

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50126528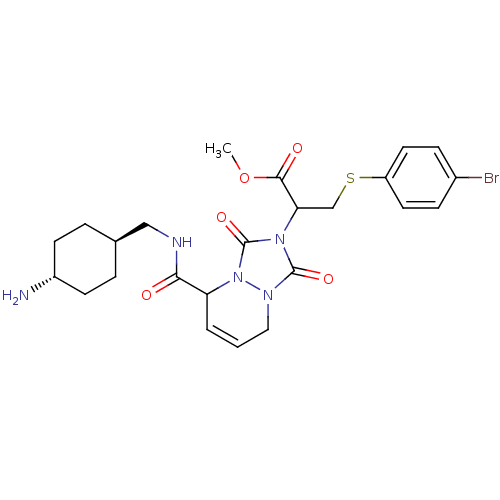 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071565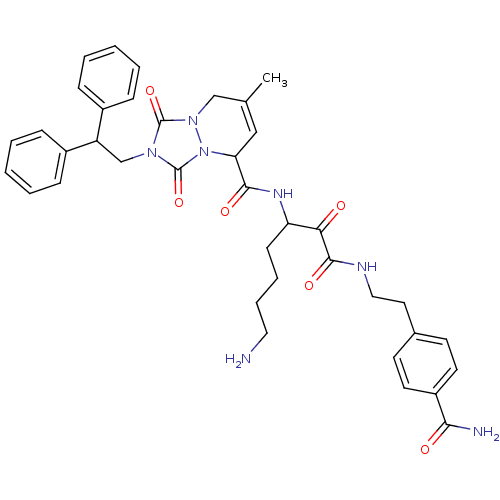 (2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126525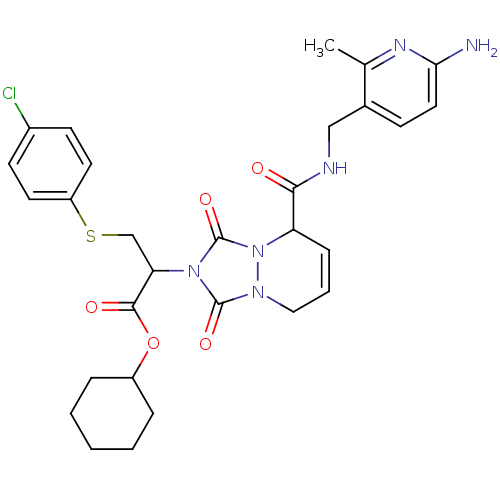 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076224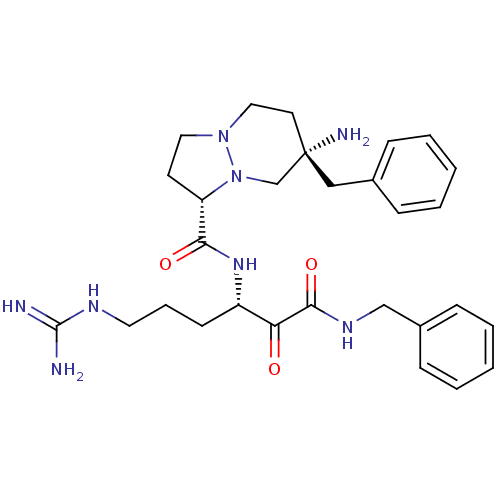 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076224 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126521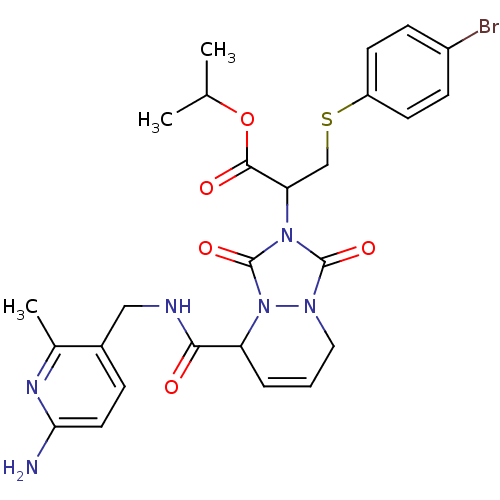 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50234795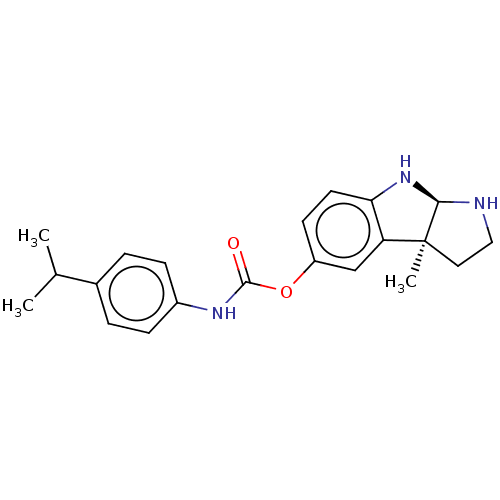 (CHEMBL4089082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071575 (2,2-Dibutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076219 ((3S,6R)-6-Amino-6-benzyl-octahydro-indolizine-3-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126508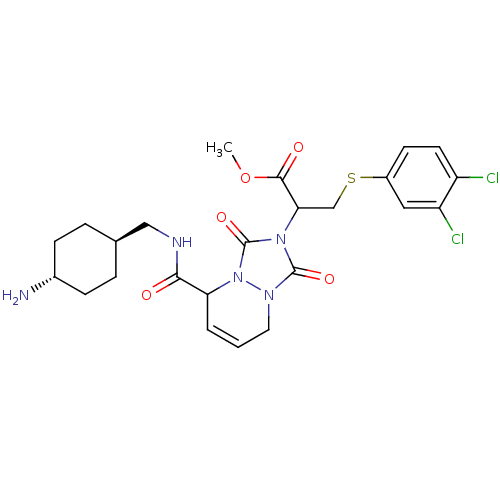 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126520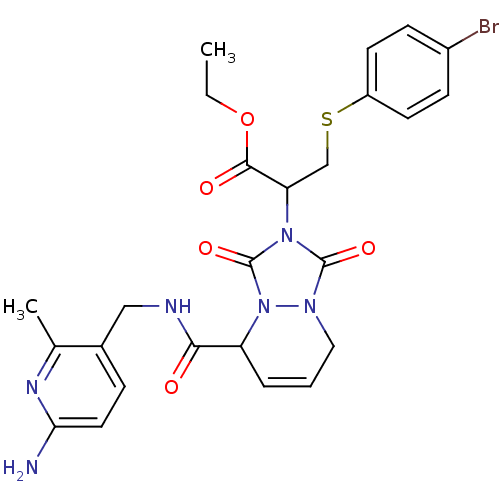 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071570 (8-Isobutyl-2-(4-methoxy-phenyl)-1,3-dioxo-2,3,5,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071573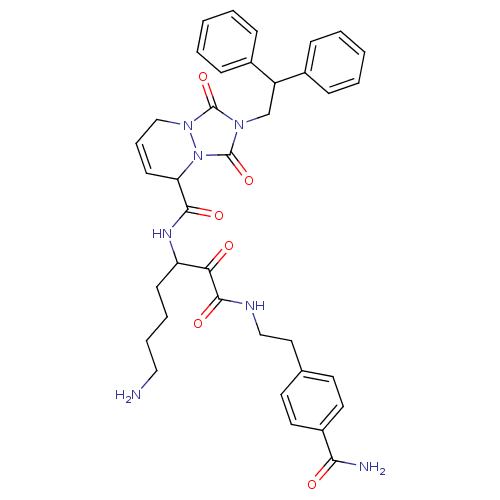 (2-(2,2-Diphenyl-ethyl)-1,3-dioxo-2,3,5,8-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076223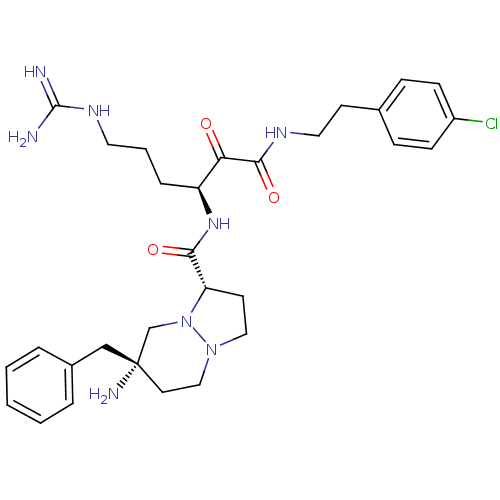 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126526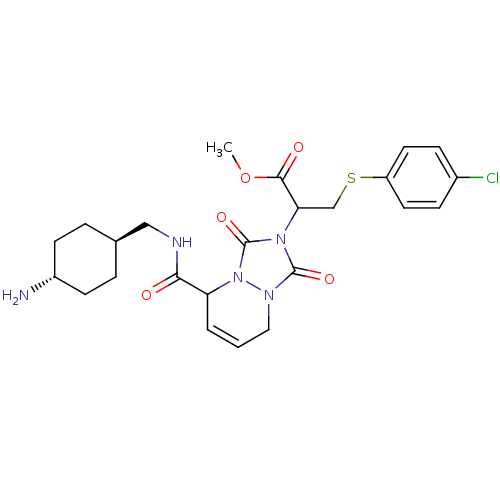 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323737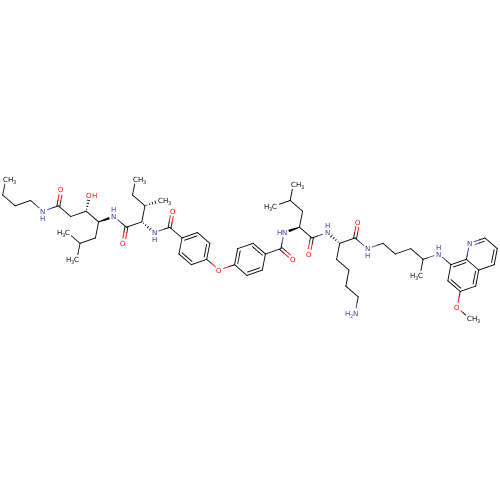 (CHEMBL1213687 | N-((2S)-1-((2S)-6-amino-1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071571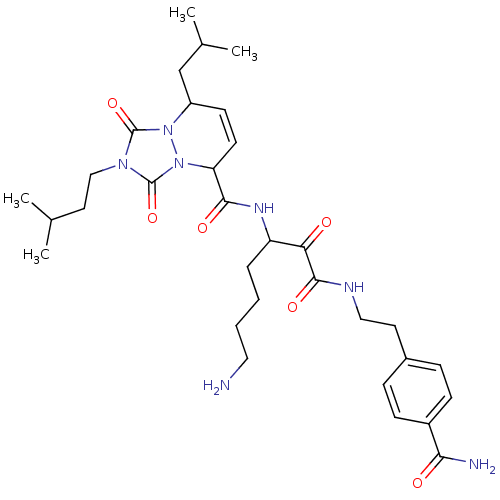 (8-Isobutyl-2-(3-methyl-butyl)-1,3-dioxo-2,3,5,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076225 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50209559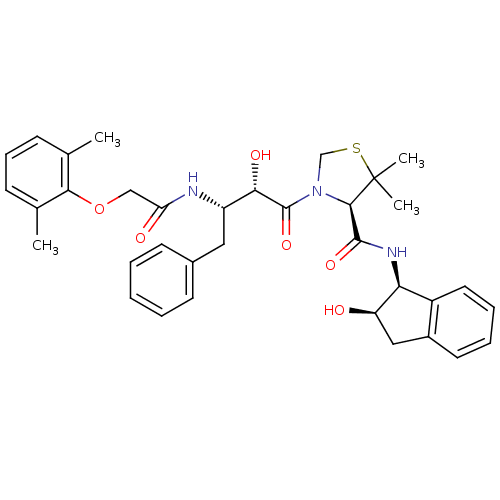 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 expressed in Escherichia coli BL21 (DE3) by fluorometric assay | Bioorg Med Chem 17: 5933-49 (2009) Article DOI: 10.1016/j.bmc.2009.06.065 BindingDB Entry DOI: 10.7270/Q2S46S0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076222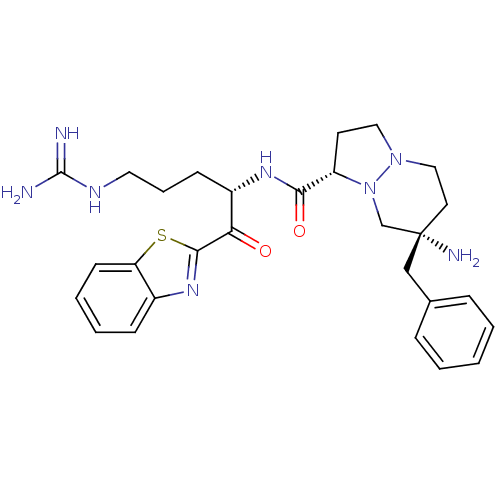 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076222 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126500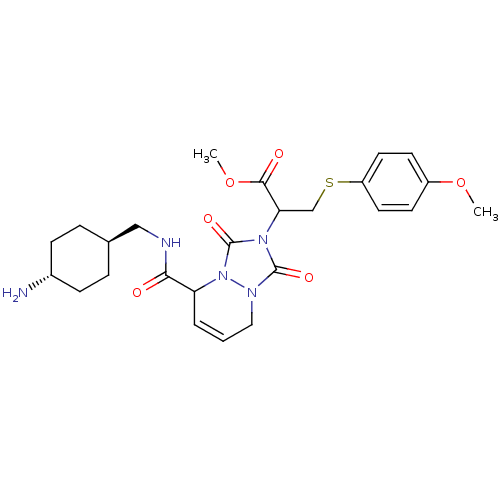 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076219 ((3S,6R)-6-Amino-6-benzyl-octahydro-indolizine-3-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076219 ((3S,6R)-6-Amino-6-benzyl-octahydro-indolizine-3-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390636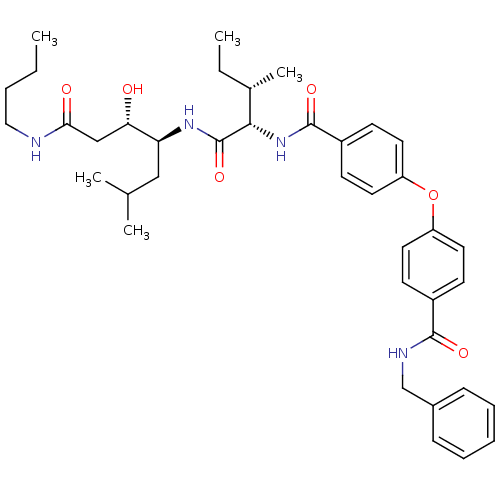 (CHEMBL2069615) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126514 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126523 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071568 (2-Amino-2-benzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071567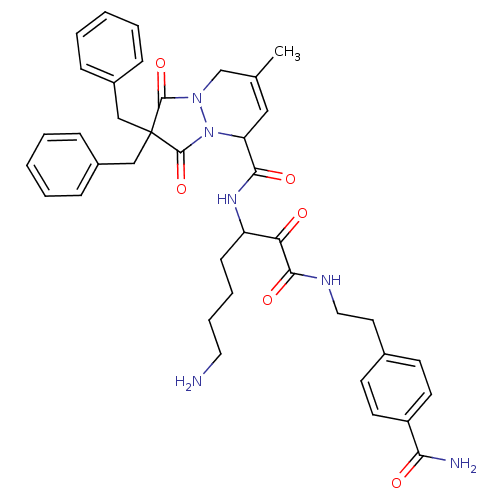 (2,2-Dibenzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390637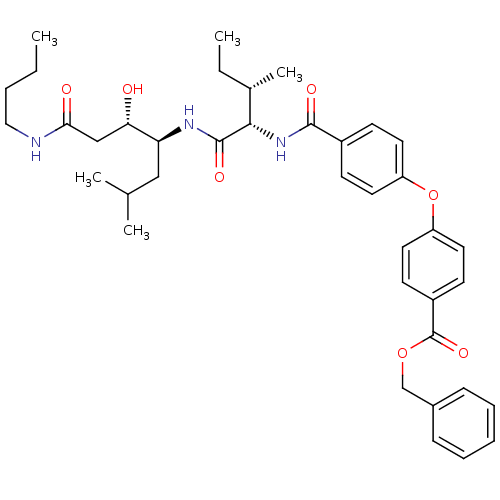 (CHEMBL2069616) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM642921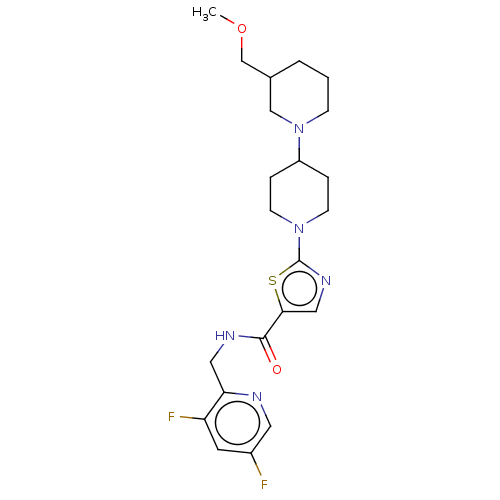 (US20240000767, Example 100 | US20240000767, Exampl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50209559 ((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin D | Bioorg Med Chem 17: 5933-49 (2009) Article DOI: 10.1016/j.bmc.2009.06.065 BindingDB Entry DOI: 10.7270/Q2S46S0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390633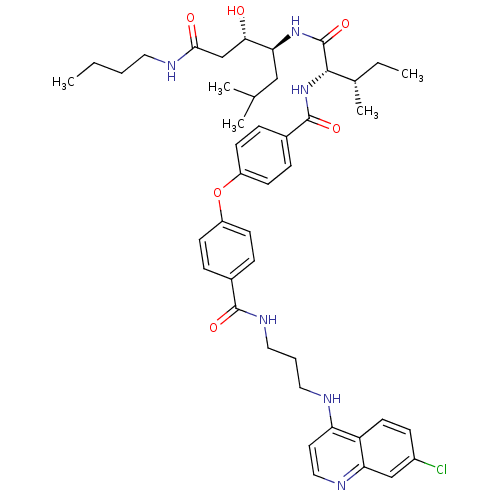 (CHEMBL2069612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076228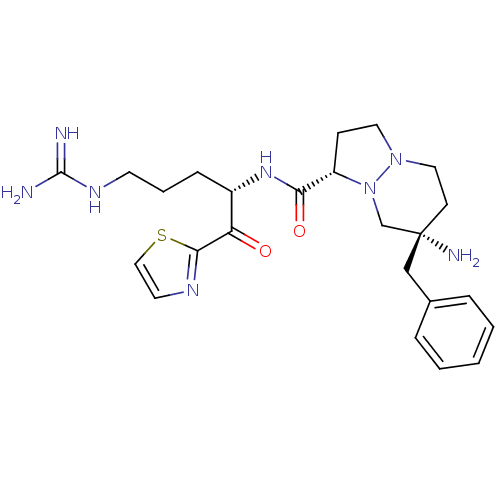 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against thrombin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Asparagine synthetase [glutamine-hydrolyzing] (Homo sapiens (Human)) | BDBM50378579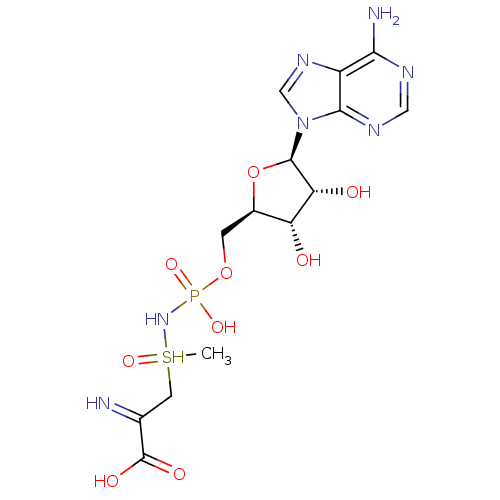 (CHEMBL1627258) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of human recombinant ASNS expressed in Sf9 cells assessed as assessed as overall inhibition constant for ammonia dependent production of i... | Bioorg Med Chem 20: 5915-27 (2012) Article DOI: 10.1016/j.bmc.2012.07.047 BindingDB Entry DOI: 10.7270/Q2MC914M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390635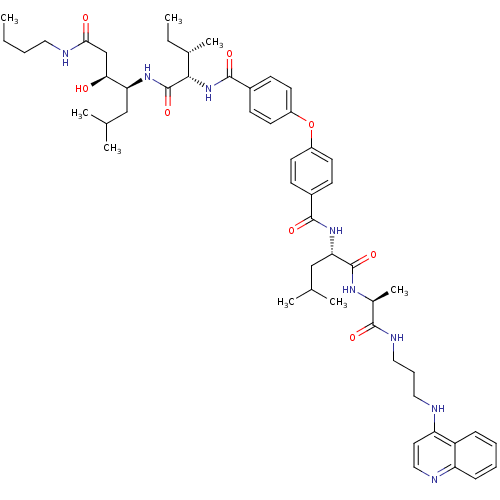 (CHEMBL2069614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126529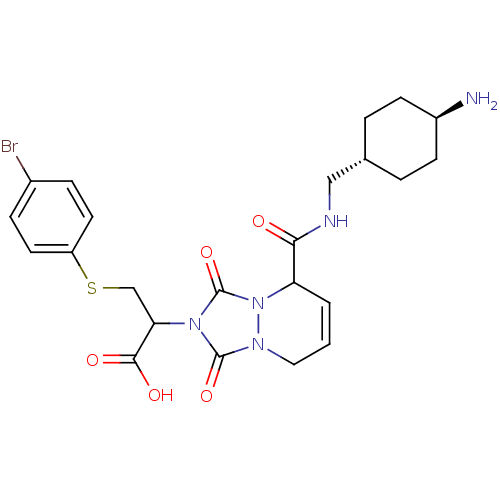 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126532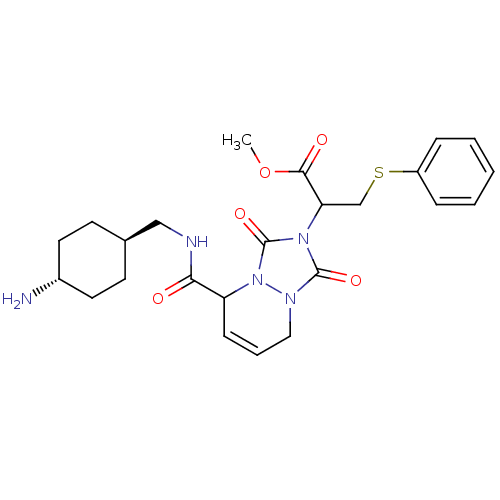 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390634 (CHEMBL2069613) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071566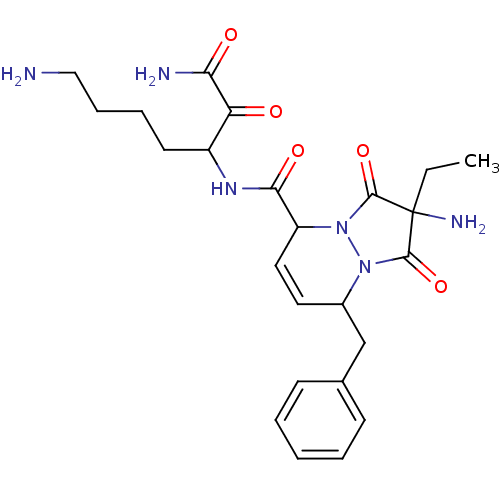 (2-Amino-8-benzyl-2-ethyl-1,3-dioxo-2,3,5,8-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126534 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071574 (2,2-Diisobutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126505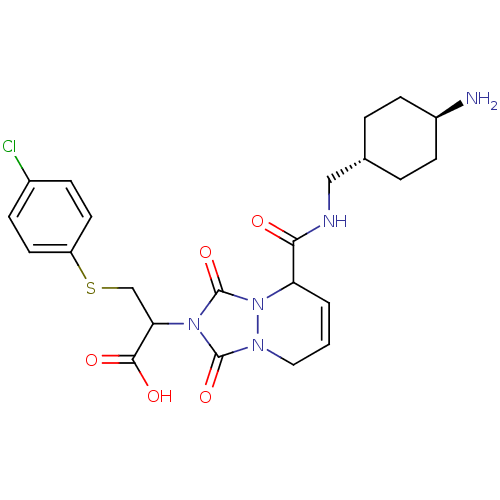 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50216213 (CHEMBL306744) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the tryptase | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50393088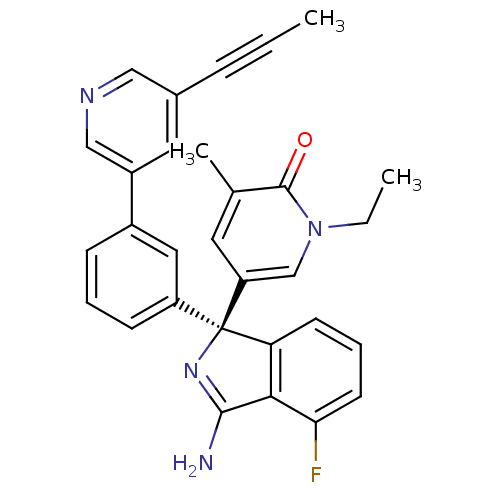 (CHEMBL2152903) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE2 using (europium)CEVNLDAEFK(Qsy7) as substrate incubated for 10 mins prior to substrate addition measured after ... | J Med Chem 55: 9346-61 (2012) Article DOI: 10.1021/jm3009025 BindingDB Entry DOI: 10.7270/Q2WW7JTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126519 (2-[1-(4-Chloro-phenylsulfanylmethyl)-2-morpholin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Asparagine synthetase [glutamine-hydrolyzing] (Homo sapiens (Human)) | BDBM50392270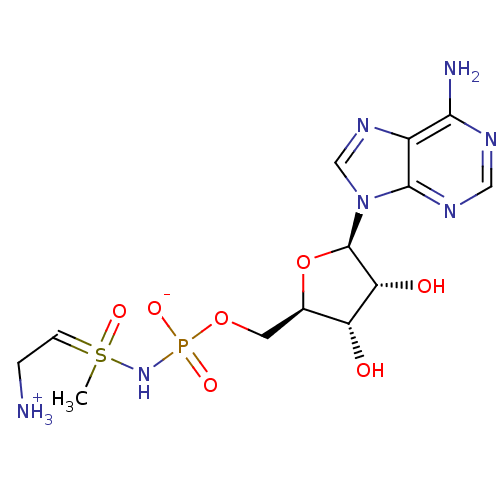 (CHEMBL2153507) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of human recombinant ASNS expressed in Sf9 cells assessed as overall inhibition constant for glutamine dependent production of inorganic p... | Bioorg Med Chem 20: 5915-27 (2012) Article DOI: 10.1016/j.bmc.2012.07.047 BindingDB Entry DOI: 10.7270/Q2MC914M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50342656 (CHEMBL1770735 | benzo[b]thiophen-2-ylmethanamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Eastern Finland Curated by ChEMBL | Assay Description Inhibition of human CYP2A6 in baculovirus-infected insect cell system using coumarin 7 as substrate preincubated for 10 mins by fluorescence assay | Bioorg Med Chem 22: 6655-64 (2015) Article DOI: 10.1016/j.bmc.2014.10.001 BindingDB Entry DOI: 10.7270/Q2V40WT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2791 total ) | Next | Last >> |