 Found 121 hits with Last Name = 'zhang' and Initial = 'ln'
Found 121 hits with Last Name = 'zhang' and Initial = 'ln' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25743

(1-cycloheptyl-3-(1-acetylpiperidin-4-yl)urea | US8...)Show InChI InChI=1S/C15H27N3O2/c1-12(19)18-10-8-14(9-11-18)17-15(20)16-13-6-4-2-3-5-7-13/h13-14H,2-11H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335965
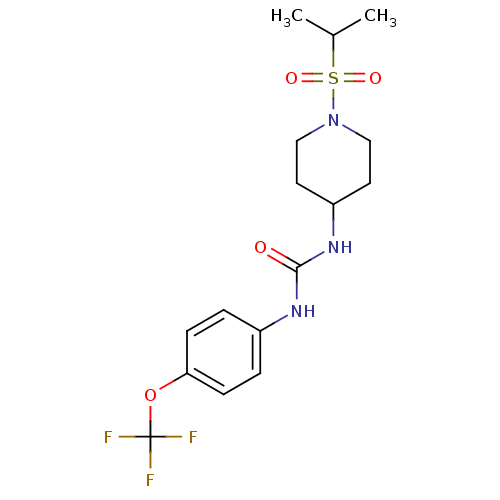
(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H22F3N3O4S/c1-11(2)27(24,25)22-9-7-13(8-10-22)21-15(23)20-12-3-5-14(6-4-12)26-16(17,18)19/h3-6,11,13H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335964

(1-(1-nicotinoylpiperidin-4-yl)-3-(4-(trifluorometh...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H19F3N4O3/c20-19(21,22)29-16-5-3-14(4-6-16)24-18(28)25-15-7-10-26(11-8-15)17(27)13-2-1-9-23-12-13/h1-6,9,12,15H,7-8,10-11H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335966
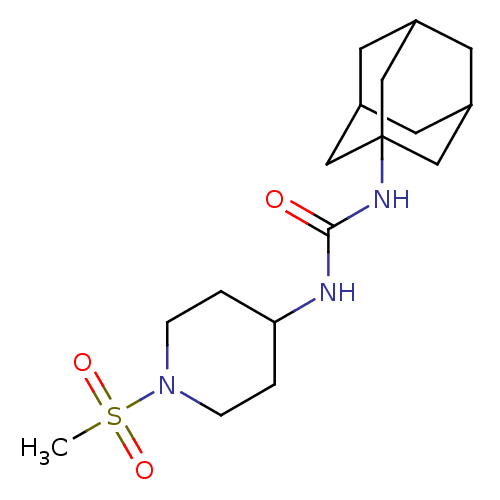
(1-Adamantan-1-yl-3-(1-methanesulfonyl-piperidin-4-...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:16.21.15:22,THB:19:18:15:21.20.22,19:20:17.18.23:15,17:16:18.19.23:22| Show InChI InChI=1S/C17H29N3O3S/c1-24(22,23)20-4-2-15(3-5-20)18-16(21)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,2-11H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335967
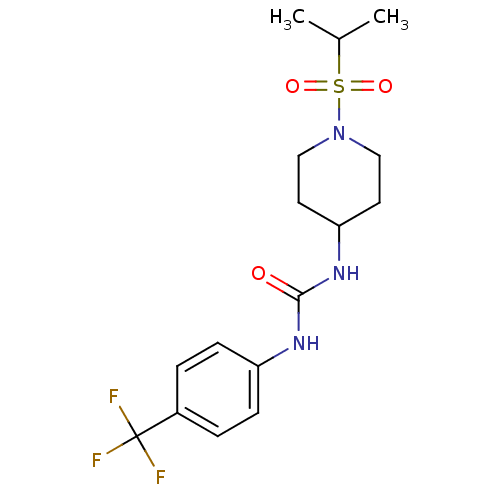
(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H22F3N3O3S/c1-11(2)26(24,25)22-9-7-14(8-10-22)21-15(23)20-13-5-3-12(4-6-13)16(17,18)19/h3-6,11,14H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50191854
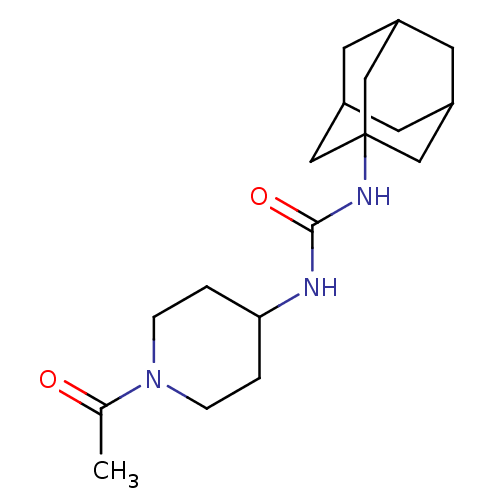
(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27707
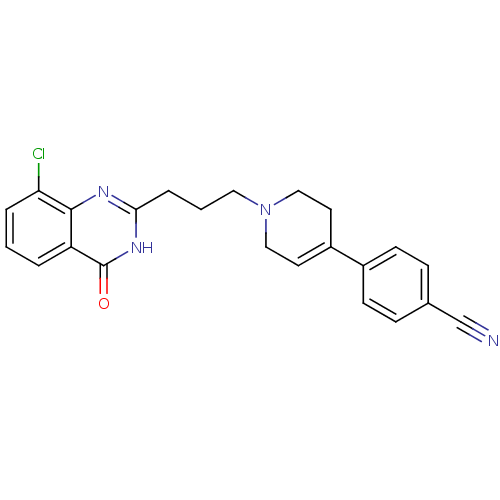
(4-{1-[3-(8-chloro-4-oxo-3,4-dihydroquinazolin-2-yl...)Show SMILES Clc1cccc2c1nc(CCCN1CCC(=CC1)c1ccc(cc1)C#N)[nH]c2=O |c:16| Show InChI InChI=1S/C23H21ClN4O/c24-20-4-1-3-19-22(20)26-21(27-23(19)29)5-2-12-28-13-10-18(11-14-28)17-8-6-16(15-25)7-9-17/h1,3-4,6-10H,2,5,11-14H2,(H,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335968
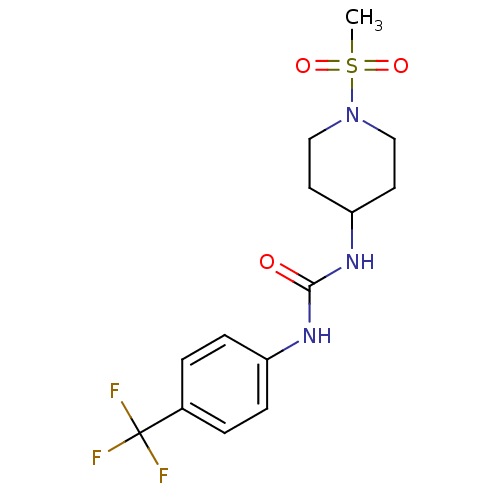
(1-(1-(methylsulfonyl)piperidin-4-yl)-3-(4-(trifluo...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C14H18F3N3O3S/c1-24(22,23)20-8-6-12(7-9-20)19-13(21)18-11-4-2-10(3-5-11)14(15,16)17/h2-5,12H,6-9H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165496
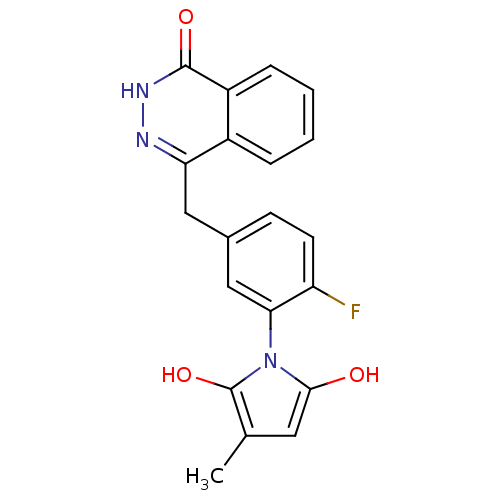
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Cc1cc(O)n(c1O)-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F |(2.63,-45.05,;1.1,-44.9,;.32,-43.57,;-1.18,-43.9,;-2.34,-42.88,;-1.33,-45.44,;.08,-46.05,;.41,-47.56,;-2.66,-46.22,;-3.99,-45.46,;-5.31,-46.24,;-6.65,-45.47,;-6.65,-43.93,;-5.32,-43.16,;-5.32,-41.61,;-6.67,-40.84,;-6.68,-39.3,;-8,-41.62,;-9.34,-40.86,;-10.66,-41.63,;-10.67,-43.18,;-9.33,-43.95,;-7.99,-43.17,;-5.31,-47.78,;-3.98,-48.54,;-2.65,-47.77,;-1.31,-48.53,)| Show InChI InChI=1S/C20H16FN3O3/c1-11-8-18(25)24(20(11)27)17-10-12(6-7-15(17)21)9-16-13-4-2-3-5-14(13)19(26)23-22-16/h2-8,10,25,27H,9H2,1H3,(H,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335966

(1-Adamantan-1-yl-3-(1-methanesulfonyl-piperidin-4-...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:16.21.15:22,THB:19:18:15:21.20.22,19:20:17.18.23:15,17:16:18.19.23:22| Show InChI InChI=1S/C17H29N3O3S/c1-24(22,23)20-4-2-15(3-5-20)18-16(21)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,2-11H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165488
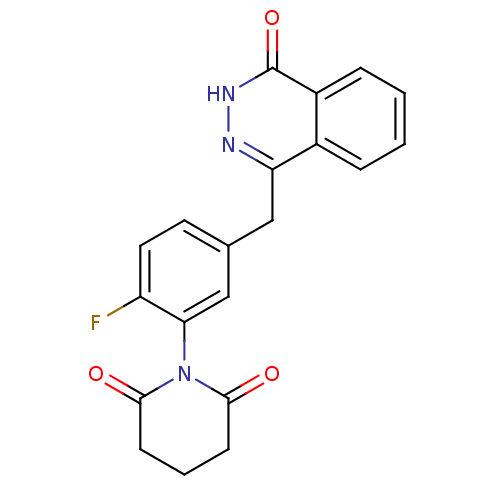
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1N1C(=O)CCCC1=O Show InChI InChI=1S/C20H16FN3O3/c21-15-9-8-12(11-17(15)24-18(25)6-3-7-19(24)26)10-16-13-4-1-2-5-14(13)20(27)23-22-16/h1-2,4-5,8-9,11H,3,6-7,10H2,(H,23,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165486
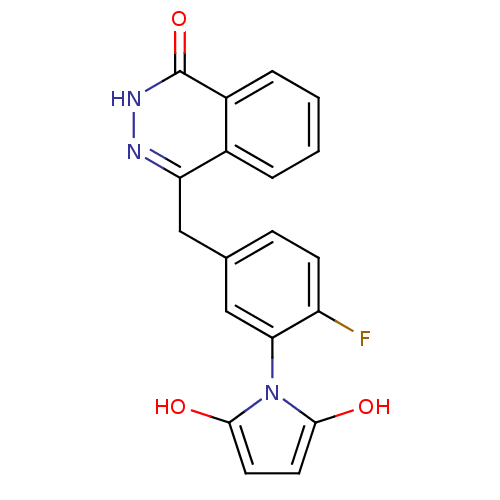
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Oc1ccc(O)n1-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F |(35.28,-33.78,;34.95,-32.28,;35.97,-31.13,;35.19,-29.8,;33.69,-30.13,;32.54,-29.11,;33.55,-31.67,;32.22,-32.45,;30.88,-31.69,;29.56,-32.47,;28.22,-31.7,;28.22,-30.16,;29.56,-29.39,;29.55,-27.84,;28.2,-27.07,;28.19,-25.53,;26.87,-27.85,;25.53,-27.09,;24.21,-27.86,;24.21,-29.4,;25.54,-30.17,;26.88,-29.4,;29.56,-34,;30.89,-34.77,;32.23,-33.99,;33.56,-34.76,)| Show InChI InChI=1S/C19H14FN3O3/c20-14-6-5-11(10-16(14)23-17(24)7-8-18(23)25)9-15-12-3-1-2-4-13(12)19(26)22-21-15/h1-8,10,24-25H,9H2,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335969

(1-(1-acetylpiperidin-4-yl)-3-(4,4-dimethylcyclohex...)Show InChI InChI=1S/C16H29N3O2/c1-12(20)19-10-6-14(7-11-19)18-15(21)17-13-4-8-16(2,3)9-5-13/h13-14H,4-11H2,1-3H3,(H2,17,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335970

(1-cyclohexyl-3-(1-picolinoylpiperidin-4-yl)urea | ...)Show InChI InChI=1S/C18H26N4O2/c23-17(16-8-4-5-11-19-16)22-12-9-15(10-13-22)21-18(24)20-14-6-2-1-3-7-14/h4-5,8,11,14-15H,1-3,6-7,9-10,12-13H2,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335964

(1-(1-nicotinoylpiperidin-4-yl)-3-(4-(trifluorometh...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H19F3N4O3/c20-19(21,22)29-16-5-3-14(4-6-16)24-18(28)25-15-7-10-26(11-8-15)17(27)13-2-1-9-23-12-13/h1-6,9,12,15H,7-8,10-11H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165498
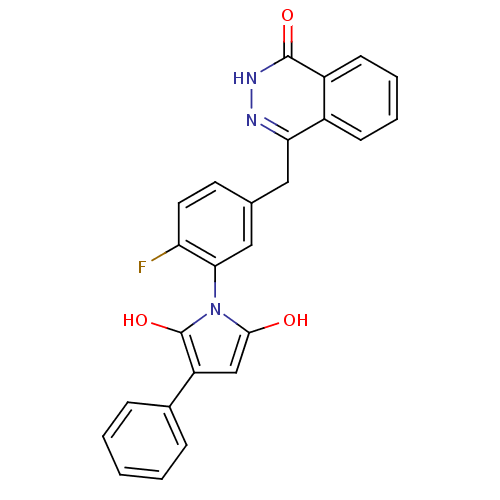
(1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Oc1cc(c(O)n1-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F)-c1ccccc1 |(14.66,-43.53,;15.81,-44.55,;17.31,-44.22,;18.09,-45.55,;17.07,-46.7,;17.4,-48.2,;15.67,-46.09,;14.34,-46.87,;13,-46.11,;11.68,-46.89,;10.34,-46.12,;10.34,-44.58,;11.68,-43.8,;11.67,-42.26,;10.32,-41.48,;10.31,-39.94,;8.99,-42.27,;7.65,-41.51,;6.33,-42.28,;6.33,-43.82,;7.66,-44.59,;9,-43.82,;11.68,-48.42,;13.01,-49.19,;14.35,-48.41,;15.68,-49.18,;19.63,-45.56,;20.38,-46.9,;21.92,-46.92,;22.71,-45.59,;21.94,-44.24,;20.41,-44.23,)| Show InChI InChI=1S/C25H18FN3O3/c26-20-11-10-15(12-21-17-8-4-5-9-18(17)24(31)28-27-21)13-22(20)29-23(30)14-19(25(29)32)16-6-2-1-3-7-16/h1-11,13-14,30,32H,12H2,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191854

(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335971
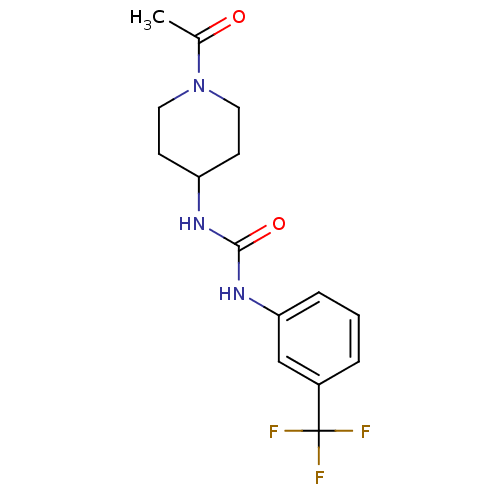
(1-(1-acetylpiperidin-4-yl)-3-(3-(trifluoromethyl)p...)Show InChI InChI=1S/C15H18F3N3O2/c1-10(22)21-7-5-12(6-8-21)19-14(23)20-13-4-2-3-11(9-13)15(16,17)18/h2-4,9,12H,5-8H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335972
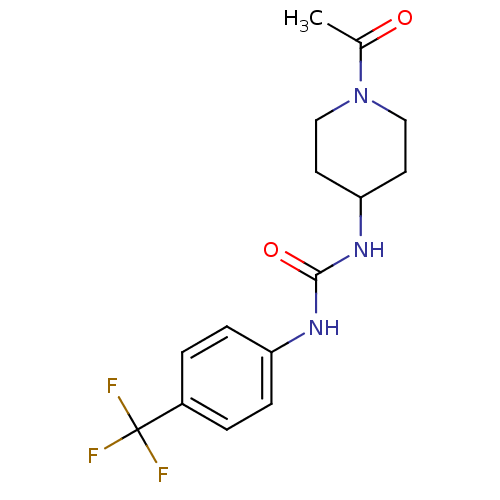
(1-(1-acetylpiperidin-4-yl)-3-(4-(trifluoromethyl)p...)Show InChI InChI=1S/C15H18F3N3O2/c1-10(22)21-8-6-13(7-9-21)20-14(23)19-12-4-2-11(3-5-12)15(16,17)18/h2-5,13H,6-9H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165478
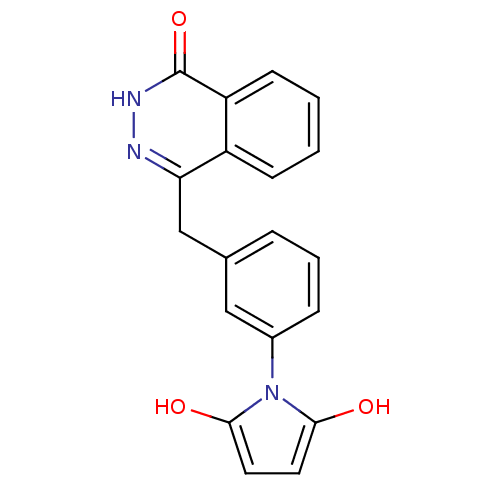
(1-(3-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)phe...)Show SMILES Oc1ccc(O)n1-c1cccc(Cc2n[nH]c(=O)c3ccccc23)c1 Show InChI InChI=1S/C19H15N3O3/c23-17-8-9-18(24)22(17)13-5-3-4-12(10-13)11-16-14-6-1-2-7-15(14)19(25)21-20-16/h1-10,23-24H,11H2,(H,21,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25744
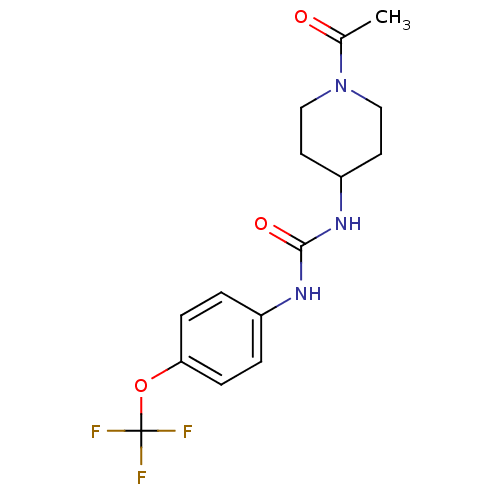
(3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...)Show InChI InChI=1S/C15H18F3N3O3/c1-10(22)21-8-6-12(7-9-21)20-14(23)19-11-2-4-13(5-3-11)24-15(16,17)18/h2-5,12H,6-9H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335973

(1-(1-acetylpiperidin-4-yl)-3-(4-tert-butylcyclohex...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC1CCC(CC1)C(C)(C)C |(1.63,-5.6,;1.57,-7.14,;.21,-7.87,;2.88,-7.96,;4.25,-7.24,;5.55,-8.05,;5.49,-9.6,;4.14,-10.32,;2.83,-9.51,;6.8,-10.41,;6.75,-11.95,;5.39,-12.68,;8.07,-12.76,;8.05,-14.31,;6.71,-15.06,;6.69,-16.59,;8.02,-17.38,;9.36,-16.62,;9.38,-15.08,;8,-18.92,;6.66,-19.67,;9.32,-19.7,;7.98,-20.45,)| Show InChI InChI=1S/C18H33N3O2/c1-13(22)21-11-9-16(10-12-21)20-17(23)19-15-7-5-14(6-8-15)18(2,3)4/h14-16H,5-12H2,1-4H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335967

(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H22F3N3O3S/c1-11(2)26(24,25)22-9-7-14(8-10-22)21-15(23)20-13-5-3-12(4-6-13)16(17,18)19/h3-6,11,14H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27708
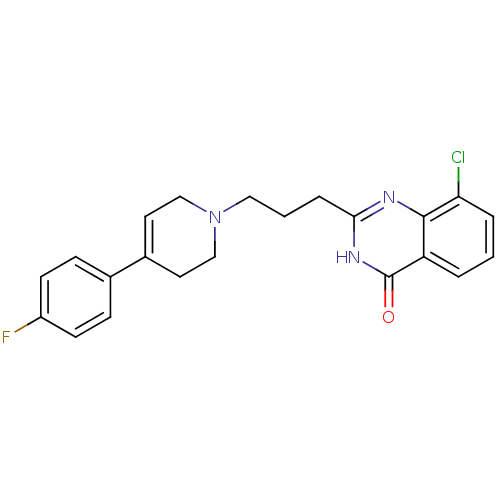
(8-chloro-2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahyd...)Show SMILES Fc1ccc(cc1)C1=CCN(CCCc2nc3c(Cl)cccc3c(=O)[nH]2)CC1 |t:8| Show InChI InChI=1S/C22H21ClFN3O/c23-19-4-1-3-18-21(19)25-20(26-22(18)28)5-2-12-27-13-10-16(11-14-27)15-6-8-17(24)9-7-15/h1,3-4,6-10H,2,5,11-14H2,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335974
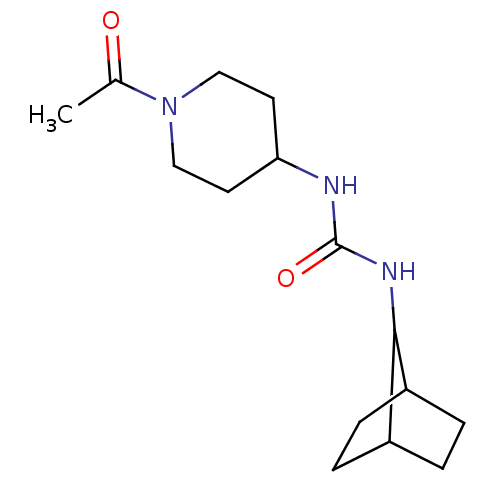
(1-(1-Acetyl-piperidin-4-yl)-3-bicyclo[2.2.1]hept-7...)Show InChI InChI=1S/C15H25N3O2/c1-10(19)18-8-6-13(7-9-18)16-15(20)17-14-11-2-3-12(14)5-4-11/h11-14H,2-9H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27709
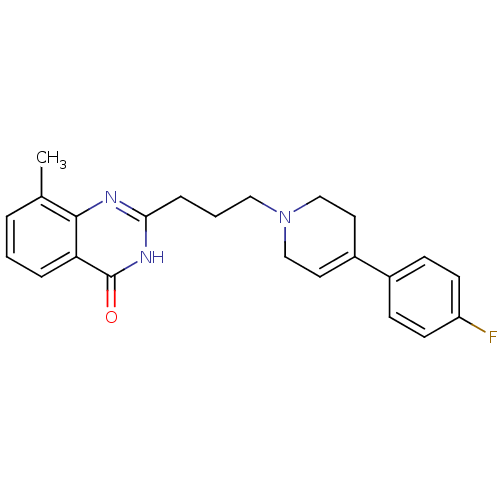
(2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahydropyridin...)Show SMILES Cc1cccc2c1nc(CCCN1CCC(=CC1)c1ccc(F)cc1)[nH]c2=O |c:16| Show InChI InChI=1S/C23H24FN3O/c1-16-4-2-5-20-22(16)25-21(26-23(20)28)6-3-13-27-14-11-18(12-15-27)17-7-9-19(24)10-8-17/h2,4-5,7-11H,3,6,12-15H2,1H3,(H,25,26,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165472
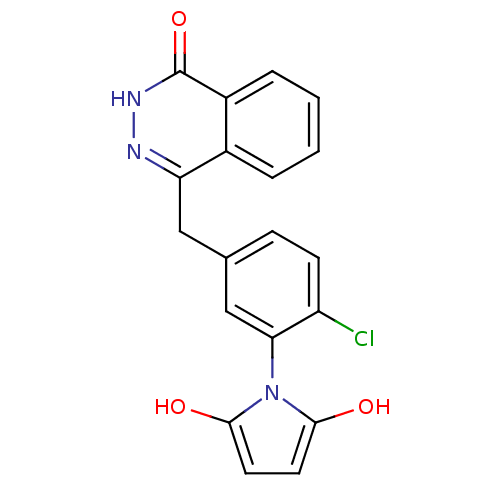
(1-(2-chloro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Oc1ccc(O)n1-c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1Cl |(18.5,-33.78,;18.16,-32.28,;19.19,-31.13,;18.41,-29.8,;16.9,-30.13,;15.75,-29.11,;16.76,-31.67,;15.43,-32.45,;14.1,-31.69,;12.77,-32.47,;11.44,-31.7,;11.43,-30.16,;12.77,-29.39,;12.76,-27.84,;11.42,-27.07,;11.41,-25.53,;10.09,-27.85,;8.75,-27.09,;7.42,-27.86,;7.42,-29.4,;8.75,-30.17,;10.1,-29.4,;12.77,-34,;14.11,-34.77,;15.44,-33.99,;16.78,-34.76,)| Show InChI InChI=1S/C19H14ClN3O3/c20-14-6-5-11(10-16(14)23-17(24)7-8-18(23)25)9-15-12-3-1-2-4-13(12)19(26)22-21-15/h1-8,10,24-25H,9H2,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165477
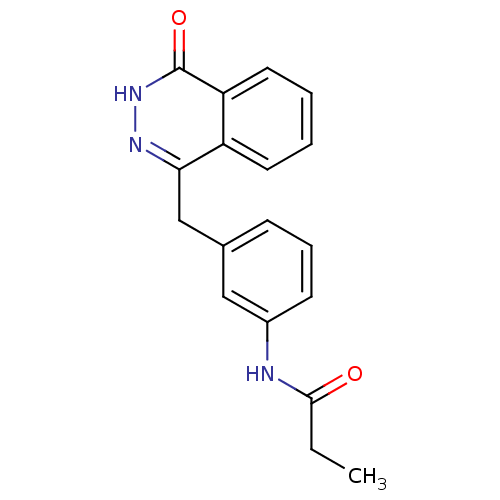
(CHEMBL371425 | N-(3-((4-oxo-3,4-dihydrophthalazin-...)Show InChI InChI=1S/C18H17N3O2/c1-2-17(22)19-13-7-5-6-12(10-13)11-16-14-8-3-4-9-15(14)18(23)21-20-16/h3-10H,2,11H2,1H3,(H,19,22)(H,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27705
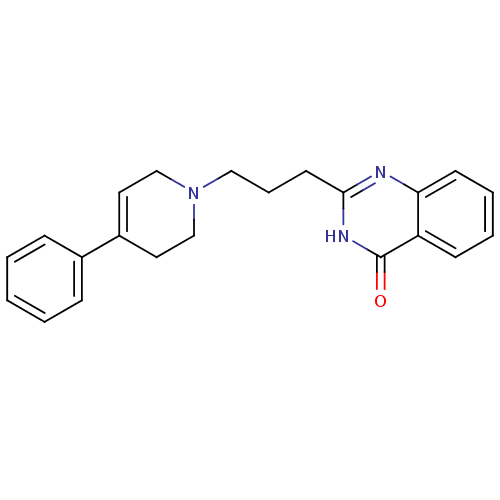
(2-[3-(4-phenyl-1,2,3,6-tetrahydropyridin-1-yl)prop...)Show SMILES O=c1[nH]c(CCCN2CCC(=CC2)c2ccccc2)nc2ccccc12 |c:10| Show InChI InChI=1S/C22H23N3O/c26-22-19-9-4-5-10-20(19)23-21(24-22)11-6-14-25-15-12-18(13-16-25)17-7-2-1-3-8-17/h1-5,7-10,12H,6,11,13-16H2,(H,23,24,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50220868
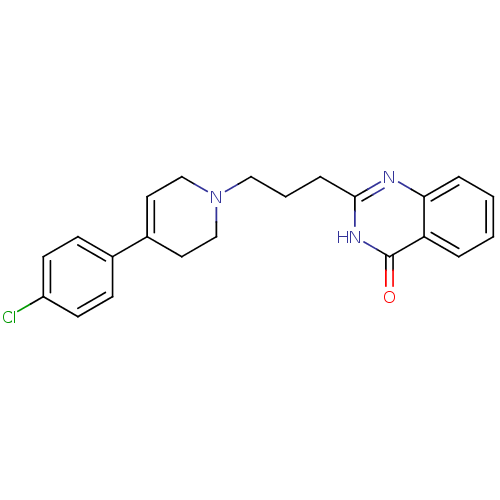
(2-(3-(4-(4-chlorophenyl)-5,6-dihydropyridin-1(2H)-...)Show SMILES Clc1ccc(cc1)C1=CCN(CCCc2nc3ccccc3c(=O)[nH]2)CC1 |t:8| Show InChI InChI=1S/C22H22ClN3O/c23-18-9-7-16(8-10-18)17-11-14-26(15-12-17)13-3-6-21-24-20-5-2-1-4-19(20)22(27)25-21/h1-2,4-5,7-11H,3,6,12-15H2,(H,24,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255501

(3-(2,4-dioxo-2,3,4,5,7,8-hexahydro-1H-thiopyrano[4...)Show SMILES O=C(CCn1c2CCSCc2c(=O)[nH]c1=O)NCC(=O)c1ccc(cc1)-c1ccnnc1 Show InChI InChI=1S/C22H21N5O4S/c28-19(15-3-1-14(2-4-15)16-5-8-24-25-11-16)12-23-20(29)6-9-27-18-7-10-32-13-17(18)21(30)26-22(27)31/h1-5,8,11H,6-7,9-10,12-13H2,(H,23,29)(H,26,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335975

(1-(1-Acetyl-piperidin-4-yl)-3-(4-methyl-bicyclo[2....)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC1CC2(C)CCC1CC2 |(19.45,7.01,;19.4,5.47,;18.03,4.74,;20.7,4.65,;22.06,5.37,;23.37,4.56,;23.3,3.02,;21.96,2.29,;20.65,3.11,;24.61,2.21,;24.57,.67,;23.21,-.06,;25.88,-.14,;25.86,-1.68,;26.33,-2.77,;26.12,-4.67,;26.14,-6.19,;27.5,-5.27,;27.19,-3.88,;25.83,-3.31,;24.39,-3.99,;24.61,-5.36,)| Show InChI InChI=1S/C17H29N3O2/c1-12(21)20-9-5-14(6-10-20)18-16(22)19-15-11-17(2)7-3-13(15)4-8-17/h13-15H,3-11H2,1-2H3,(H2,18,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50120265
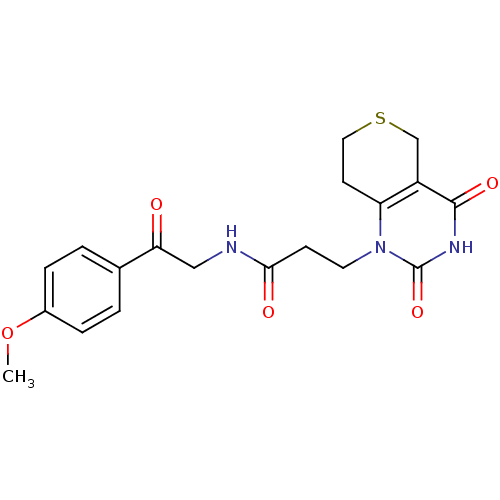
(3-(2,4-Dioxo-3,4,7,8-tetrahydro-2H,5H-thiopyrano[4...)Show SMILES COc1ccc(cc1)C(=O)CNC(=O)CCn1c2CCSCc2c(=O)[nH]c1=O Show InChI InChI=1S/C19H21N3O5S/c1-27-13-4-2-12(3-5-13)16(23)10-20-17(24)6-8-22-15-7-9-28-11-14(15)18(25)21-19(22)26/h2-5H,6-11H2,1H3,(H,20,24)(H,21,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335968

(1-(1-(methylsulfonyl)piperidin-4-yl)-3-(4-(trifluo...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C14H18F3N3O3S/c1-24(22,23)20-8-6-12(7-9-20)19-13(21)18-11-4-2-10(3-5-11)14(15,16)17/h2-5,12H,6-9H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255360
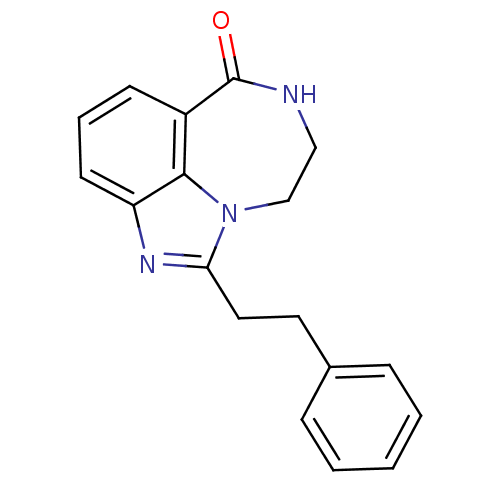
(1-Phenethyl-8,9-dihydro-7H-2,7,9a-triaza-benzo[cd]...)Show InChI InChI=1S/C18H17N3O/c22-18-14-7-4-8-15-17(14)21(12-11-19-18)16(20-15)10-9-13-5-2-1-3-6-13/h1-8H,9-12H2,(H,19,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255630
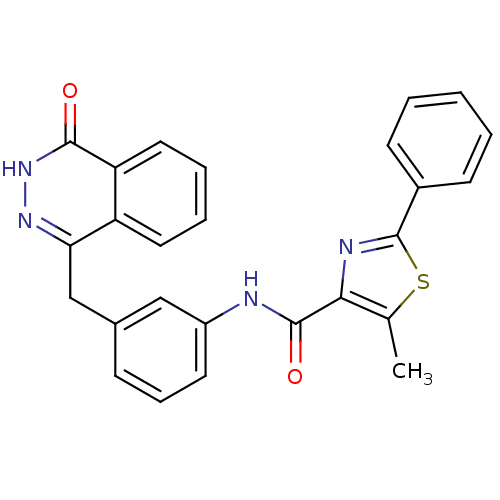
(5-methyl-N-(3-((4-oxo-3,4-dihydrophthalazin-1-yl)m...)Show SMILES Cc1sc(nc1C(=O)Nc1cccc(Cc2n[nH]c(=O)c3ccccc23)c1)-c1ccccc1 Show InChI InChI=1S/C26H20N4O2S/c1-16-23(28-26(33-16)18-9-3-2-4-10-18)25(32)27-19-11-7-8-17(14-19)15-22-20-12-5-6-13-21(20)24(31)30-29-22/h2-14H,15H2,1H3,(H,27,32)(H,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25743

(1-cycloheptyl-3-(1-acetylpiperidin-4-yl)urea | US8...)Show InChI InChI=1S/C15H27N3O2/c1-12(19)18-10-8-14(9-11-18)17-15(20)16-13-6-4-2-3-5-7-13/h13-14H,2-11H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335976
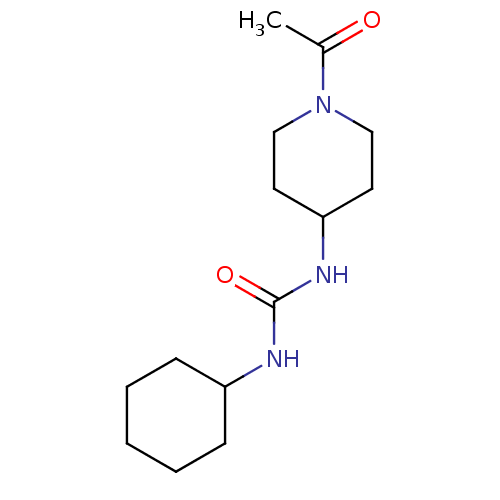
(1-(1-Acetyl-piperidin-4-yl)-3-cyclohexyl-urea | CH...)Show InChI InChI=1S/C14H25N3O2/c1-11(18)17-9-7-13(8-10-17)16-14(19)15-12-5-3-2-4-6-12/h12-13H,2-10H2,1H3,(H2,15,16,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165484
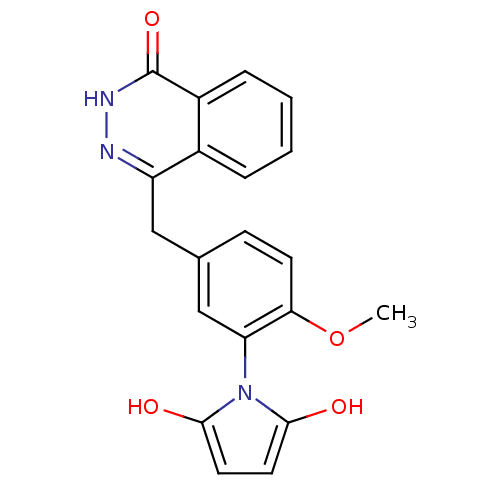
(1-(2-methoxy-5-((4-oxo-3,4-dihydrophthalazin-1-yl)...)Show SMILES COc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1-n1c(O)ccc1O |(-3.66,1.78,;-4.47,.48,;-3.75,-.88,;-2.22,-.93,;-1.48,-2.29,;-2.3,-3.61,;-1.58,-4.97,;-.04,-5.02,;.68,-6.38,;2.21,-6.44,;3.03,-5.13,;4.57,-5.19,;2.31,-3.77,;3.13,-2.47,;2.41,-1.1,;.86,-1.05,;.05,-2.35,;.77,-3.71,;-3.85,-3.55,;-4.57,-2.19,;-6.11,-2.13,;-7.06,-3.34,;-6.64,-4.82,;-8.51,-2.81,;-8.45,-1.27,;-6.97,-.85,;-6.44,.6,)| Show InChI InChI=1S/C20H17N3O4/c1-27-17-7-6-12(11-16(17)23-18(24)8-9-19(23)25)10-15-13-4-2-3-5-14(13)20(26)22-21-15/h2-9,11,24-25H,10H2,1H3,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165474
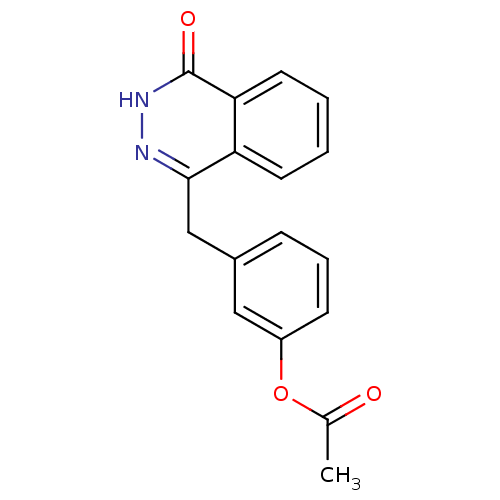
(3-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)phenyl...)Show InChI InChI=1S/C17H14N2O3/c1-11(20)22-13-6-4-5-12(9-13)10-16-14-7-2-3-8-15(14)17(21)19-18-16/h2-9H,10H2,1H3,(H,19,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335969

(1-(1-acetylpiperidin-4-yl)-3-(4,4-dimethylcyclohex...)Show InChI InChI=1S/C16H29N3O2/c1-12(20)19-10-6-14(7-11-19)18-15(21)17-13-4-8-16(2,3)9-5-13/h13-14H,4-11H2,1-3H3,(H2,17,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255572
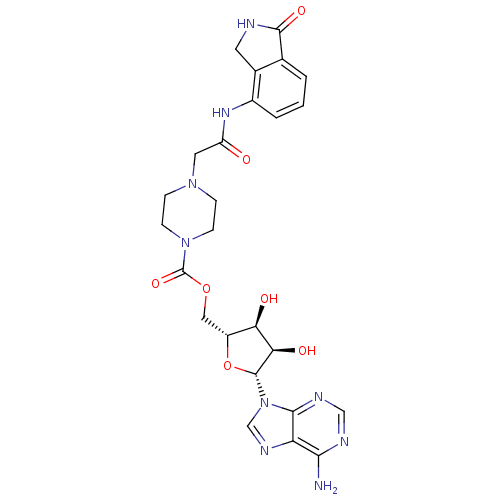
(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COC(=O)N2CCN(CC(=O)Nc3cccc4C(=O)NCc34)CC2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C25H29N9O7/c26-21-18-22(29-11-28-21)34(12-30-18)24-20(37)19(36)16(41-24)10-40-25(39)33-6-4-32(5-7-33)9-17(35)31-15-3-1-2-13-14(15)8-27-23(13)38/h1-3,11-12,16,19-20,24,36-37H,4-10H2,(H,27,38)(H,31,35)(H2,26,28,29)/t16-,19-,20-,24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255502
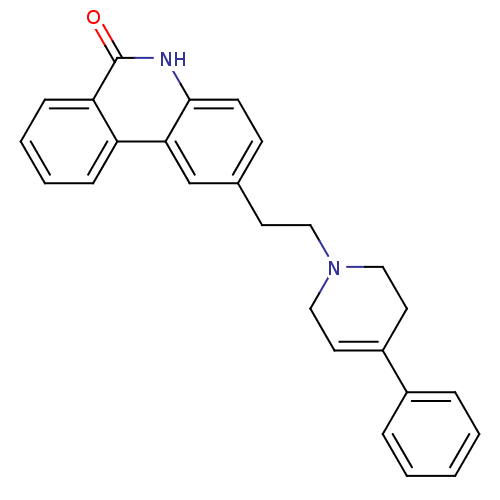
(2-(2-(4-phenyl-5,6-dihydropyridin-1(2H)-yl)ethyl)p...)Show SMILES O=c1[nH]c2ccc(CCN3CCC(=CC3)c3ccccc3)cc2c2ccccc12 |c:12| Show InChI InChI=1S/C26H24N2O/c29-26-23-9-5-4-8-22(23)24-18-19(10-11-25(24)27-26)12-15-28-16-13-21(14-17-28)20-6-2-1-3-7-20/h1-11,13,18H,12,14-17H2,(H,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165483
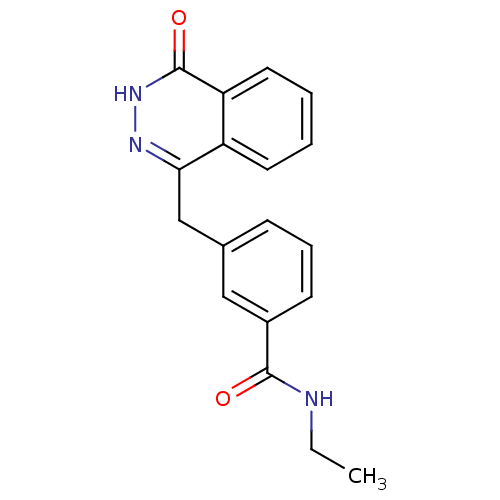
(CHEMBL381208 | N-Ethyl-3-(4-oxo-3,4-dihydro-phthal...)Show InChI InChI=1S/C18H17N3O2/c1-2-19-17(22)13-7-5-6-12(10-13)11-16-14-8-3-4-9-15(14)18(23)21-20-16/h3-10H,2,11H2,1H3,(H,19,22)(H,21,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50165471
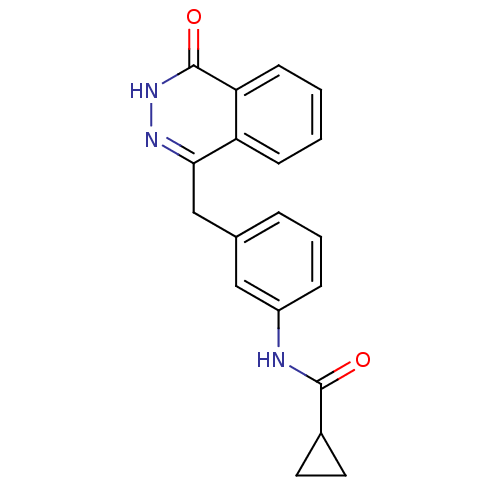
(CHEMBL196507 | Cyclopropanecarboxylic acid [3-(4-o...)Show SMILES O=C(Nc1cccc(Cc2n[nH]c(=O)c3ccccc23)c1)C1CC1 Show InChI InChI=1S/C19H17N3O2/c23-18(13-8-9-13)20-14-5-3-4-12(10-14)11-17-15-6-1-2-7-16(15)19(24)22-21-17/h1-7,10,13H,8-9,11H2,(H,20,23)(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50240980
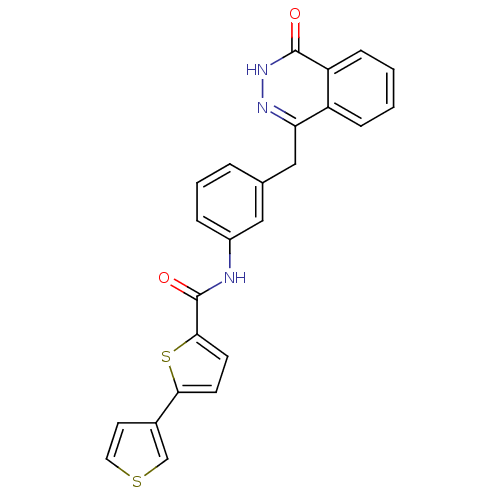
(CHEMBL372450 | N-(3-((4-oxo-3,4-dihydrophthalazin-...)Show SMILES O=C(Nc1cccc(Cc2n[nH]c(=O)c3ccccc23)c1)c1ccc(s1)-c1ccsc1 Show InChI InChI=1S/C24H17N3O2S2/c28-23-19-7-2-1-6-18(19)20(26-27-23)13-15-4-3-5-17(12-15)25-24(29)22-9-8-21(31-22)16-10-11-30-14-16/h1-12,14H,13H2,(H,25,29)(H,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191854

(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335977

(1-(1-Acetyl-piperidin-4-yl)-3-(3-fluoro-adamantan-...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(F)(CC(F)(C3)C1)C2 |TLB:16:17:23:22.15.14,18:17:23:22.15.14,18:17:20.22.23:14,21:20:17.16.24:14,THB:16:15:19.17.24:23,19:20:17.16.24:14,19:17:20.22.23:14| Show InChI InChI=1S/C18H27F2N3O2/c1-12(24)23-4-2-14(3-5-23)21-15(25)22-18-8-13-6-16(19,10-18)9-17(20,7-13)11-18/h13-14H,2-11H2,1H3,(H2,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25744

(3-(1-acetylpiperidin-4-yl)-1-[4-(trifluoromethoxy)...)Show InChI InChI=1S/C15H18F3N3O3/c1-10(22)21-8-6-12(7-9-21)20-14(23)19-11-2-4-13(5-3-11)24-15(16,17)18/h2-5,12H,6-9H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50255411
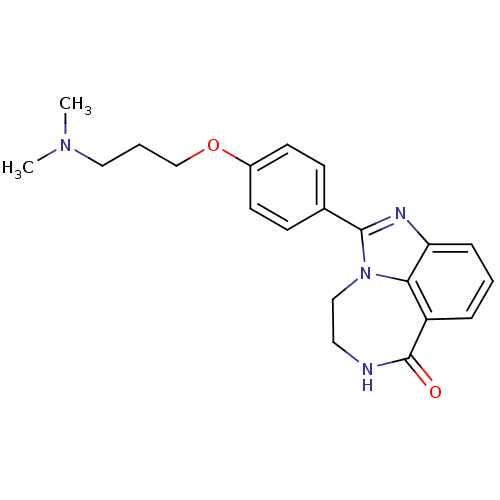
(1-[4-(3-Dimethylamino-propoxy)-phenyl]-8,9-dihydro...)Show SMILES CN(C)CCCOc1ccc(cc1)-c1nc2cccc3C(=O)NCCn1c23 Show InChI InChI=1S/C21H24N4O2/c1-24(2)12-4-14-27-16-9-7-15(8-10-16)20-23-18-6-3-5-17-19(18)25(20)13-11-22-21(17)26/h3,5-10H,4,11-14H2,1-2H3,(H,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PARP1 |
J Med Chem 52: 718-25 (2009)
Article DOI: 10.1021/jm800902t
BindingDB Entry DOI: 10.7270/Q2C53KPW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data