 Found 625 hits with Last Name = 'hrabinova' and Initial = 'm'
Found 625 hits with Last Name = 'hrabinova' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389383

(CHEMBL2064464)Show SMILES CN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C29H43N5O/c1-32(23-9-3-2-4-10-23)17-15-28(35)34-21-19-33(20-22-34)18-16-30-29-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)29/h5,7,11,13,23H,2-4,6,8-10,12,14-22H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.318 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389383

(CHEMBL2064464)Show SMILES CN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C29H43N5O/c1-32(23-9-3-2-4-10-23)17-15-28(35)34-21-19-33(20-22-34)18-16-30-29-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)29/h5,7,11,13,23H,2-4,6,8-10,12,14-22H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50555835
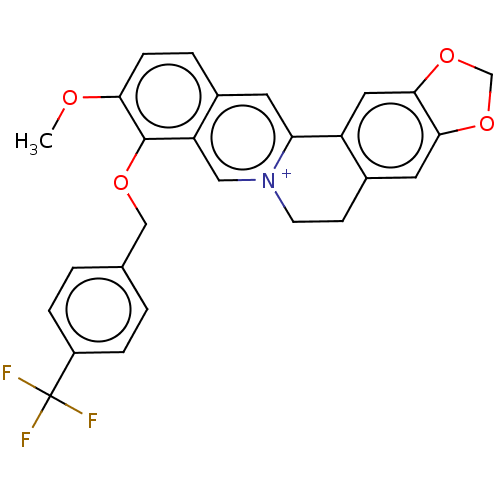
(CHEMBL4752175)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc(cc1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Noncompetitive inhibition of human acetylcholinesterase assessed as affinity towards free enzyme using acetylthiocholine iodide as substrate measured... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389382
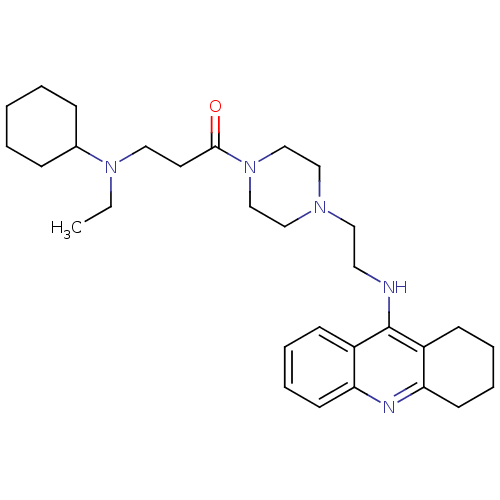
(CHEMBL2064465)Show SMILES CCN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C30H45N5O/c1-2-34(24-10-4-3-5-11-24)18-16-29(36)35-22-20-33(21-23-35)19-17-31-30-25-12-6-8-14-27(25)32-28-15-9-7-13-26(28)30/h6,8,12,14,24H,2-5,7,9-11,13,15-23H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human erythrocyte AChE assessed as enzyme-substrate-inhibitor complex using varying levels of acetylthiocholine iodide as su... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human erythrocyte AChE assessed as enzyme-inhibitor complex using varying levels of acetylthiocholine iodide as substrate pr... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389382

(CHEMBL2064465)Show SMILES CCN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C30H45N5O/c1-2-34(24-10-4-3-5-11-24)18-16-29(36)35-22-20-33(21-23-35)19-17-31-30-25-12-6-8-14-27(25)32-28-15-9-7-13-26(28)30/h6,8,12,14,24H,2-5,7,9-11,13,15-23H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056134
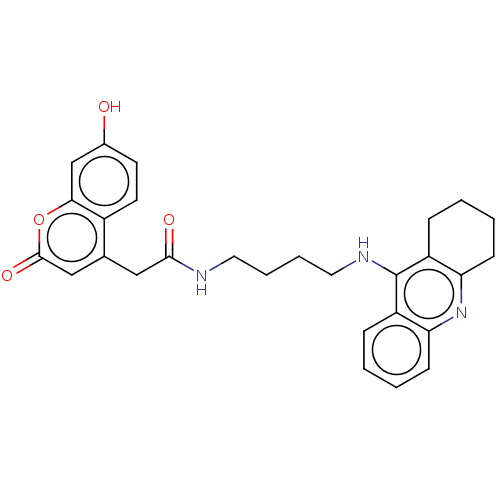
(CHEMBL3322160)Show SMILES Oc1ccc2c(CC(=O)NCCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C28H29N3O4/c32-19-11-12-20-18(16-27(34)35-25(20)17-19)15-26(33)29-13-5-6-14-30-28-21-7-1-3-9-23(21)31-24-10-4-2-8-22(24)28/h1,3,7,9,11-12,16-17,32H,2,4-6,8,10,13-15H2,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Dissociation constant for enzyme-inhibitor complex of human recombinant AChE by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 811-8 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.011
BindingDB Entry DOI: 10.7270/Q2PR7W8D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Dissociation constant for enzyme-inhibitor-substrate complex of human recombinant AChE by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 811-8 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.011
BindingDB Entry DOI: 10.7270/Q2PR7W8D |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50336116

(Ambenonium dichloride | CHEMBL1669479)Show SMILES C[N+](C)(CCNC(=O)C(=O)NCC[N+](C)(C)Cc1ccccc1Cl)Cc1ccccc1Cl Show InChI InChI=1S/C24H32Cl2N4O2/c1-29(2,17-19-9-5-7-11-21(19)25)15-13-27-23(31)24(32)28-14-16-30(3,4)18-20-10-6-8-12-22(20)26/h5-12H,13-18H2,1-4H3/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50555837
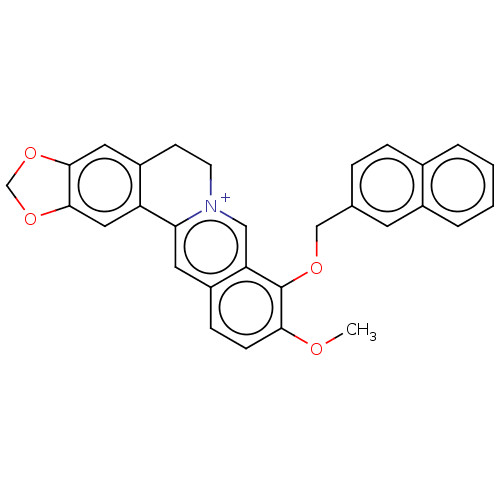
(CHEMBL4747577)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc2ccccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of human butyrylcholinesterase assessed as affinity towards free enzyme using butyrylthiocholine iodide as substrate measured by Ell... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Dissociation constant for enzyme-inhibitor complex of human recombinant AChE by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 811-8 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.011
BindingDB Entry DOI: 10.7270/Q2PR7W8D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389380

(CHEMBL2064468)Show SMILES CN(CCCN1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C29H45N5/c1-32(24-10-3-2-4-11-24)17-9-18-33-20-22-34(23-21-33)19-16-30-29-25-12-5-7-14-27(25)31-28-15-8-6-13-26(28)29/h5,7,12,14,24H,2-4,6,8-11,13,15-23H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389381
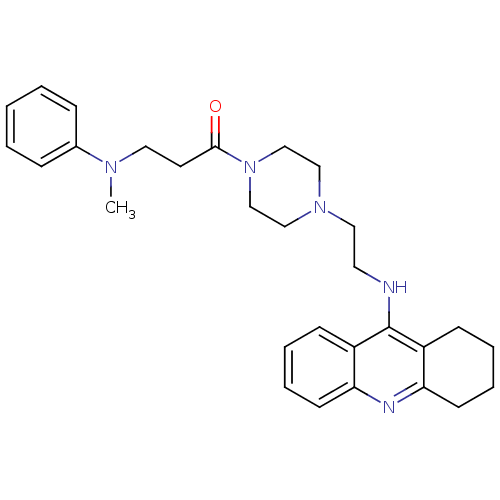
(CHEMBL2064466)Show SMILES CN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)c1ccccc1 Show InChI InChI=1S/C29H37N5O/c1-32(23-9-3-2-4-10-23)17-15-28(35)34-21-19-33(20-22-34)18-16-30-29-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)29/h2-5,7,9-11,13H,6,8,12,14-22H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389384

(CHEMBL2064404)Show SMILES O=C(CCN1CCCCC1)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C27H39N5O/c33-26(12-16-30-14-6-1-7-15-30)32-20-18-31(19-21-32)17-13-28-27-22-8-2-4-10-24(22)29-25-11-5-3-9-23(25)27/h2,4,8,10H,1,3,5-7,9,11-21H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005114

(CHEMBL3093804)Show SMILES [Br-].[Br-].C(CCCCCC[n+]1cccc2ccccc12)CCCCC[n+]1ccccc1 Show InChI InChI=1S/C26H36N2.2BrH/c1(3-5-7-12-20-27-21-13-9-14-22-27)2-4-6-8-15-23-28-24-16-18-25-17-10-11-19-26(25)28;;/h9-11,13-14,16-19,21-22,24H,1-8,12,15,20,23H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119798
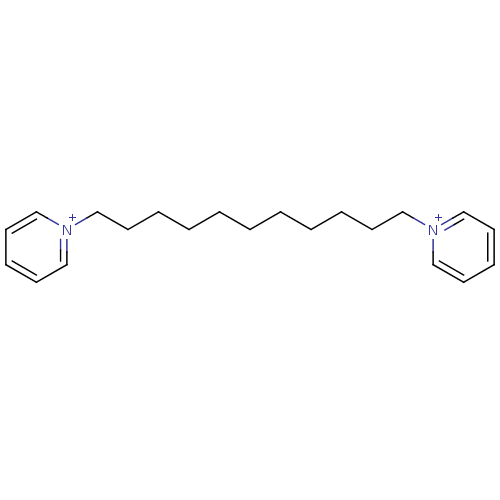
(1,11-bis(pyridinium)-undecane dibromide | 1,11-di(...)Show InChI InChI=1S/C21H32N2/c1(2-4-6-10-16-22-18-12-8-13-19-22)3-5-7-11-17-23-20-14-9-15-21-23/h8-9,12-15,18-21H,1-7,10-11,16-17H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis for enzyme-inhibitor complex |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056134

(CHEMBL3322160)Show SMILES Oc1ccc2c(CC(=O)NCCCCNc3c4CCCCc4nc4ccccc34)cc(=O)oc2c1 Show InChI InChI=1S/C28H29N3O4/c32-19-11-12-20-18(16-27(34)35-25(20)17-19)15-26(33)29-13-5-6-14-30-28-21-7-1-3-9-23(21)31-24-10-4-2-8-22(24)28/h1,3,7,9,11-12,16-17,32H,2,4-6,8,10,13-15H2,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50571189
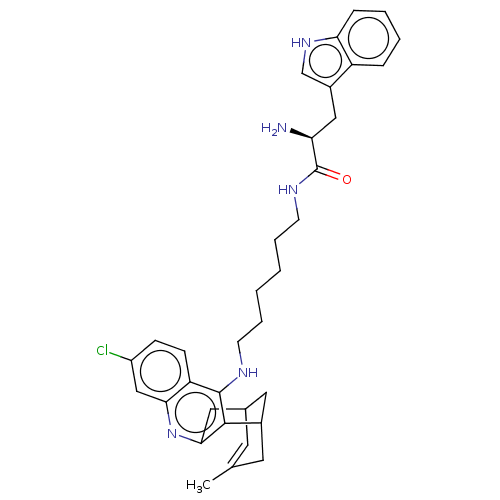
(CHEMBL4856400)Show SMILES Cl.Cl.CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCNC(=O)[C@@H](N)Cc1c[nH]c2ccccc12 |r,t:1,TLB:20:9:6:4.8.3,THB:2:3:6:11.10.9| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Non competitive type inhibition of human AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by double recipr... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389379
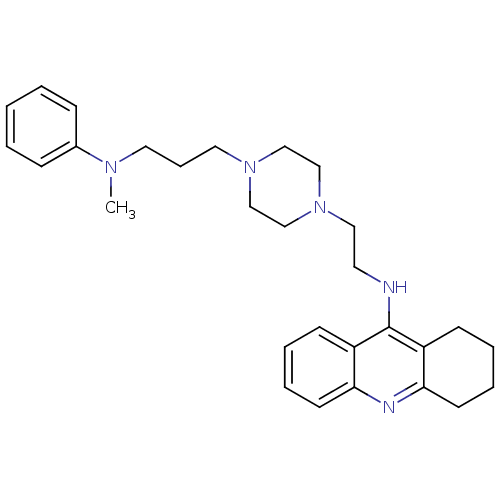
(CHEMBL2064470)Show SMILES CN(CCCN1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)c1ccccc1 Show InChI InChI=1S/C29H39N5/c1-32(24-10-3-2-4-11-24)17-9-18-33-20-22-34(23-21-33)19-16-30-29-25-12-5-7-14-27(25)31-28-15-8-6-13-26(28)29/h2-5,7,10-12,14H,6,8-9,13,15-23H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50119798

(1,11-bis(pyridinium)-undecane dibromide | 1,11-di(...)Show InChI InChI=1S/C21H32N2/c1(2-4-6-10-16-22-18-12-8-13-19-22)3-5-7-11-17-23-20-14-9-15-21-23/h8-9,12-15,18-21H,1-7,10-11,16-17H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389380

(CHEMBL2064468)Show SMILES CN(CCCN1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)C1CCCCC1 Show InChI InChI=1S/C29H45N5/c1-32(24-10-3-2-4-11-24)17-9-18-33-20-22-34(23-21-33)19-16-30-29-25-12-5-7-14-27(25)31-28-15-8-6-13-26(28)29/h5,7,12,14,24H,2-4,6,8-11,13,15-23H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473
(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Dissociation constant for enzyme-inhibitor-substrate complex of human recombinant AChE by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 811-8 (2011)
Article DOI: 10.1016/j.ejmech.2010.12.011
BindingDB Entry DOI: 10.7270/Q2PR7W8D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50555837

(CHEMBL4747577)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc2ccccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of human butyrylcholinesterase assessed as affinity towards enzyme-substrate complex using butyrylthiocholine iodide as substrate me... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389384

(CHEMBL2064404)Show SMILES O=C(CCN1CCCCC1)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C27H39N5O/c33-26(12-16-30-14-6-1-7-15-30)32-20-18-31(19-21-32)17-13-28-27-22-8-2-4-10-24(22)29-25-11-5-3-9-23(25)27/h2,4,8,10H,1,3,5-7,9,11-21H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056126
(CHEMBL3322141)Show SMILES CCN(CCC(=O)NCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C27H40N4O/c1-2-31(21-11-4-3-5-12-21)20-17-26(32)28-18-10-19-29-27-22-13-6-8-15-24(22)30-25-16-9-7-14-23(25)27/h6,8,13,15,21H,2-5,7,9-12,14,16-20H2,1H3,(H,28,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389379

(CHEMBL2064470)Show SMILES CN(CCCN1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)c1ccccc1 Show InChI InChI=1S/C29H39N5/c1-32(24-10-3-2-4-11-24)17-9-18-33-20-22-34(23-21-33)19-16-30-29-25-12-5-7-14-27(25)31-28-15-8-6-13-26(28)29/h2-5,7,10-12,14H,6,8-9,13,15-23H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056126

(CHEMBL3322141)Show SMILES CCN(CCC(=O)NCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C27H40N4O/c1-2-31(21-11-4-3-5-12-21)20-17-26(32)28-18-10-19-29-27-22-13-6-8-15-24(22)30-25-16-9-7-14-23(25)27/h6,8,13,15,21H,2-5,7,9-12,14,16-20H2,1H3,(H,28,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50389381

(CHEMBL2064466)Show SMILES CN(CCC(=O)N1CCN(CCNc2c3CCCCc3nc3ccccc23)CC1)c1ccccc1 Show InChI InChI=1S/C29H37N5O/c1-32(23-9-3-2-4-10-23)17-15-28(35)34-21-19-33(20-22-34)18-16-30-29-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)29/h2-5,7,9-11,13H,6,8,12,14-22H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334300
(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50334303

(1,1'-bis(4-tert.butylpyridinium)-naphtyl-3,6-dimet...)Show SMILES CC(C)(C)c1cc[n+](\C=C2\CC3=C\C(CC=C3C=C2)=C\[n+]2ccc(cc2)C(C)(C)C)cc1 |c:15,18,t:11| Show InChI InChI=1S/C30H36N2/c1-29(2,3)27-11-15-31(16-12-27)21-23-7-9-25-10-8-24(20-26(25)19-23)22-32-17-13-28(14-18-32)30(4,5)6/h7,9-18,20-22H,8,19H2,1-6H3/q+2/b23-21+,24-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex using acetylthiocholine substrate prein... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50313093
(1,1'-bis(pyridinium)-naphtyl-3,6-dimethylene dibro...)Show InChI InChI=1S/C22H20N2/c1-3-11-23(12-4-1)17-19-7-9-21-10-8-20(16-22(21)15-19)18-24-13-5-2-6-14-24/h1-16H,17-18H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis for enzyme-inhibitor complex |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50313093

(1,1'-bis(pyridinium)-naphtyl-3,6-dimethylene dibro...)Show InChI InChI=1S/C22H20N2/c1-3-11-23(12-4-1)17-19-7-9-21-10-8-20(16-22(21)15-19)18-24-13-5-2-6-14-24/h1-16H,17-18H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Military Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE after 5 mins by noncompetitive Lineweaver-burk plot analysis |
Bioorg Med Chem Lett 20: 1763-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.034
BindingDB Entry DOI: 10.7270/Q2BK1CGS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056127
(CHEMBL3322142)Show SMILES CCN(CCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C28H42N4O/c1-2-32(22-12-4-3-5-13-22)21-18-27(33)29-19-10-11-20-30-28-23-14-6-8-16-25(23)31-26-17-9-7-15-24(26)28/h6,8,14,16,22H,2-5,7,9-13,15,17-21H2,1H3,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50334300

(1,8-bis(4-tert.butylpyridinium)-oct-1,8-diyl dibro...)Show SMILES CC(C)(C)c1cc[n+](CCCCCCCC[n+]2ccc(cc2)C(C)(C)C)cc1 Show InChI InChI=1S/C26H42N2/c1-25(2,3)23-13-19-27(20-14-23)17-11-9-7-8-10-12-18-28-21-15-24(16-22-28)26(4,5)6/h13-16,19-22H,7-12,17-18H2,1-6H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
Bioorg Med Chem Lett 21: 150-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.051
BindingDB Entry DOI: 10.7270/Q2542PK6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex using acetylthiocholine substrate preincubated fo... |
Eur J Med Chem 55: 23-31 (2012)
Article DOI: 10.1016/j.ejmech.2012.06.051
BindingDB Entry DOI: 10.7270/Q2HH6M4Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50056127

(CHEMBL3322142)Show SMILES CCN(CCC(=O)NCCCCNc1c2CCCCc2nc2ccccc12)C1CCCCC1 Show InChI InChI=1S/C28H42N4O/c1-2-32(22-12-4-3-5-13-22)21-18-27(33)29-19-10-11-20-30-28-23-14-6-8-16-25(23)31-26-17-9-7-15-24(26)28/h6,8,14,16,22H,2-5,7,9-13,15,17-21H2,1H3,(H,29,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 328 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
P. J. Safarik University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE assessed as dissociation constant for enzyme-inhibitor-substrate complex by Lineweaver-Burk plot |
J Med Chem 57: 7073-84 (2014)
Article DOI: 10.1021/jm5008648
BindingDB Entry DOI: 10.7270/Q23T9JWH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005115
(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005115

(CHEMBL3093802)Show SMILES [Br-].[Br-].C(CCCCC[n+]1cccc2ccccc12)CCCC[n+]1ccccc1 Show InChI InChI=1S/C24H32N2.2BrH/c1(3-5-10-18-25-19-11-7-12-20-25)2-4-6-13-21-26-22-14-16-23-15-8-9-17-24(23)26;;/h7-9,11-12,14-17,19-20,22H,1-6,10,13,18,21H2;2*1H/q+2;;/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University in Prague
Curated by ChEMBL
| Assay Description
Non competitive inhibition of human recombinant AChE using ATChCl as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 6663-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.043
BindingDB Entry DOI: 10.7270/Q2ZG6TR6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data