

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50020217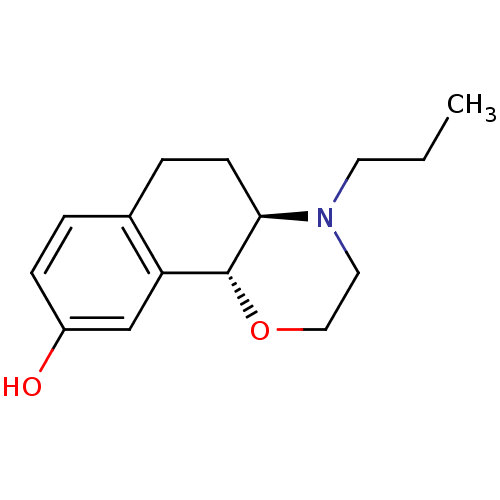 ((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells | J Med Chem 43: 2871-82 (2000) BindingDB Entry DOI: 10.7270/Q2X067R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50312840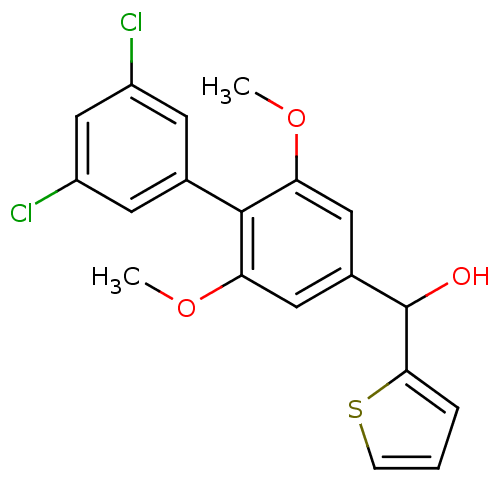 (CHEMBL1076680 | US9139546, 16 | [11C](3',5'-dichlo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to rat brain CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163592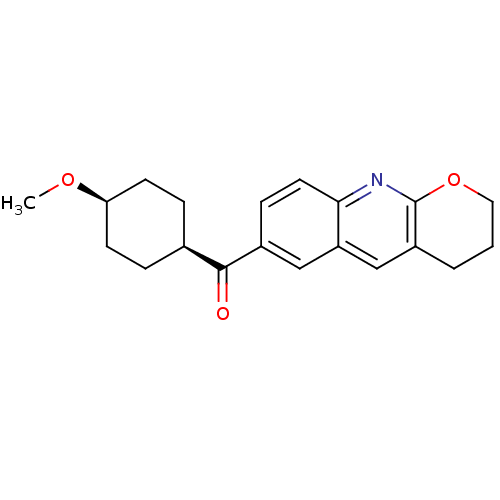 ((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50241089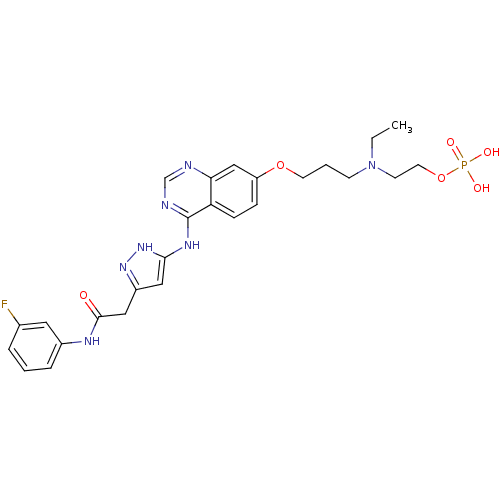 (2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical College Curated by ChEMBL | Assay Description Inhibition of aurora B (unknown origin) | Bioorg Med Chem Lett 23: 3523-30 (2013) Article DOI: 10.1016/j.bmcl.2013.04.039 BindingDB Entry DOI: 10.7270/Q26111Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50301822 (5-(1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 by PET analysis | Bioorg Med Chem Lett 21: 2998-3001 (2011) Article DOI: 10.1016/j.bmcl.2011.03.046 BindingDB Entry DOI: 10.7270/Q2WH2Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50301822 (5-(1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical College Curated by ChEMBL | Assay Description Inhibition of aurora A (unknown origin) | Bioorg Med Chem Lett 23: 3523-30 (2013) Article DOI: 10.1016/j.bmcl.2013.04.039 BindingDB Entry DOI: 10.7270/Q26111Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266889 (CHEMBL513922 | N-benzyl-N-ethyl-2-(7-methyl-8-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20286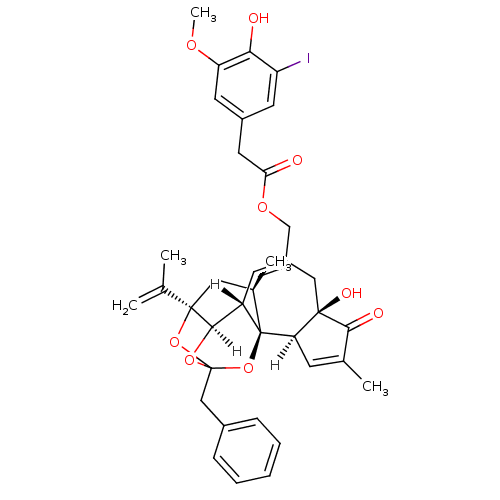 (5-I-RTX | 5-iodoresiniferatoxin | [(1R,2R,6R,10S,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -54.7 | n/a | n/a | 12.2 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50054139 ((R)1-(2-Chloro-phenyl)-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50333368 (CHEMBL1645348 | [11C]-cis-(3-ethyl-2-methylquinoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 by PET analysis | Bioorg Med Chem Lett 21: 2998-3001 (2011) Article DOI: 10.1016/j.bmcl.2011.03.046 BindingDB Entry DOI: 10.7270/Q2WH2Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50333368 (CHEMBL1645348 | [11C]-cis-(3-ethyl-2-methylquinoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50090828 ((-)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4-oxa-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Tested for agonist binding affinity by measuring displacement of [3H]NPA from Human Dopamine receptor D2L expressed in CHO K-1 cells | J Med Chem 43: 2871-82 (2000) BindingDB Entry DOI: 10.7270/Q2X067R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50092173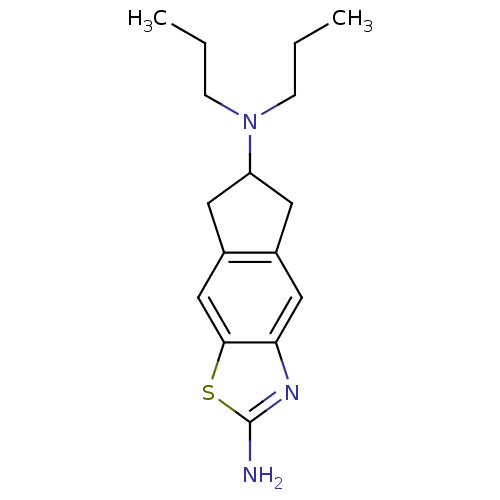 (CHEMBL325710 | N*6*,N*6*-Dipropyl-6,7-dihydro-5H-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maastricht Curated by ChEMBL | Assay Description in vitro binding affinity was determined on human Dopamine receptor D3 expressed in chinese hamster ovary(CHO) K-1 cells using [3H]spiperone as radio... | J Med Chem 43: 3549-57 (2000) BindingDB Entry DOI: 10.7270/Q2B27W0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50010586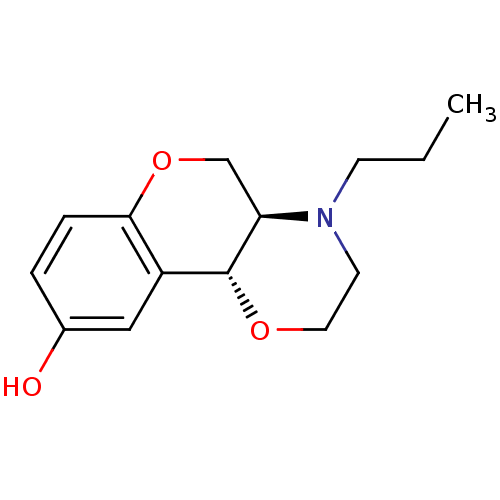 ((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells | J Med Chem 43: 2871-82 (2000) BindingDB Entry DOI: 10.7270/Q2X067R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50010586 ((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells | J Med Chem 43: 2871-82 (2000) BindingDB Entry DOI: 10.7270/Q2X067R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535166 (WO2022013684, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50090829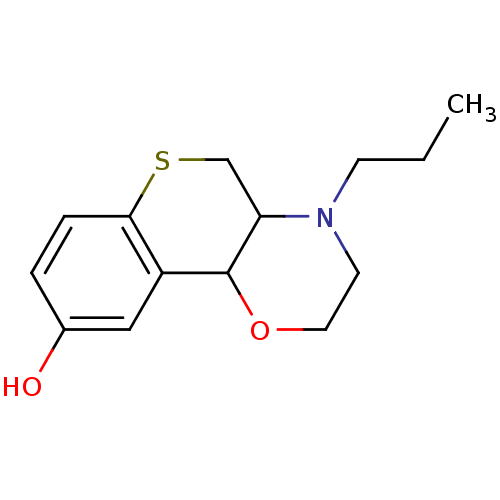 ((trans)1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4-oxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells | J Med Chem 43: 2871-82 (2000) BindingDB Entry DOI: 10.7270/Q2X067R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20314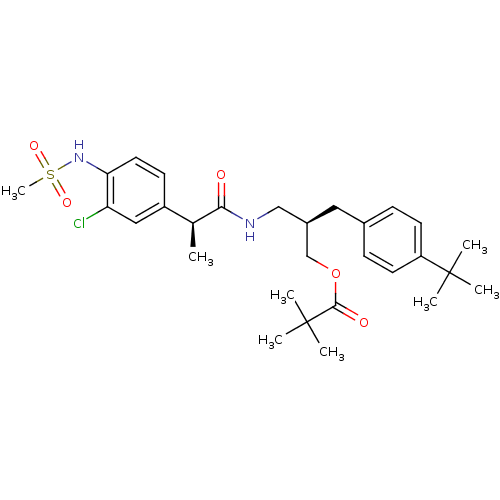 ((2R)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.83 | -51.9 | n/a | n/a | 5.20 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50273942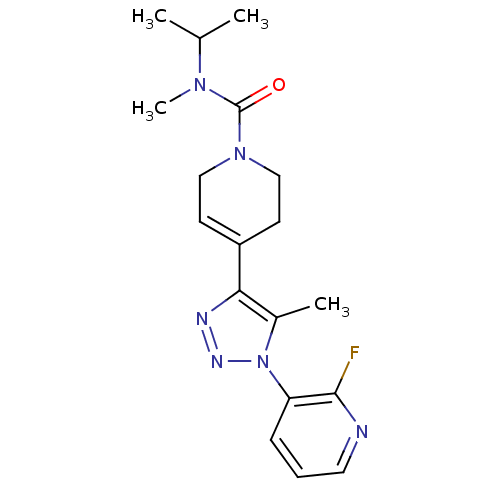 (4-(1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 by PET analysis | Bioorg Med Chem Lett 21: 2998-3001 (2011) Article DOI: 10.1016/j.bmcl.2011.03.046 BindingDB Entry DOI: 10.7270/Q2WH2Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017594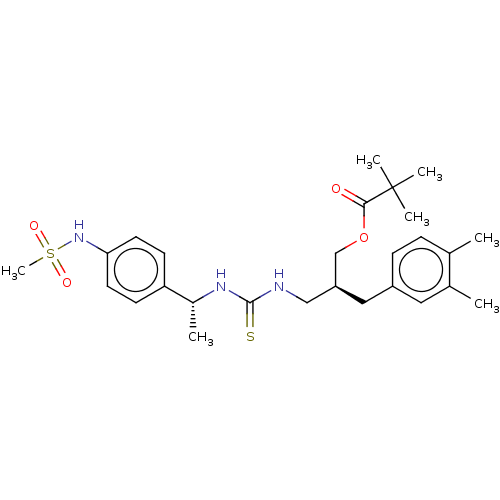 (CHEMBL3288626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50312841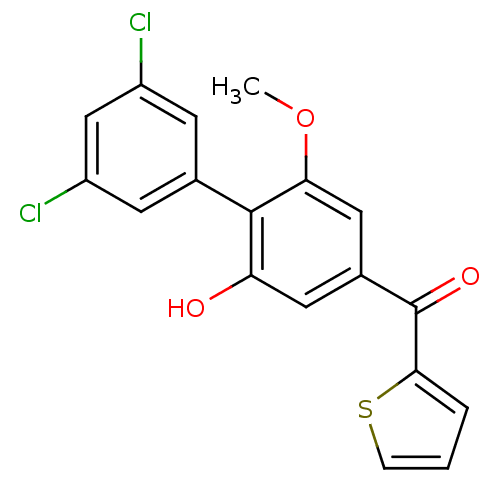 (CHEMBL1081609 | US9139546, 30 | [11C](3',5'-dichlo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to rat brain CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM50395192 (CHEMBL2164336) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to 5-HT7 in rat brain | Bioorg Med Chem 21: 5316-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.020 BindingDB Entry DOI: 10.7270/Q2H9985B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50090828 ((-)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4-oxa-9-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells | J Med Chem 43: 2871-82 (2000) BindingDB Entry DOI: 10.7270/Q2X067R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20311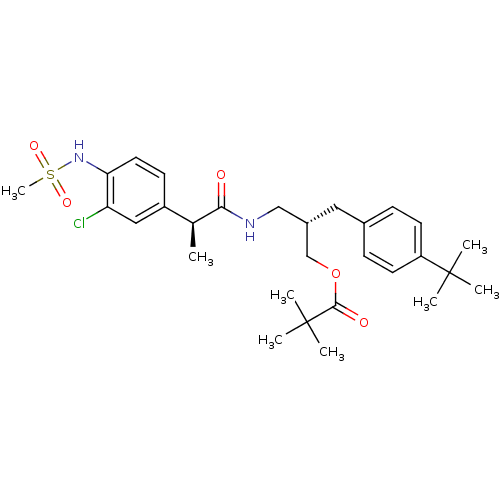 ((2S)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.29 | -50.4 | n/a | n/a | 12.1 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20330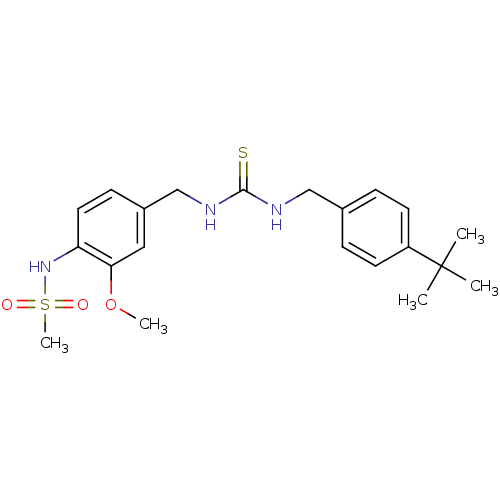 (3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity towards rat TRPV1 expressed in CHO cells | Bioorg Med Chem Lett 15: 4143-50 (2005) Article DOI: 10.1016/j.bmcl.2005.06.006 BindingDB Entry DOI: 10.7270/Q2JH3KQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20330 (3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells assessed as capsaicin-stimulated 45Ca2+ uptake | Eur J Med Chem 44: 322-31 (2008) Article DOI: 10.1016/j.ejmech.2008.02.026 BindingDB Entry DOI: 10.7270/Q2R49QJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20330 (3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity for rat TRPV1 expressed in CHO cells | Bioorg Med Chem Lett 15: 4136-42 (2005) Article DOI: 10.1016/j.bmcl.2005.06.009 BindingDB Entry DOI: 10.7270/Q23B5ZN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50090832 ((cis)1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4-oxa-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells | J Med Chem 43: 2871-82 (2000) BindingDB Entry DOI: 10.7270/Q2X067R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20291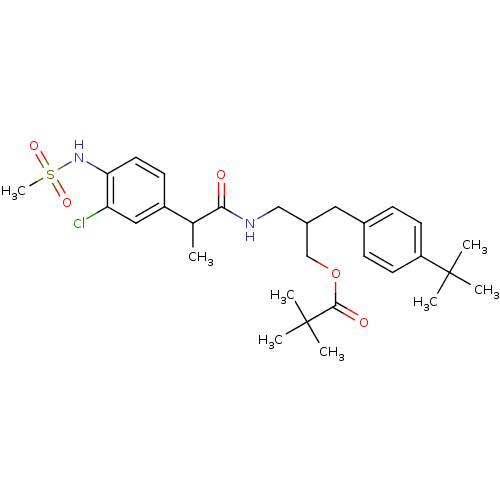 (2-[(4-tert-butylphenyl)methyl]-3-[2-(3-chloro-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60 | -50.1 | n/a | n/a | 12.3 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50273942 (4-(1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM50237707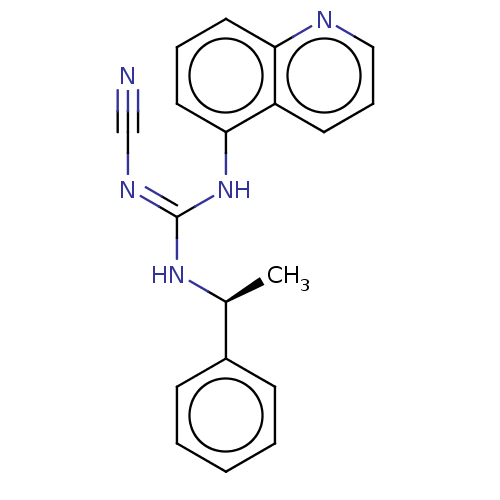 (A-804598 | CHEMBL1628690) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-A-804598 from rat P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02249 BindingDB Entry DOI: 10.7270/Q2862MC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20313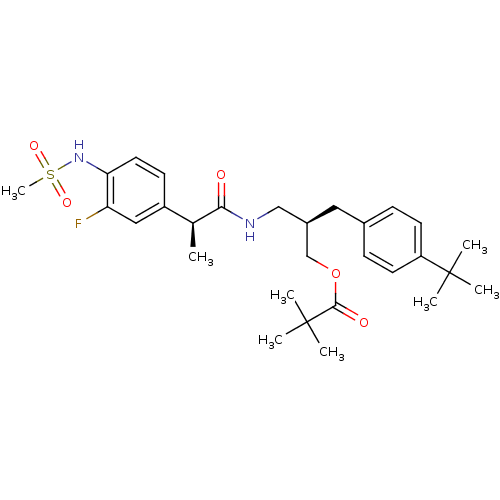 ((2R)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.12 | -49.8 | n/a | n/a | 0.580 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50107877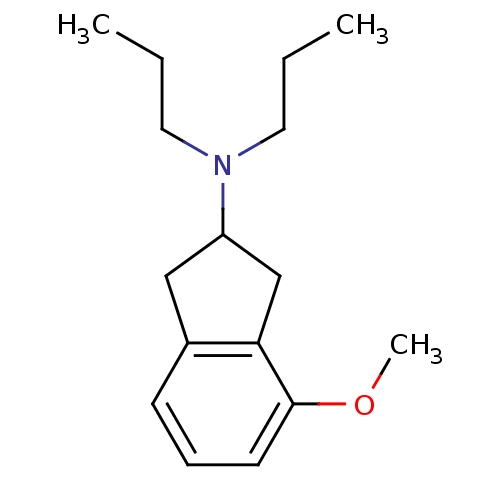 ((4-Methoxy-indan-2-yl)-dipropyl-amine | CHEMBL4219...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description Affinity at D2 dopamine receptor on CHO cell membranes by [3H]-PNU-86170 displacement. | J Med Chem 44: 4716-32 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50312838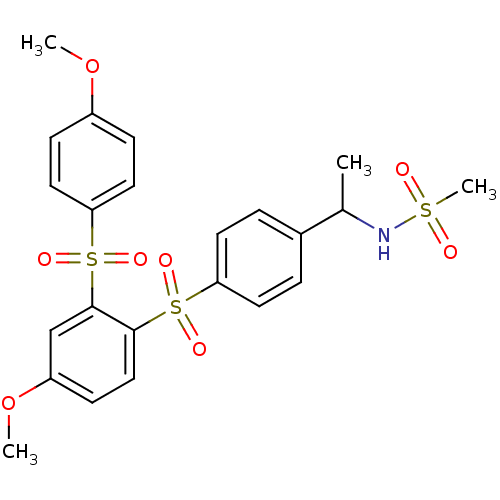 (CHEMBL1081623 | SCH-225336 | [11C]N-(1-(4-(4-metho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xuzhou Medical College Curated by ChEMBL | Assay Description Inhibition of aurora C (unknown origin) | Bioorg Med Chem Lett 23: 3523-30 (2013) Article DOI: 10.1016/j.bmcl.2013.04.039 BindingDB Entry DOI: 10.7270/Q26111Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50333371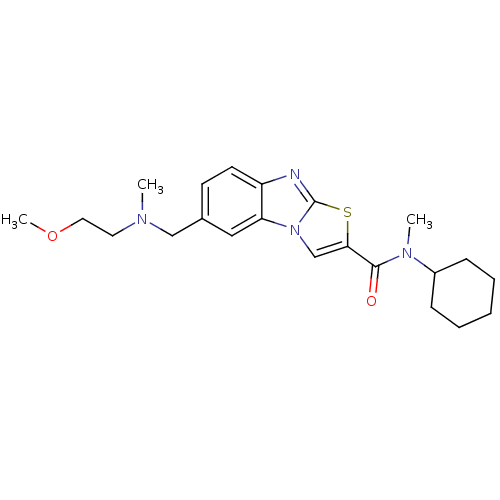 (CHEMBL1645351 | CHEMBL1771388 | [11C]-6-N-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 by PET analysis | Bioorg Med Chem Lett 21: 2998-3001 (2011) Article DOI: 10.1016/j.bmcl.2011.03.046 BindingDB Entry DOI: 10.7270/Q2WH2Q9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50333371 (CHEMBL1645351 | CHEMBL1771388 | [11C]-6-N-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50107877 ((4-Methoxy-indan-2-yl)-dipropyl-amine | CHEMBL4219...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-hydroxytryptamine 1A receptor | J Med Chem 44: 4716-32 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50401000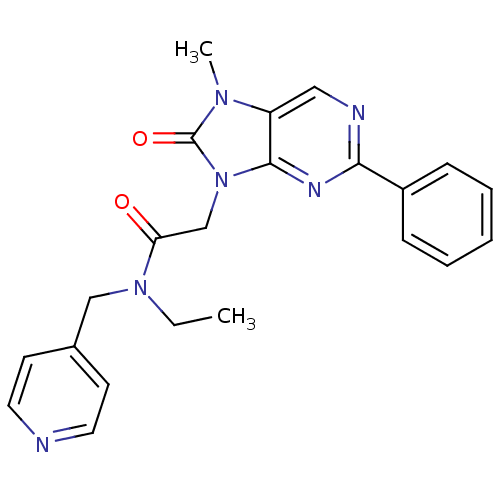 (CHEMBL2206282) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50107867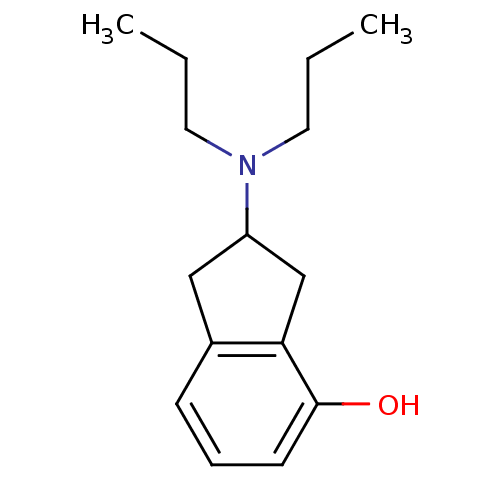 ((R)-2-Dipropylamino-indan-4-ol | (S)-2-Dipropylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Curated by ChEMBL | Assay Description Affinity at D2 dopamine receptor on CHO cell membranes by [3H]-PNU-86170 displacement. | J Med Chem 44: 4716-32 (2001) BindingDB Entry DOI: 10.7270/Q2ZP45D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50301520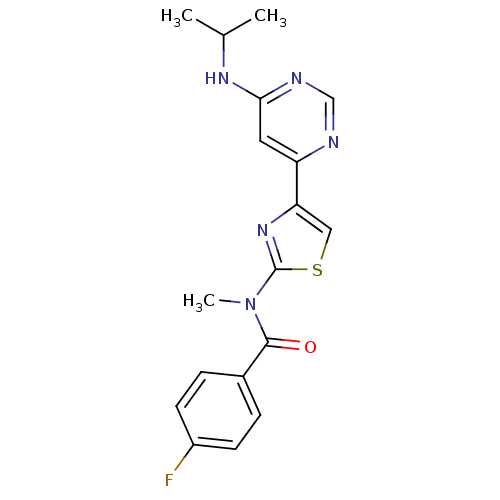 (4-fluoro-N-(4-(6-(isopropylamino)pyrimidin-4-yl)th...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of 4-[18F]fluoro-N-[4-(6-(isopropylamino)pyrimidin-4-yl)-1,3-thiazol-2-yl]-N-methylbenzamide from mGluR1 in Sprague-Dawley rat brain hom... | J Med Chem 55: 2342-52 (2012) Article DOI: 10.1021/jm201590g BindingDB Entry DOI: 10.7270/Q2S46T1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM198970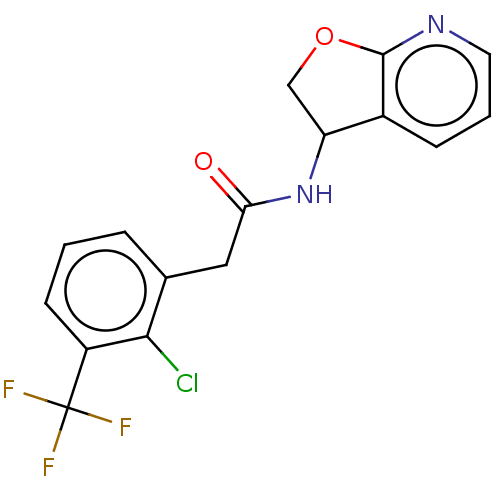 (BDBM199009 | BDBM199010 | US9221832, 34) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02249 BindingDB Entry DOI: 10.7270/Q2862MC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50416603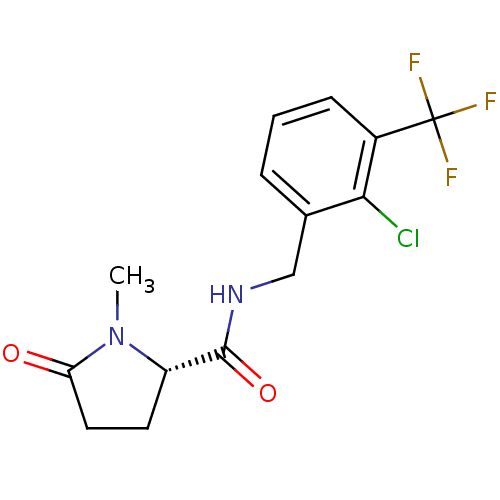 (CHEMBL1222883) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-A-804598 from human P2X7 receptor expressed in HEK293 cell membrane by in vitro binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02249 BindingDB Entry DOI: 10.7270/Q2862MC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535159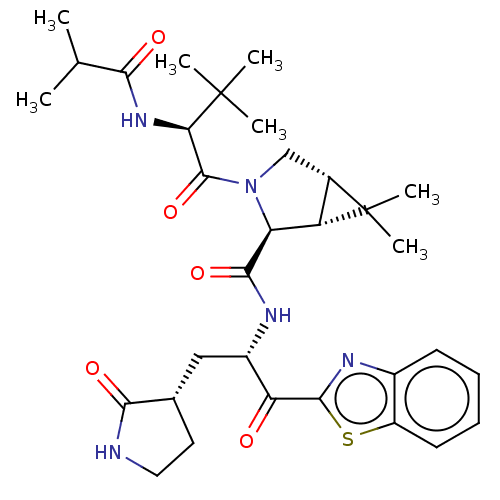 (WO2022013684, Example 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017592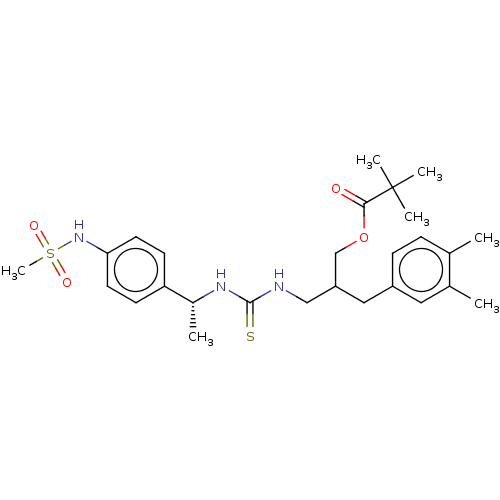 (CHEMBL3288624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535160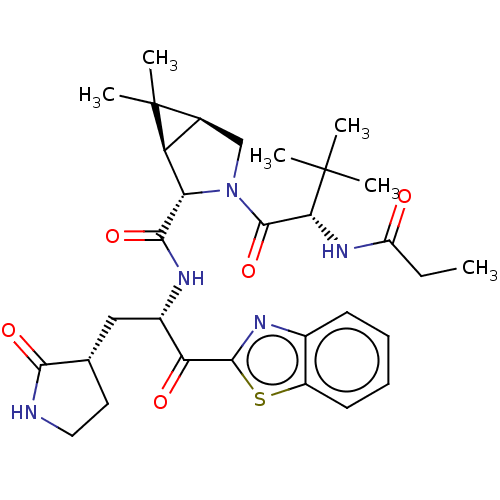 (WO2022013684, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50020217 ((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Human Dopamine receptor D2L affinities to determine agonist activity, using [3H]spip as radioligand | J Med Chem 43: 2871-82 (2000) BindingDB Entry DOI: 10.7270/Q2X067R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20310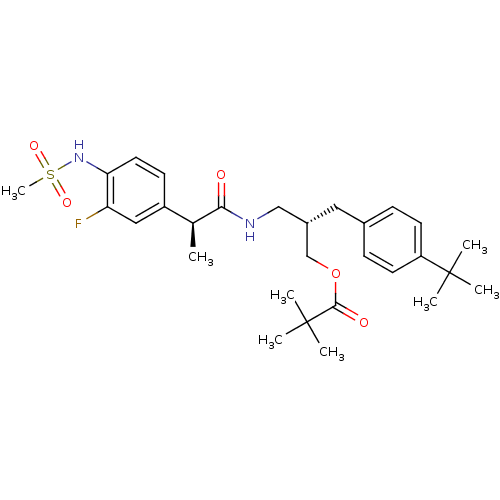 ((2S)-2-[(4-tert-butylphenyl)methyl]-3-[(2S)-2-(3-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.26 | -48.7 | n/a | n/a | 10.9 | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... | J Med Chem 51: 57-67 (2008) Article DOI: 10.1021/jm701049p BindingDB Entry DOI: 10.7270/Q2222S1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50017581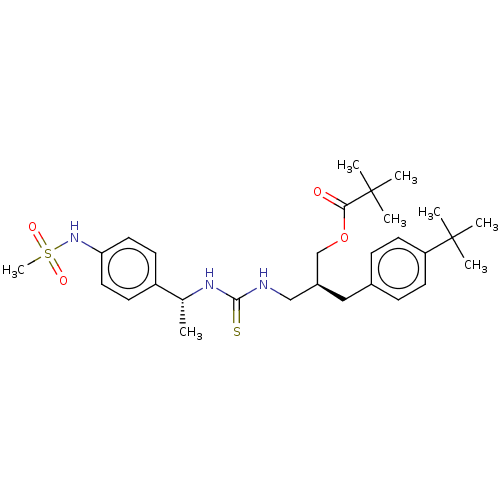 (CHEMBL3288631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from rat TRPV1 after 60 mins by competitive binding assay | Bioorg Med Chem Lett 24: 2685-8 (2014) Article DOI: 10.1016/j.bmcl.2014.04.054 BindingDB Entry DOI: 10.7270/Q26111VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2056 total ) | Next | Last >> |