-8-3-15(14-30-23)21-12-16(25(27,28)29)4-6-18(21)19-9-11-36-22-13-17(5-7-20(19)22)37(34,35)33-24-2-1-10-31-32-24/h1-8,10,12-14,19H,9,11H2,(H,32,33)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
| Assay Description
HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30000 cells/well and incubated at 37° C./5% CO2 f... |
J Med Chem 51: 545-52 (2008)
BindingDB Entry DOI: 10.7270/Q2NG4SZX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371523

((R)-N-(6-Fluoropyridin-2-yl)-4-(2-(1-methyl-1H-pyr...)Show SMILES Cn1nccc1-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1cccc(F)n1)C(F)(F)F Show InChI InChI=1S/C25H20F4N4O3S/c1-33-21(9-11-30-33)20-13-15(25(27,28)29)5-7-17(20)18-10-12-36-22-14-16(6-8-19(18)22)37(34,35)32-24-4-2-3-23(26)31-24/h2-9,11,13-14,18H,10,12H2,1H3,(H,31,32)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
| Assay Description
HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30000 cells/well and incubated at 37° C./5% CO2 f... |
J Med Chem 51: 545-52 (2008)
BindingDB Entry DOI: 10.7270/Q2NG4SZX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371635

((R)-4-(2-(1H-1,2,3-Triazol-1-yl)-4-(trifluoromethy...)Show SMILES Fc1cccc(NS(=O)(=O)c2ccc3[C@H](CCOc3c2)c2ccc(cc2-n2ccnn2)C(F)(F)F)n1 Show InChI InChI=1S/C23H17F4N5O3S/c24-21-2-1-3-22(29-21)30-36(33,34)15-5-7-18-16(8-11-35-20(18)13-15)17-6-4-14(23(25,26)27)12-19(17)32-10-9-28-31-32/h1-7,9-10,12-13,16H,8,11H2,(H,29,30)/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
| Assay Description
HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30000 cells/well and incubated at 37° C./5% CO2 f... |
J Med Chem 51: 545-52 (2008)
BindingDB Entry DOI: 10.7270/Q2NG4SZX |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545560

(CHEMBL4634421)Show SMILES Fc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F |r| Show InChI InChI=1S/C24H17F4N3O3S2/c25-22-6-1-14(13-30-22)20-11-15(24(26,27)28)2-4-17(20)18-7-9-34-21-12-16(3-5-19(18)21)36(32,33)31-23-29-8-10-35-23/h1-6,8,10-13,18H,7,9H2,(H,29,31)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545552

(CHEMBL4646742)Show SMILES Cn1nccc1-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F |r| Show InChI InChI=1S/C23H19F3N4O3S2/c1-30-20(6-8-28-30)19-12-14(23(24,25)26)2-4-16(19)17-7-10-33-21-13-15(3-5-18(17)21)35(31,32)29-22-27-9-11-34-22/h2-6,8-9,11-13,17H,7,10H2,1H3,(H,27,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545563
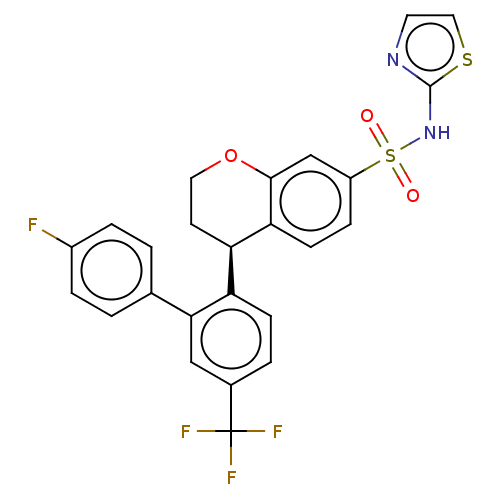
(CHEMBL4636838)Show SMILES Fc1ccc(cc1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1nccs1)C(F)(F)F |r| Show InChI InChI=1S/C25H18F4N2O3S2/c26-17-4-1-15(2-5-17)22-13-16(25(27,28)29)3-7-19(22)20-9-11-34-23-14-18(6-8-21(20)23)36(32,33)31-24-30-10-12-35-24/h1-8,10,12-14,20H,9,11H2,(H,30,31)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50545558

(CHEMBL4642748)Show SMILES FC(F)(F)c1ccc([C@H]2CCOc3cc(ccc23)S(=O)(=O)Nc2nccs2)c(c1)-n1ccnn1 |r| Show InChI InChI=1S/C21H16F3N5O3S2/c22-21(23,24)13-1-3-16(18(11-13)29-8-6-26-28-29)15-5-9-32-19-12-14(2-4-17(15)19)34(30,31)27-20-25-7-10-33-20/h1-4,6-8,10-12,15H,5,9H2,(H,25,27)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lupin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.7 (unknown origin) expressed in HEK293 cells assessed as reduction in veratridine-induced depolarization preincubated for 15 to 20... |
J Med Chem 63: 6107-6133 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00361
BindingDB Entry DOI: 10.7270/Q2V69P5C |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM371776
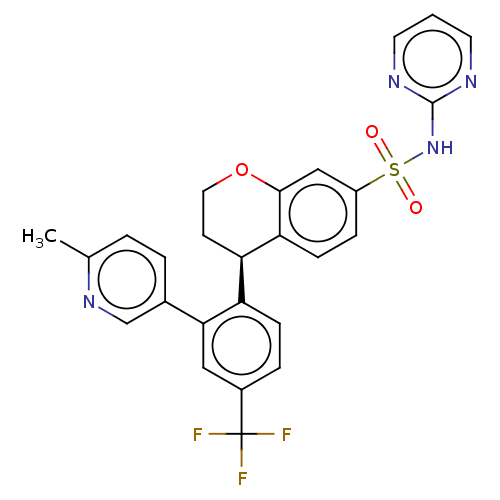
((R)-4-(2-(6-methylpyridin-3-yl)-4-(trifluoromethyl...)Show SMILES Cc1ccc(cn1)-c1cc(ccc1[C@H]1CCOc2cc(ccc12)S(=O)(=O)Nc1ncccn1)C(F)(F)F |r| Show InChI InChI=1S/C26H21F3N4O3S/c1-16-3-4-17(15-32-16)23-13-18(26(27,28)29)5-7-20(23)21-9-12-36-24-14-19(6-8-22(21)24)37(34,35)33-25-30-10-2-11-31-25/h2-8,10-11,13-15,21H,9,12H2,1H3,(H,30,31,33)/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at San Francisco
| Assay Description
HEK-293 cells overexpressing the channel of interest were seeded in a 96-well plate at a density of 30000 cells/well and incubated at 37° C./5% CO2 f... |
J Med Chem 51: 545-52 (2008)
BindingDB Entry DOI: 10.7270/Q2NG4SZX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data