

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259295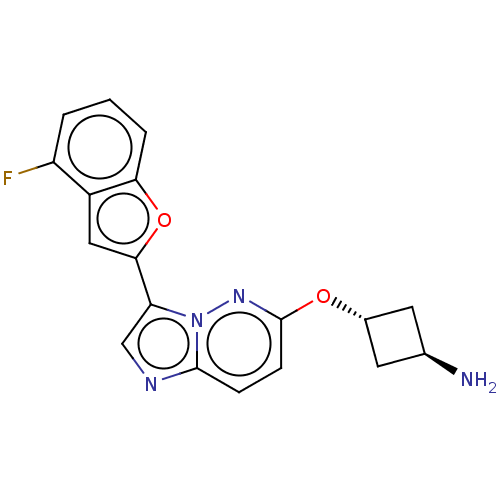 (US9499547, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1 (Homo sapiens (Human)) | BDBM50134989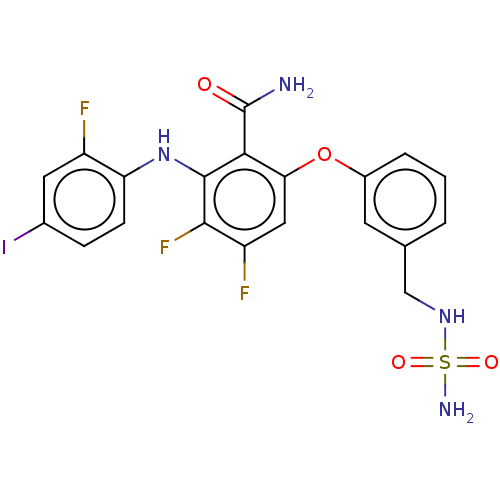 (CHEMBL3746640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of MEK1 in human HeLa-MaTu matched pair cells assessed as reduction in ERK phosphorylation | Bioorg Med Chem Lett 26: 186-93 (2015) Article DOI: 10.1016/j.bmcl.2015.11.004 BindingDB Entry DOI: 10.7270/Q26M38NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259295 (US9499547, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description MKNK1-inhibitory activity at high ATP of compounds of the present invention after their preincubation with MKNK1 was quantified employing the TR-FRET... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378995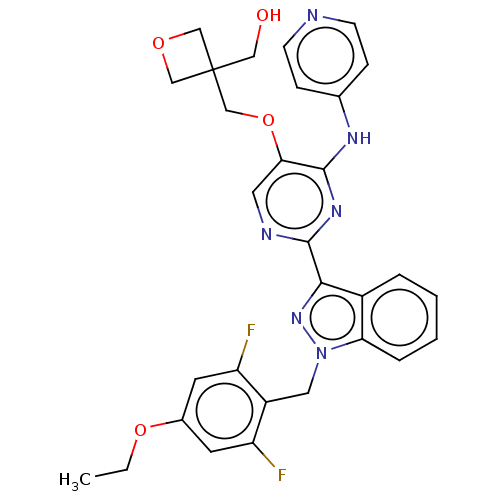 (Preparation of {3-[({2-[1-(4-ethoxy-2,6-difluorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415317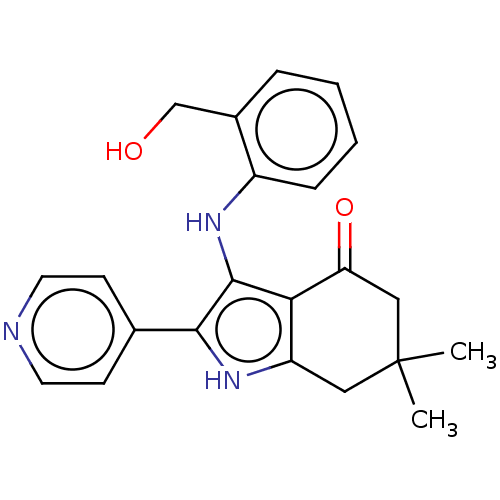 (Preparation of 3-{[2-(hydroxymethyl)phenyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.58 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378915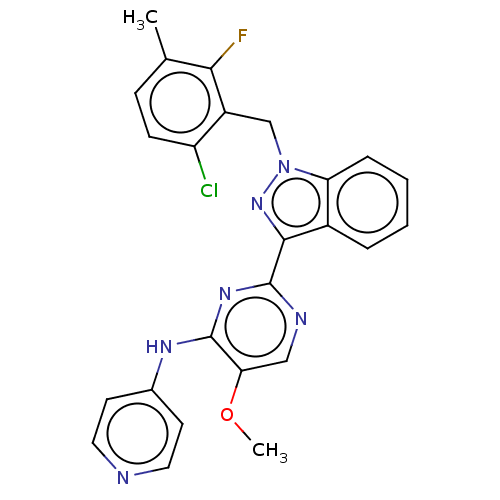 (2-[1-(6-chloro-2-fluoro-3-methylbenzyl)-1H-indazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378932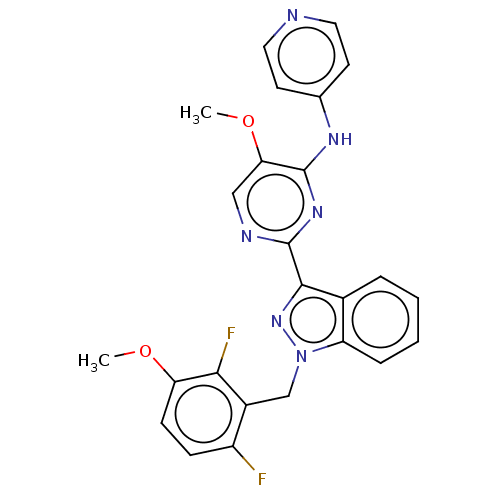 (2-[1-(6-chloro-2- fluoro-3- methoxybenzyl)- 1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259297 (US9499547, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259298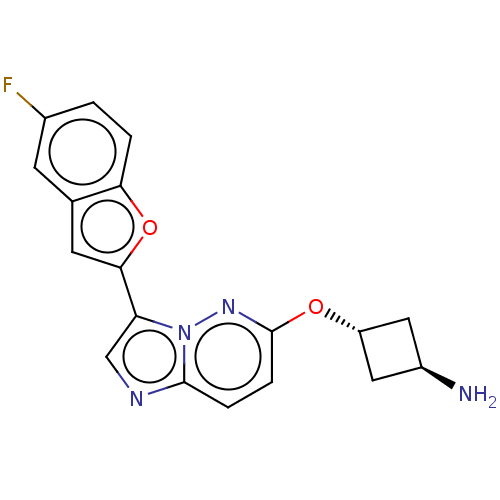 (US9499547, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259260 (US9499547, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379026 (Preparation of 1-[3,5-difluoro-4-({3-[5-methoxy-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379006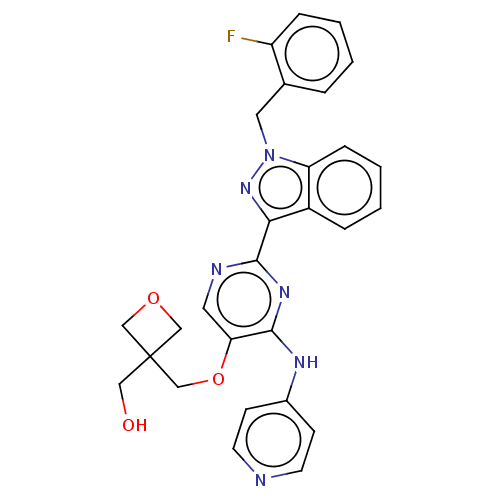 (US10266548, Example 4-2-1 | {3[({2-[1-(2- fluorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379042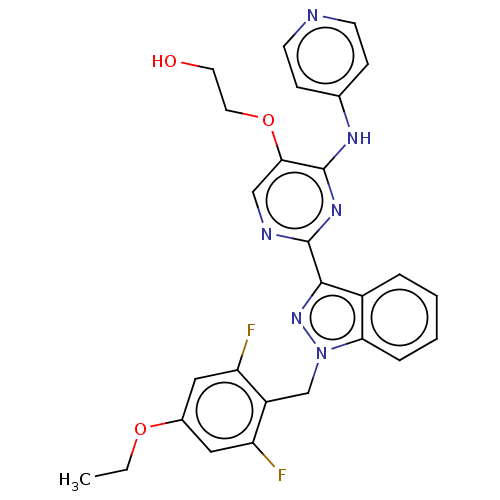 (Preparation of 2-({2-[1-(4-ethoxy-2,6-difluorobenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415374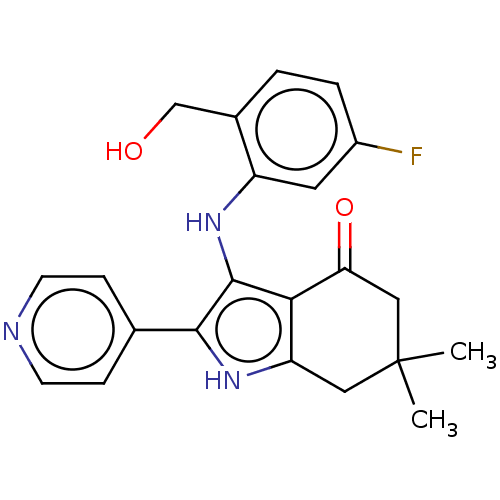 (Preparation of 3-{[5-fluoro-2-(hydroxymethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379107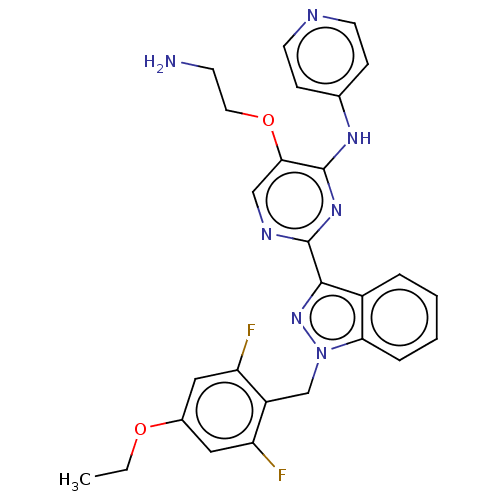 (Preparation of 5-(2-aminoethoxy)-2-[1-(4-ethoxy-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379108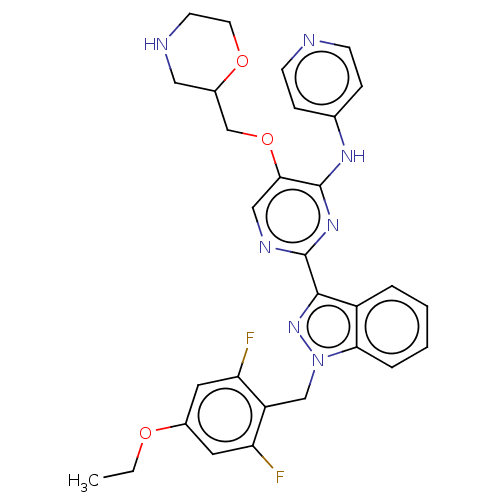 (2-[1-(4-ethoxy-2,6- difluorobenzyl)-1H- indazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415373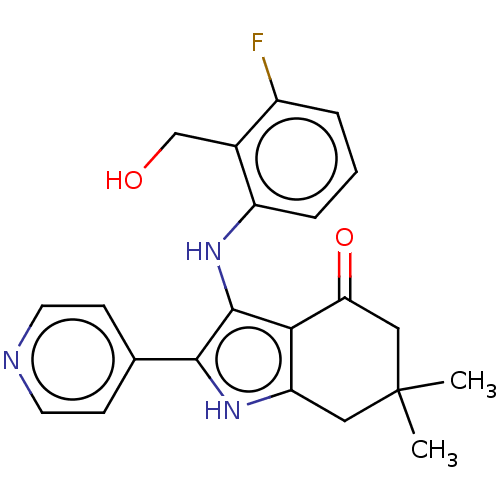 (Preparation of 3-{[3-fluoro-2-(hydroxymethyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415303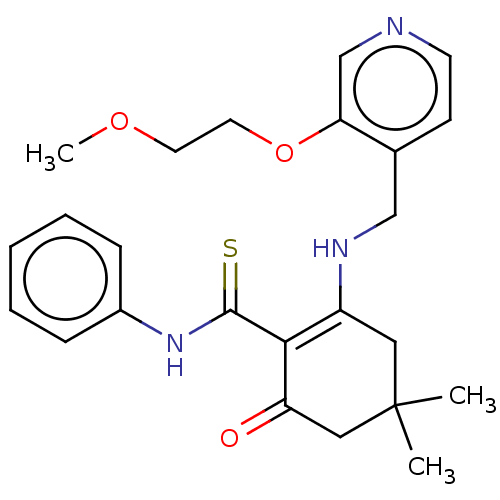 (Intermediate 1-2-69 | US10428044, Example 410) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379024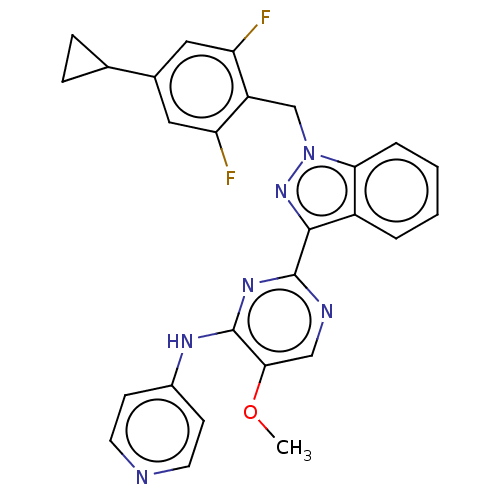 (Preparation of 2-[1-(4-cyclopropyl-2,6-difluoroben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415283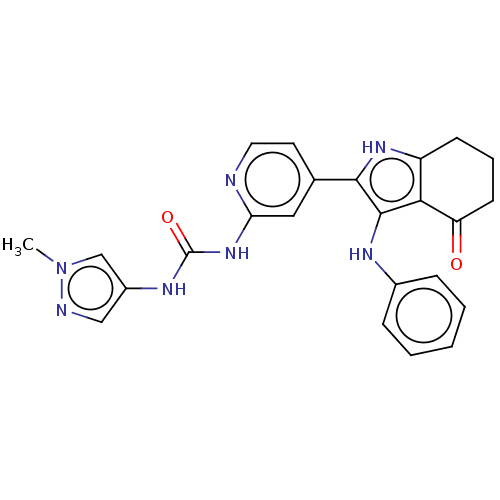 (Preparation of 1-(1-methyl-1H-pyrazol-4-yl)-3-{4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259262 (US9499547, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259279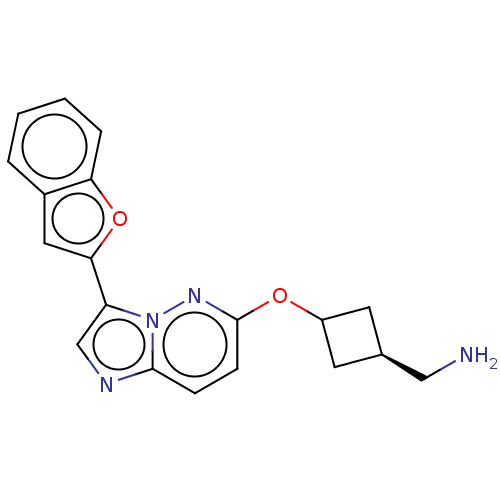 (US9499547, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259296 (US9499547, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM414909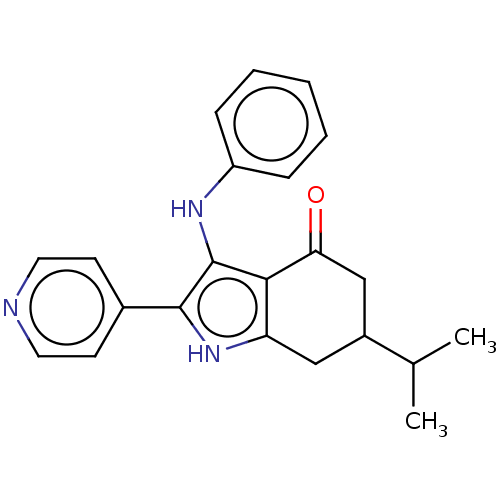 (Preparation of 3-(phenylamino)-6-(propan-2-yl)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415365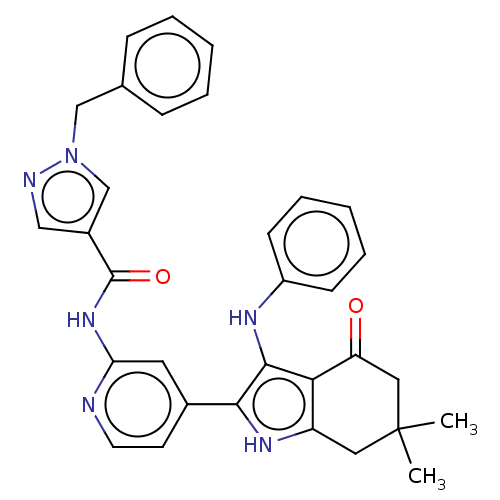 (Preparation of 1-benzyl-N-{4-[6,6-dimethyl-4-oxo-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415336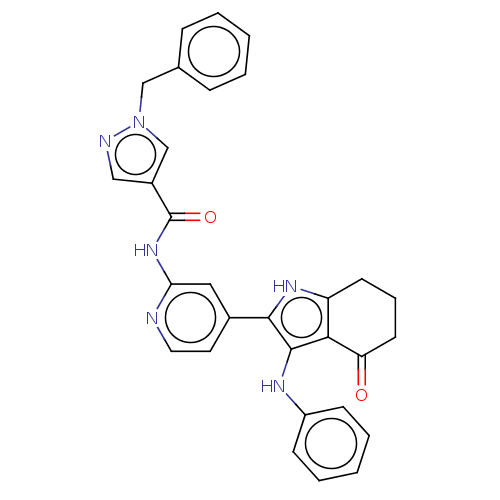 (Preparation of 1-benzyl-N-{4-[4-oxo-3-(phenylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378946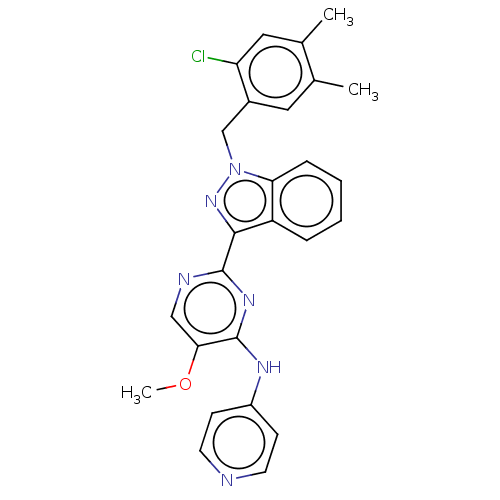 (2-[1-(2-chloro- 4,5- dimethylbenzyl)- 1H-indazol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415357 (Preparation of N-{4-[4′-oxo-3′-(phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378960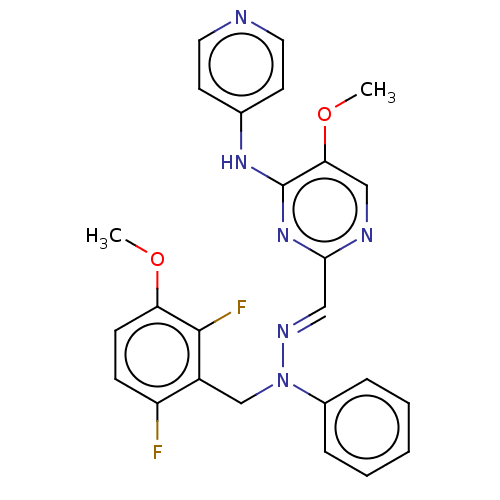 (2-[1-(2,6- difluoro-3- methoxybenzyl)- 1H-indazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415284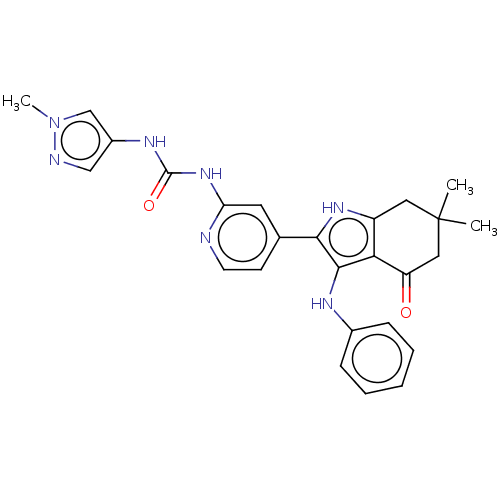 (Preparation of 1-{4-[6,6-dimethyl-4-oxo-3-(phenyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379040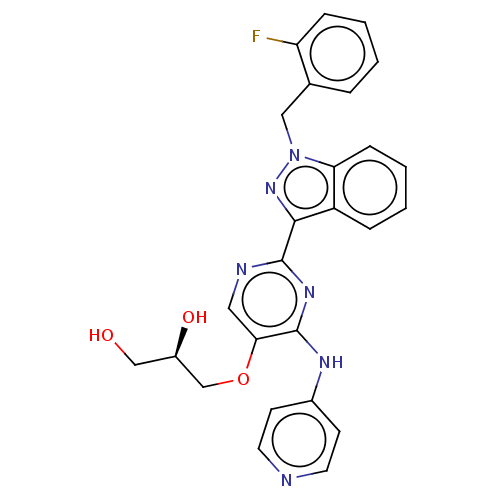 (Preparation of (2R)-3-({2-[1-(2-fluorobenzyl)-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379040 (Preparation of (2R)-3-({2-[1-(2-fluorobenzyl)-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378973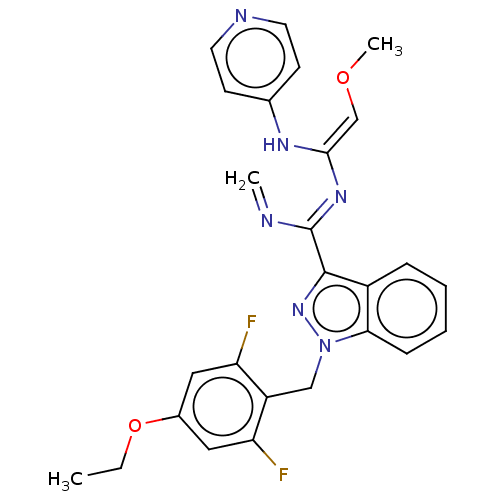 (2-[1-(4-ethoxy- 2,6- difluorobenzyl)- 1H-indazol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415331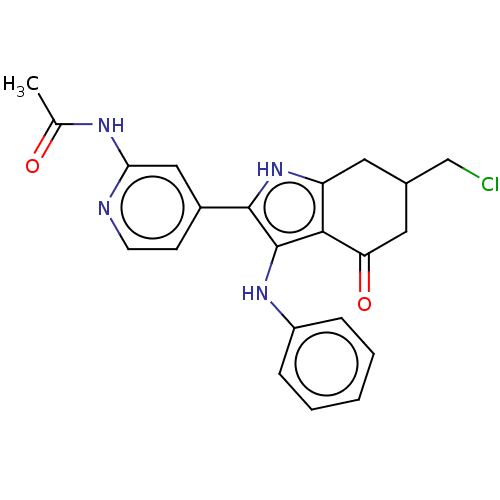 (2-{[(2-aminopyridin-4-yl)methyl]amino}-4-({[tert-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM341151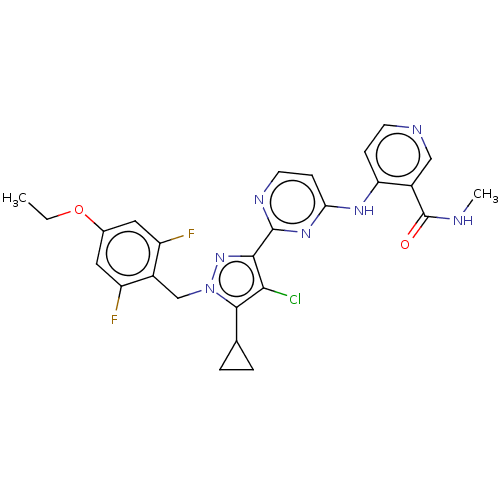 (4-({2-[4-chloro-5-cyclopropyl-1-(4-ethoxy-2,6-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US9765058 (2017) BindingDB Entry DOI: 10.7270/Q2JQ1347 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378980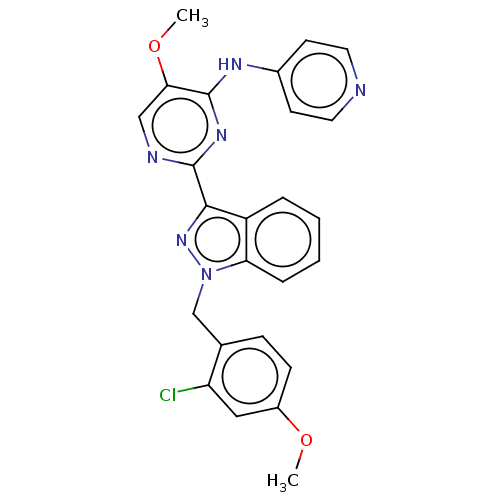 (2-[1-(2-chloro-4- methoxybenzyl)- 1H-indazol-3-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378991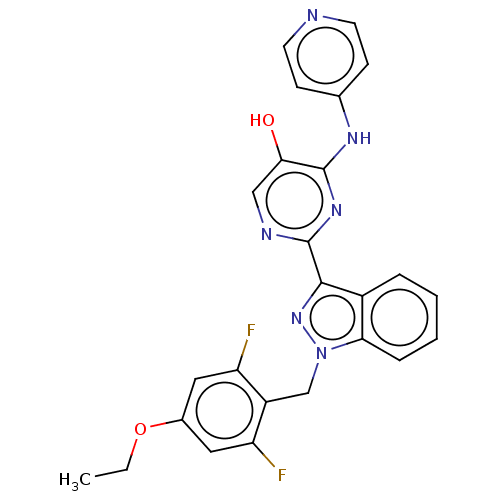 (Preparation of 2-[1-(4-ethoxy-2,6-difluorobenzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379045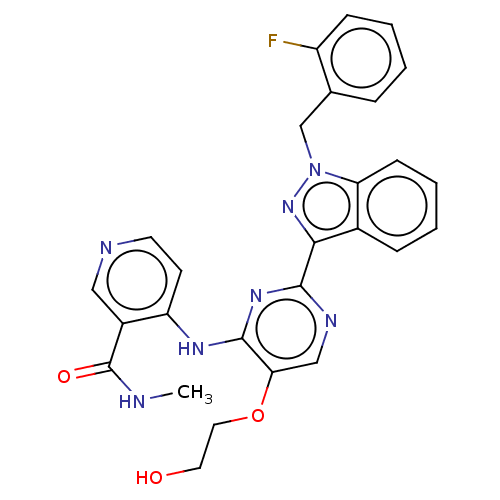 (4-({2-[1-(2- fluorobenzyl)- 1H-indazol-3-yl]- 5-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378982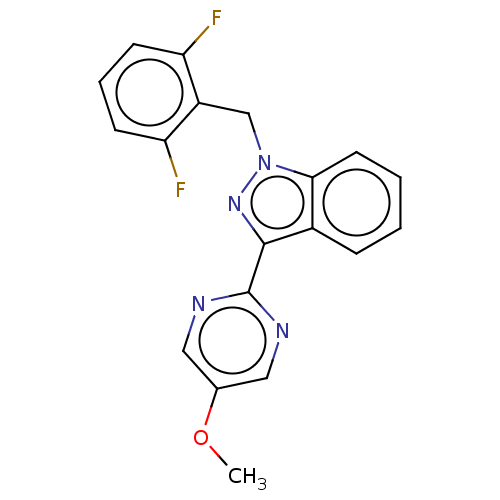 (2-[1-(2,6- difluorobenzyl)- 1H-indazol-3-yl]- 5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379041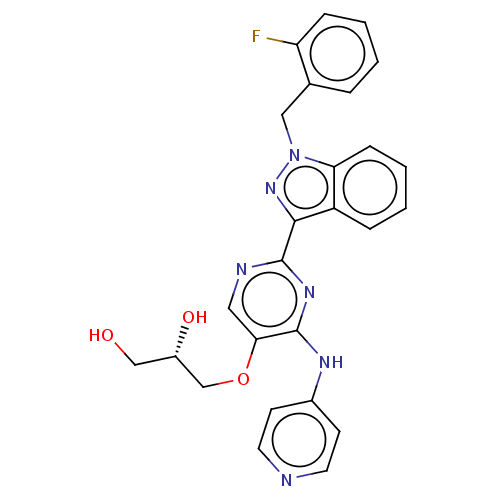 ((2S)-3-({2-[1-(2- fluorobenzyl)- 1H-indazol-3-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379103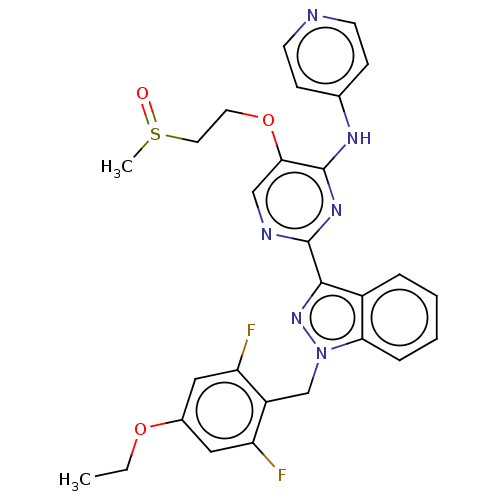 (Preparation of 2-[1-(4-ethoxy-2,6-difluorobenzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259260 (US9499547, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description MKNK1-inhibitory activity at high ATP of compounds of the present invention after their preincubation with MKNK1 was quantified employing the TR-FRET... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259261 (US9499547, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259263 (US9499547, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259296 (US9499547, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description MKNK1-inhibitory activity at high ATP of compounds of the present invention after their preincubation with MKNK1 was quantified employing the TR-FRET... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415353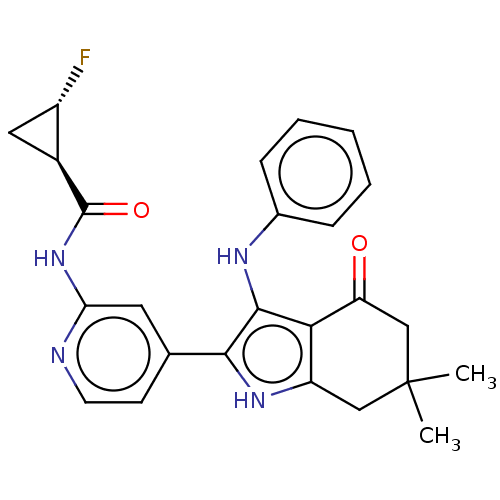 (Preparation of rel-(R,S)-2-fluoro-N-{4-[4′-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379085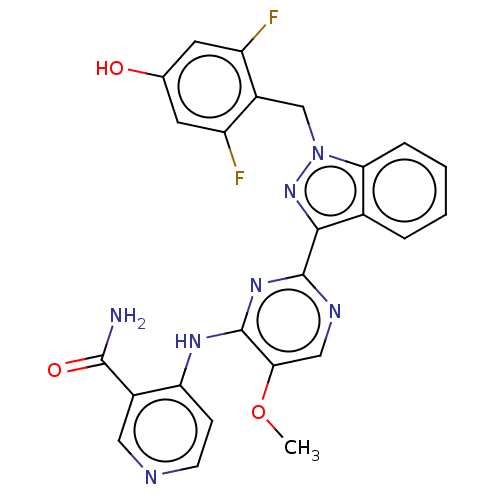 (4-({2-[1-(2,6- difluoro-4- hydroxybenzyl)-1H- inda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415359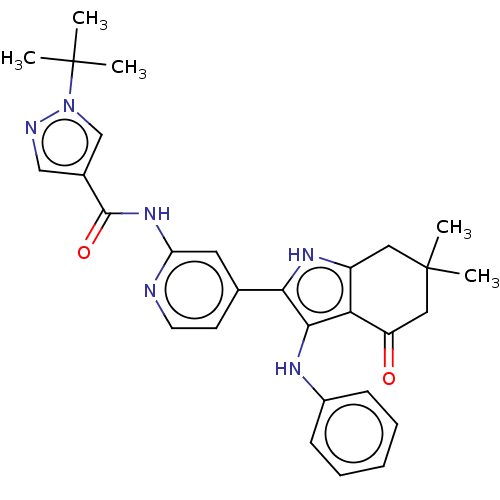 (Preparation of 1-tert-butyl-N-{4-[6,6-dimethyl-4-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415330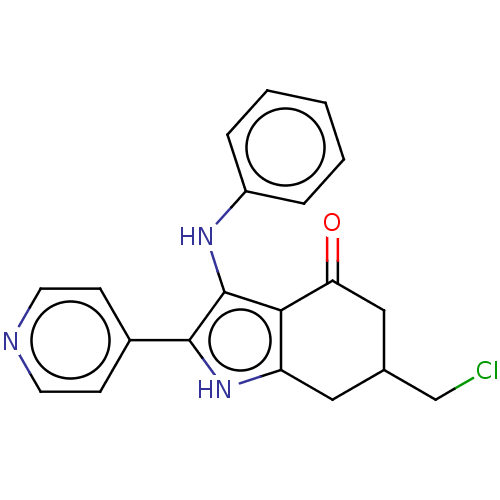 (Preparation of 6-(chloromethyl)-3-(phenylamino)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM415366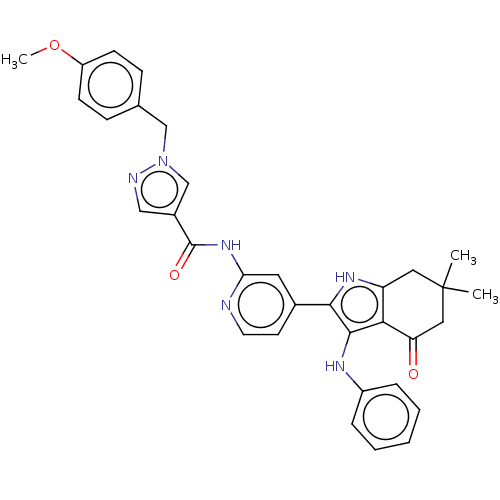 (Preparation of N-{4-[6,6-dimethyl-4-oxo-3-(phenyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μ... | US Patent US10428044 (2019) BindingDB Entry DOI: 10.7270/Q2QF8W7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 953 total ) | Next | Last >> |