

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50126528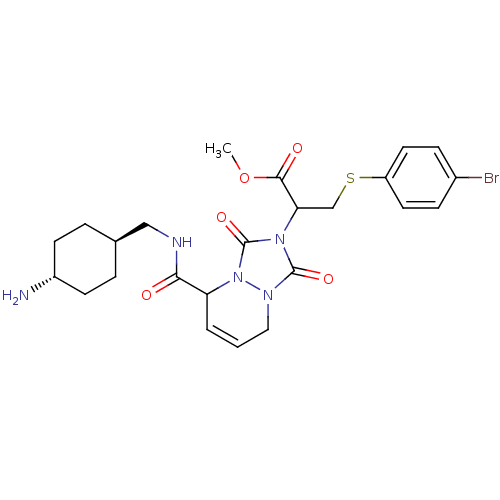 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198754 (CHEMBL3924888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071565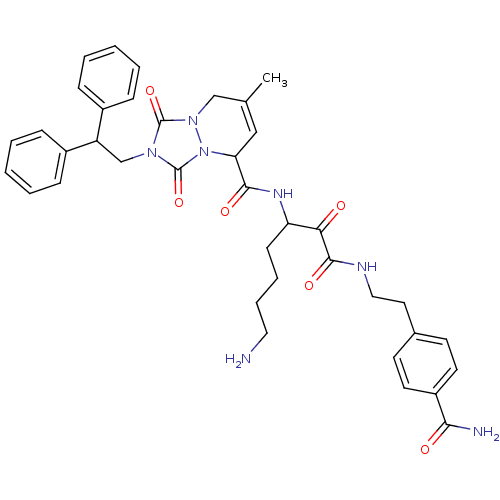 (2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126525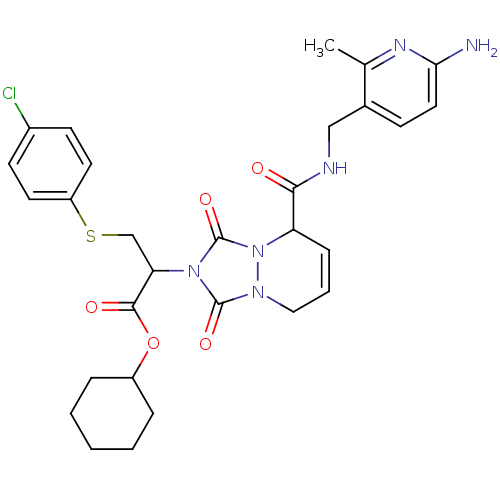 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198760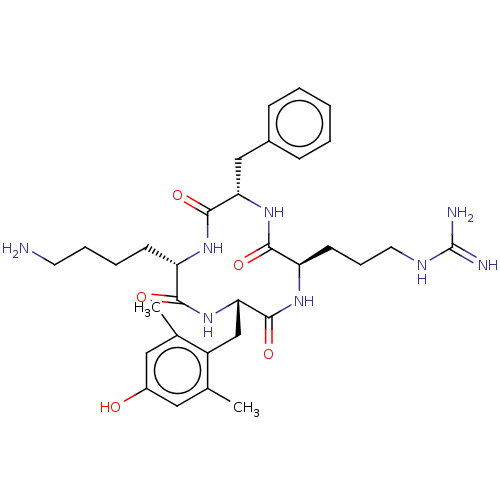 (CHEMBL3897031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198758 (CHEMBL3908315) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50010704 (CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0869 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity was measured on opioid receptor kappa 1 | J Med Chem 44: 3048-53 (2001) BindingDB Entry DOI: 10.7270/Q28K79SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126521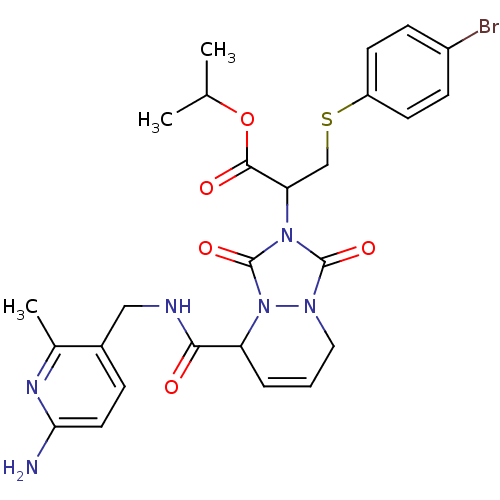 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM466723 (US10800761, Example 42 | US10800761, Example 55 | ...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of human carbonic anhydrase II (0.1 nM). | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001157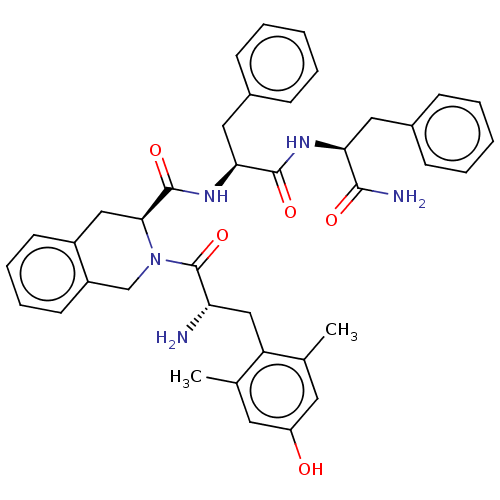 (CHEMBL538700) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001157 (CHEMBL538700) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198755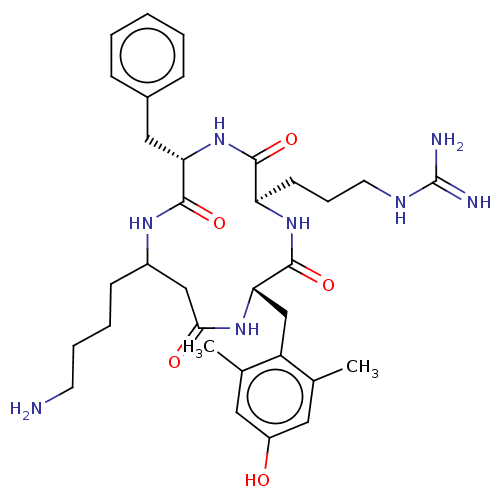 (CHEMBL3979449) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM85731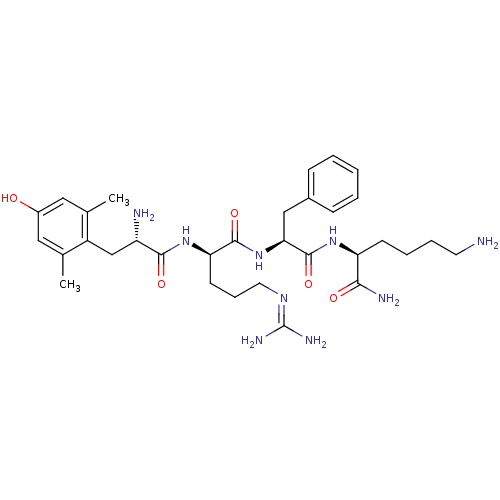 ([Dmt1]DALDA) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by PDSP Ki Database | Eur J Med Chem 35: 895-901 (2000) Article DOI: 10.1016/s0223-5234(00)01171-5 BindingDB Entry DOI: 10.7270/Q23X856N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50198757 (CHEMBL363142) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in rat brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50493134 (CHEMBL3038184) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50493138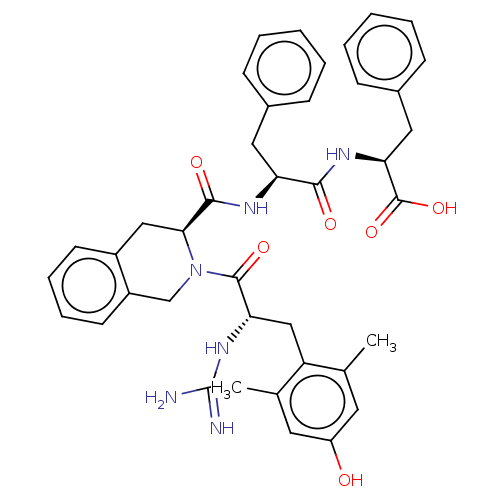 (CHEMBL3038186) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM85736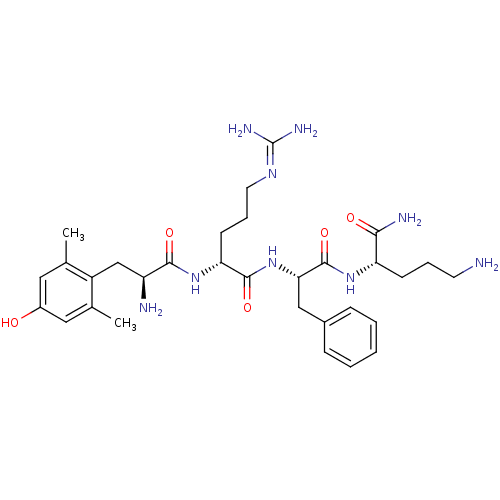 (Dmt-d-Arg-Phe-Orn-NH2 | H-Dmt-D-Arg-Phe-Orn-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by PDSP Ki Database | Eur J Med Chem 35: 895-901 (2000) Article DOI: 10.1016/s0223-5234(00)01171-5 BindingDB Entry DOI: 10.7270/Q23X856N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Tested for binding affinity against delta opioid receptor by displacing [3H]- DSLET radioligand from rat brain membrane preparations | J Med Chem 36: 3182-7 (1993) BindingDB Entry DOI: 10.7270/Q2F190BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM85732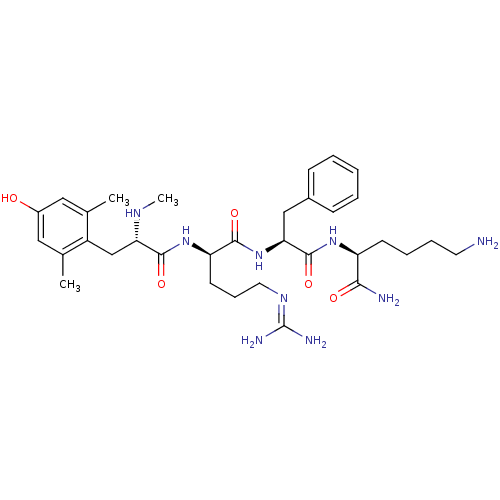 (Tmt-D-Arg-Phe-Lys-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by PDSP Ki Database | Eur J Med Chem 35: 895-901 (2000) Article DOI: 10.1016/s0223-5234(00)01171-5 BindingDB Entry DOI: 10.7270/Q23X856N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071575 (2,2-Dibutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM85734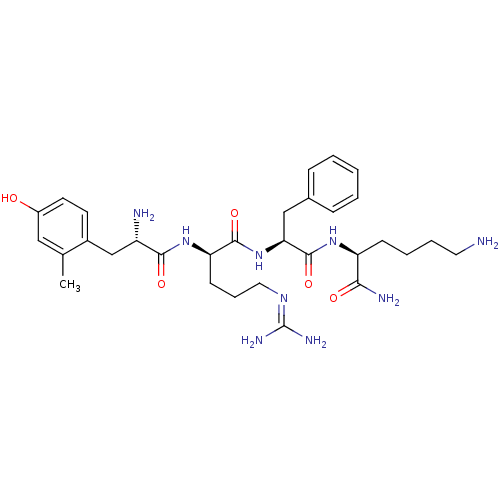 (H-Hmt-D-Arg-Phe-Lys-NH2 I | H-Hmt-D-Arg-Phe-Lys-NH...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by PDSP Ki Database | Eur J Med Chem 35: 895-901 (2000) Article DOI: 10.1016/s0223-5234(00)01171-5 BindingDB Entry DOI: 10.7270/Q23X856N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126508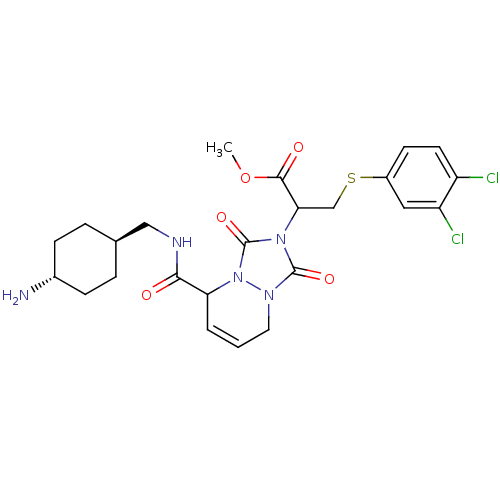 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50299557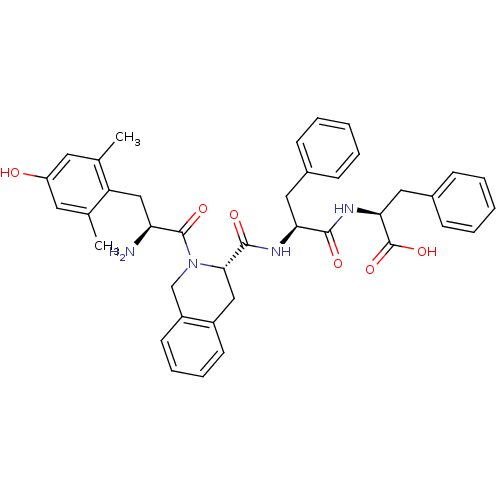 ((S)-2-((S)-2-((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126520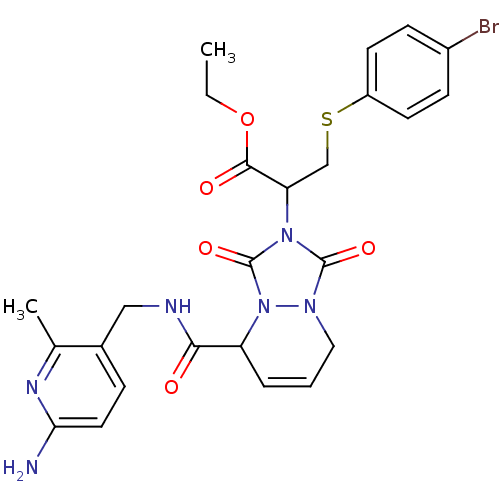 (2-{5-[(6-Amino-2-methyl-pyridin-3-ylmethyl)-carbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50021325 (4-methyl-(1S,5R,13R,14S)-12-oxa-4-azapentacyclo[9....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Peptides 19: 1091-8 (1998) Article DOI: 10.1016/s0196-9781(98)00023-0 BindingDB Entry DOI: 10.7270/Q2B27STB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071570 (8-Isobutyl-2-(4-methoxy-phenyl)-1,3-dioxo-2,3,5,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071573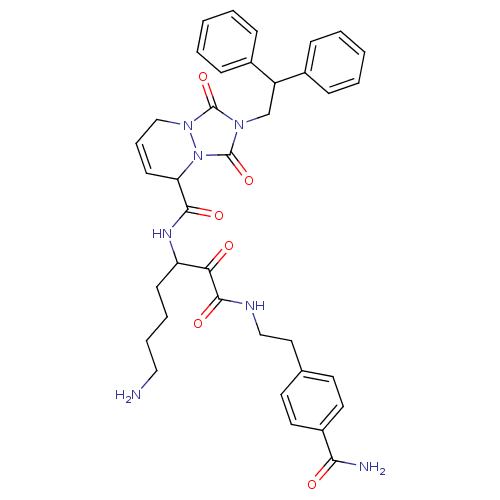 (2-(2,2-Diphenyl-ethyl)-1,3-dioxo-2,3,5,8-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50493137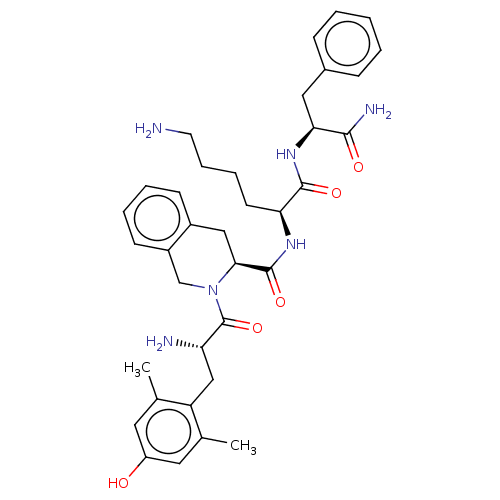 (CHEMBL3038180) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.306 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068664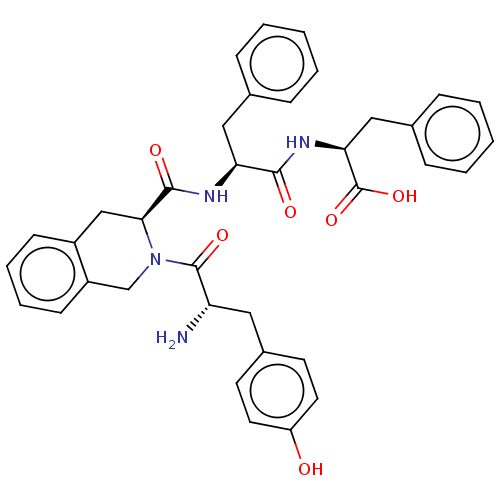 (2-[2-({(S)-2-[2-Amino-3-(4-hydroxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Tested for binding affinity against delta opioid receptor by displacing [3H]- DSLET radioligand from rat brain membrane preparations | J Med Chem 36: 3182-7 (1993) BindingDB Entry DOI: 10.7270/Q2F190BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM85328 (TIPP-PSI | TIPPpsi) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068664 (2-[2-({(S)-2-[2-Amino-3-(4-hydroxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Tested for binding affinity against delta opioid receptor by displacing [3H]- DSLET radioligand from rat brain membrane preparations | J Med Chem 36: 3182-7 (1993) BindingDB Entry DOI: 10.7270/Q2F190BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126526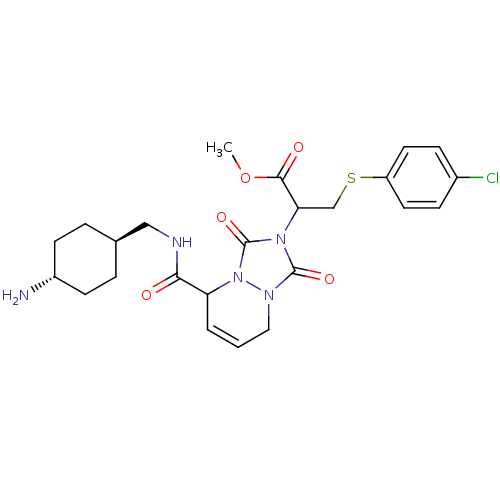 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50103978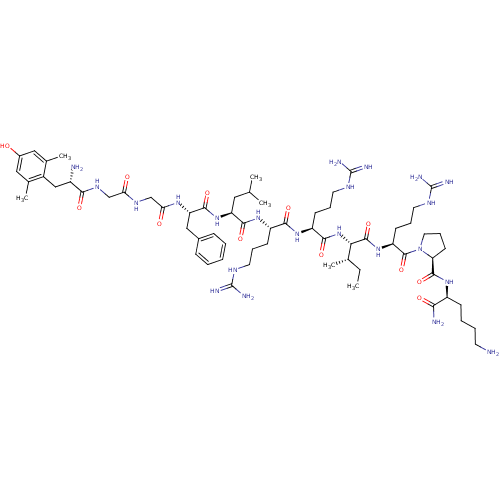 (CHEMBL436911 | [Dmt1]Dyn A(1-11)-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity was measured on opioid receptor kappa 1 | J Med Chem 44: 3048-53 (2001) BindingDB Entry DOI: 10.7270/Q28K79SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50493132 (CHEMBL3038179) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50003571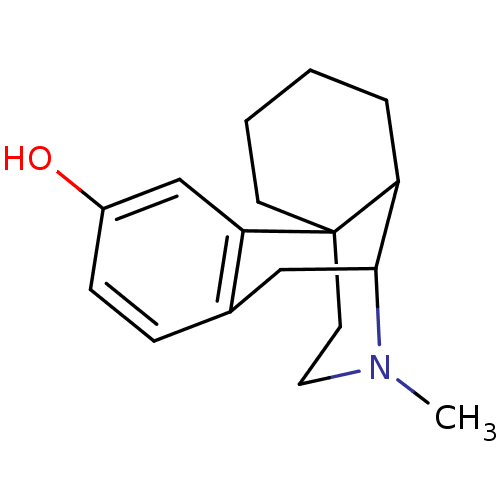 ((-)-3-Hydroxy-N-methylmorphinan hydrochloride (lev...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Peptides 19: 1091-8 (1998) Article DOI: 10.1016/s0196-9781(98)00023-0 BindingDB Entry DOI: 10.7270/Q2B27STB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50071571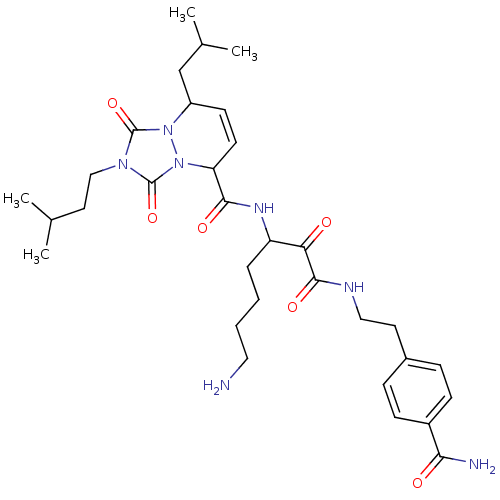 (8-Isobutyl-2-(3-methyl-butyl)-1,3-dioxo-2,3,5,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the trypsin enzyme | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50103978 (CHEMBL436911 | [Dmt1]Dyn A(1-11)-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity was measured on mu opioid receptor | J Med Chem 44: 3048-53 (2001) BindingDB Entry DOI: 10.7270/Q28K79SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080453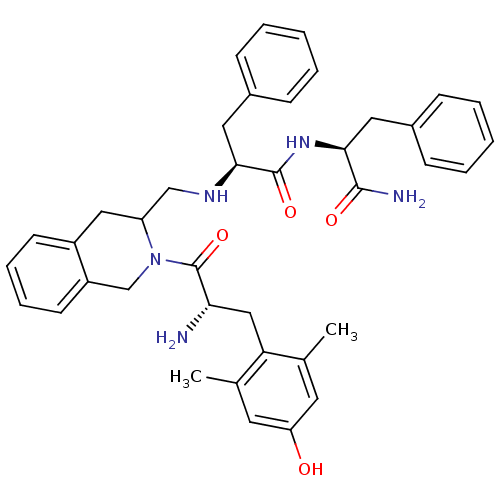 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080453 ((S)-2-({2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 determined by displacing [3H]-DSLET from rat brain membrane binding sites | J Med Chem 42: 3520-6 (1999) Article DOI: 10.1021/jm980724+ BindingDB Entry DOI: 10.7270/Q2SJ1JT1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085050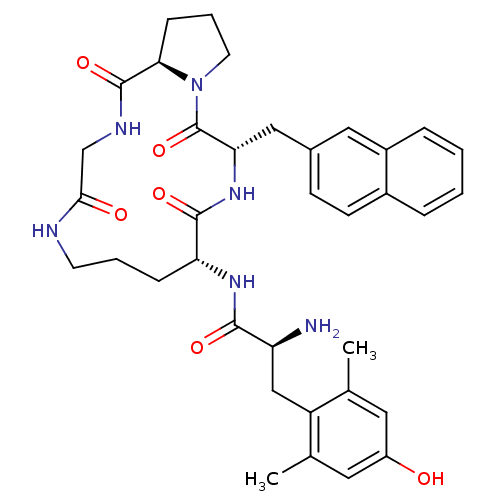 (CHEMBL152690 | H-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50085050 (CHEMBL152690 | H-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-]) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al Curated by ChEMBL | Assay Description Binding affinity was determined by displacement of [3H]- DAMGO at Opioid receptor mu 1 in rat brain membrane homogenates | J Med Chem 43: 551-9 (2000) BindingDB Entry DOI: 10.7270/Q2WQ04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50003571 ((-)-3-Hydroxy-N-methylmorphinan hydrochloride (lev...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Peptides 19: 1091-8 (1998) Article DOI: 10.1016/s0196-9781(98)00023-0 BindingDB Entry DOI: 10.7270/Q2B27STB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50493134 (CHEMBL3038184) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50021325 (4-methyl-(1S,5R,13R,14S)-12-oxa-4-azapentacyclo[9....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences Curated by PDSP Ki Database | Peptides 19: 1091-8 (1998) Article DOI: 10.1016/s0196-9781(98)00023-0 BindingDB Entry DOI: 10.7270/Q2B27STB | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50010704 (CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.653 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity was measured on mu opioid receptor | J Med Chem 44: 3048-53 (2001) BindingDB Entry DOI: 10.7270/Q28K79SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001456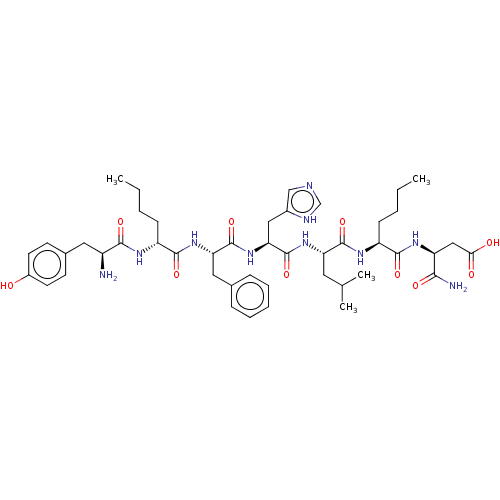 (3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.665 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Inhibition of [3H]- ]DSLET binding to delta receptor from rat brain membrane | J Med Chem 35: 3956-61 (1992) BindingDB Entry DOI: 10.7270/Q2H70DRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198754 (CHEMBL3924888) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126500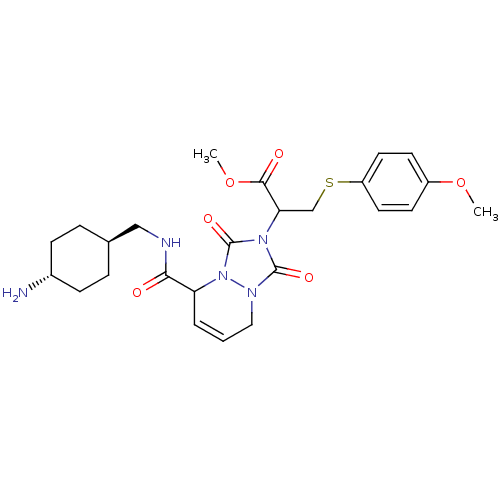 (2-{5-[(4-Amino-cyclohexylmethyl)-carbamoyl]-1,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin | Bioorg Med Chem Lett 13: 1445-9 (2003) BindingDB Entry DOI: 10.7270/Q23F4P0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50198755 (CHEMBL3979449) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.786 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in guinea pig brain membrane measured after 2 hrs | J Med Chem 59: 9243-9254 (2016) Article DOI: 10.1021/acs.jmedchem.6b01200 BindingDB Entry DOI: 10.7270/Q23B623F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50493133 (CHEMBL3038185) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.789 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Displacement of [3H]DSLET from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 23: 5082-5 (2013) Article DOI: 10.1016/j.bmcl.2013.07.036 BindingDB Entry DOI: 10.7270/Q2RN3BSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2354 total ) | Next | Last >> |