 Found 2750 hits with Last Name = 'di francesco' and Initial = 'me'
Found 2750 hits with Last Name = 'di francesco' and Initial = 'me' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Falcipain 2
(Plasmodium falciparum) | BDBM50371561
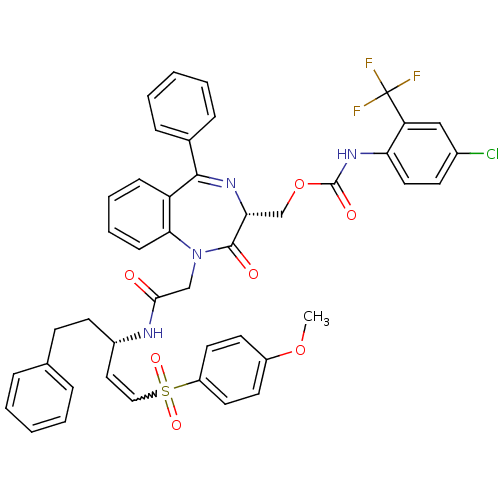
(CHEMBL405741)Show SMILES COc1ccc(cc1)S(=O)(=O)C=C[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:11.11,c:36| Show InChI InChI=1S/C44H38ClF3N4O7S/c1-58-33-19-21-34(22-20-33)60(56,57)25-24-32(18-16-29-10-4-2-5-11-29)49-40(53)27-52-39-15-9-8-14-35(39)41(30-12-6-3-7-13-30)50-38(42(52)54)28-59-43(55)51-37-23-17-31(45)26-36(37)44(46,47)48/h2-15,17,19-26,32,38H,16,18,27-28H2,1H3,(H,49,53)(H,51,55)/t32-,38+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50371567
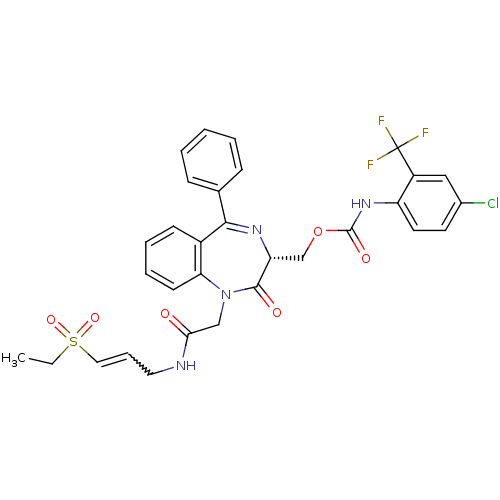
(CHEMBL270278)Show SMILES CCS(=O)(=O)C=CCNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:6.6,c:20| Show InChI InChI=1S/C31H28ClF3N4O6S/c1-2-46(43,44)16-8-15-36-27(40)18-39-26-12-7-6-11-22(26)28(20-9-4-3-5-10-20)37-25(29(39)41)19-45-30(42)38-24-14-13-21(32)17-23(24)31(33,34)35/h3-14,16-17,25H,2,15,18-19H2,1H3,(H,36,40)(H,38,42)/t25-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50371562
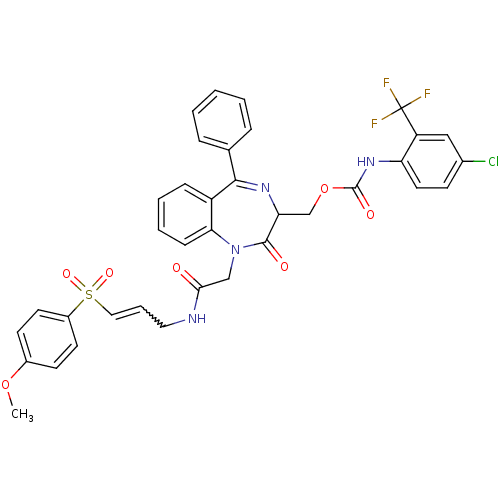
(CHEMBL271992)Show SMILES COc1ccc(cc1)S(=O)(=O)C=CCNC(=O)CN1c2ccccc2C(=NC(COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:12.13,c:27| Show InChI InChI=1S/C36H30ClF3N4O7S/c1-50-25-13-15-26(16-14-25)52(48,49)19-7-18-41-32(45)21-44-31-11-6-5-10-27(31)33(23-8-3-2-4-9-23)42-30(34(44)46)22-51-35(47)43-29-17-12-24(37)20-28(29)36(38,39)40/h2-17,19-20,30H,18,21-22H2,1H3,(H,41,45)(H,43,47) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50371568
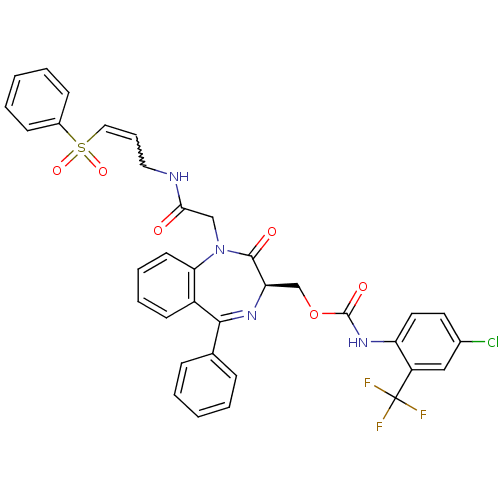
(CHEMBL408071)Show SMILES FC(F)(F)c1cc(Cl)ccc1NC(=O)OC[C@H]1N=C(c2ccccc2)c2ccccc2N(CC(=O)NCC=CS(=O)(=O)c2ccccc2)C1=O |w:37.39,t:18| Show InChI InChI=1S/C35H28ClF3N4O6S/c36-24-16-17-28(27(20-24)35(37,38)39)42-34(46)49-22-29-33(45)43(21-31(44)40-18-9-19-50(47,48)25-12-5-2-6-13-25)30-15-8-7-14-26(30)32(41-29)23-10-3-1-4-11-23/h1-17,19-20,29H,18,21-22H2,(H,40,44)(H,42,46)/t29-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50371564
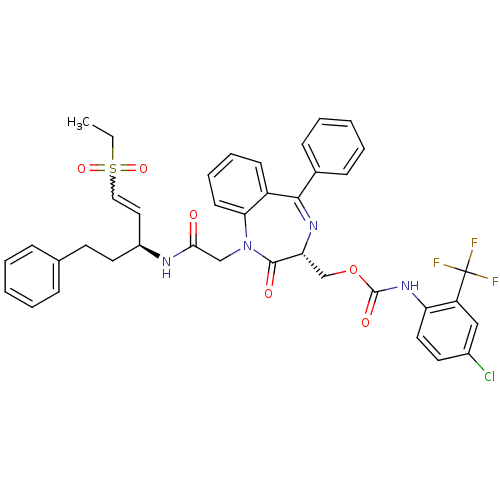
(CHEMBL403991)Show SMILES CCS(=O)(=O)C=C[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:5.4,c:29| Show InChI InChI=1S/C39H36ClF3N4O6S/c1-2-54(51,52)22-21-29(19-17-26-11-5-3-6-12-26)44-35(48)24-47-34-16-10-9-15-30(34)36(27-13-7-4-8-14-27)45-33(37(47)49)25-53-38(50)46-32-20-18-28(40)23-31(32)39(41,42)43/h3-16,18,20-23,29,33H,2,17,19,24-25H2,1H3,(H,44,48)(H,46,50)/t29-,33+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50371566
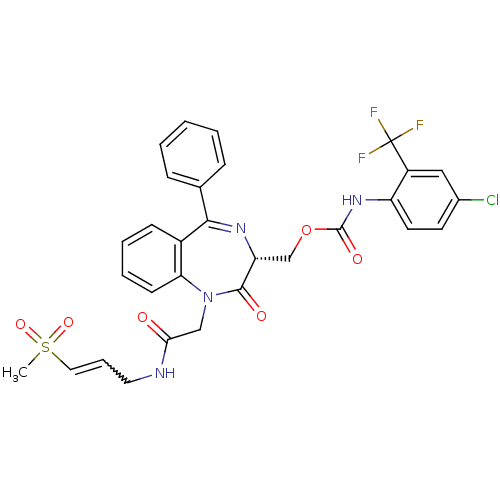
(CHEMBL407354)Show SMILES CS(=O)(=O)C=CCNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:5.5,c:19| Show InChI InChI=1S/C30H26ClF3N4O6S/c1-45(42,43)15-7-14-35-26(39)17-38-25-11-6-5-10-21(25)27(19-8-3-2-4-9-19)36-24(28(38)40)18-44-29(41)37-23-13-12-20(31)16-22(23)30(32,33)34/h2-13,15-16,24H,14,17-18H2,1H3,(H,35,39)(H,37,41)/t24-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50371563
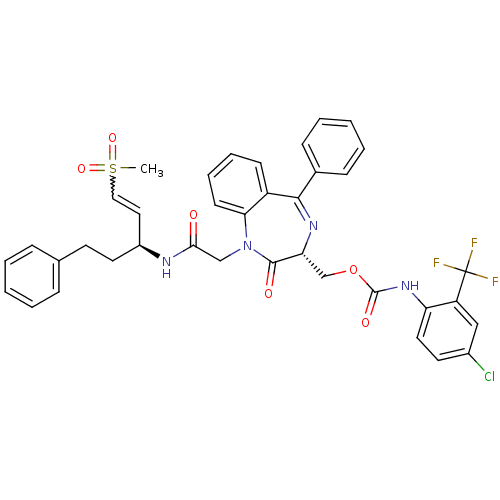
(CHEMBL272015)Show SMILES CS(=O)(=O)C=C[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:4.3,c:28| Show InChI InChI=1S/C38H34ClF3N4O6S/c1-53(50,51)21-20-28(18-16-25-10-4-2-5-11-25)43-34(47)23-46-33-15-9-8-14-29(33)35(26-12-6-3-7-13-26)44-32(36(46)48)24-52-37(49)45-31-19-17-27(39)22-30(31)38(40,41)42/h2-15,17,19-22,28,32H,16,18,23-24H2,1H3,(H,43,47)(H,45,49)/t28-,32+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50371565
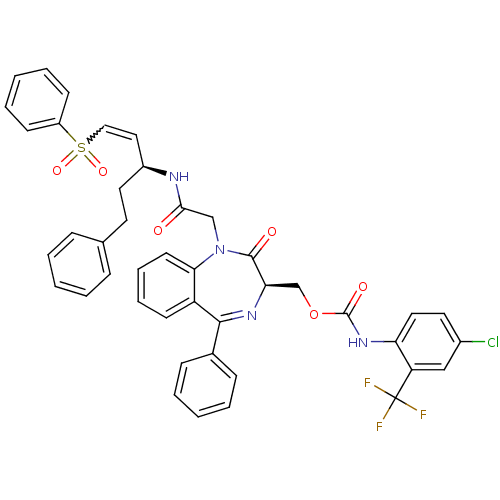
(CHEMBL402455)Show SMILES FC(F)(F)c1cc(Cl)ccc1NC(=O)OC[C@H]1N=C(c2ccccc2)c2ccccc2N(CC(=O)N[C@@H](CCc2ccccc2)C=CS(=O)(=O)c2ccccc2)C1=O |w:46.50,t:18| Show InChI InChI=1S/C43H36ClF3N4O6S/c44-31-21-23-36(35(26-31)43(45,46)47)50-42(54)57-28-37-41(53)51(38-19-11-10-18-34(38)40(49-37)30-14-6-2-7-15-30)27-39(52)48-32(22-20-29-12-4-1-5-13-29)24-25-58(55,56)33-16-8-3-9-17-33/h1-19,21,23-26,32,37H,20,22,27-28H2,(H,48,52)(H,50,54)/t32-,37+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain-2 |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50257017
((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES CN(C)C(=O)\C=C\CNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,c:20| Show InChI InChI=1S/C32H29ClF3N5O5/c1-40(2)28(43)13-8-16-37-27(42)18-41-26-12-7-6-11-22(26)29(20-9-4-3-5-10-20)38-25(30(41)44)19-46-31(45)39-24-15-14-21(33)17-23(24)32(34,35)36/h3-15,17,25H,16,18-19H2,1-2H3,(H,37,42)(H,39,45)/b13-8+/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50257017

((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES CN(C)C(=O)\C=C\CNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,c:20| Show InChI InChI=1S/C32H29ClF3N5O5/c1-40(2)28(43)13-8-16-37-27(42)18-41-26-12-7-6-11-22(26)29(20-9-4-3-5-10-20)38-25(30(41)44)19-46-31(45)39-24-15-14-21(33)17-23(24)32(34,35)36/h3-15,17,25H,16,18-19H2,1-2H3,(H,37,42)(H,39,45)/b13-8+/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50257015
((4S)-4-{2-[3-(4-Chloro-2-trifluoromethyl-phenylcar...)Show SMILES COC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,c:28| Show InChI InChI=1S/C39H34ClF3N4O6/c1-52-35(49)21-19-28(18-16-25-10-4-2-5-11-25)44-34(48)23-47-33-15-9-8-14-29(33)36(26-12-6-3-7-13-26)45-32(37(47)50)24-53-38(51)46-31-20-17-27(40)22-30(31)39(41,42)43/h2-15,17,19-22,28,32H,16,18,23-24H2,1H3,(H,44,48)(H,46,51)/b21-19+/t28-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50371565

(CHEMBL402455)Show SMILES FC(F)(F)c1cc(Cl)ccc1NC(=O)OC[C@H]1N=C(c2ccccc2)c2ccccc2N(CC(=O)N[C@@H](CCc2ccccc2)C=CS(=O)(=O)c2ccccc2)C1=O |w:46.50,t:18| Show InChI InChI=1S/C43H36ClF3N4O6S/c44-31-21-23-36(35(26-31)43(45,46)47)50-42(54)57-28-37-41(53)51(38-19-11-10-18-34(38)40(49-37)30-14-6-2-7-15-30)27-39(52)48-32(22-20-29-12-4-1-5-13-29)24-25-58(55,56)33-16-8-3-9-17-33/h1-19,21,23-26,32,37H,20,22,27-28H2,(H,48,52)(H,50,54)/t32-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50257019

((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES FC(F)(F)c1cc(Cl)ccc1NC(=O)OC[C@H]1N=C(c2ccccc2)c2ccccc2N(CC(=O)NC\C=C\C#N)C1=O |r,t:18| Show InChI InChI=1S/C30H23ClF3N5O4/c31-20-12-13-23(22(16-20)30(32,33)34)38-29(42)43-18-24-28(41)39(17-26(40)36-15-7-6-14-35)25-11-5-4-10-21(25)27(37-24)19-8-2-1-3-9-19/h1-13,16,24H,15,17-18H2,(H,36,40)(H,38,42)/b7-6+/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50371561

(CHEMBL405741)Show SMILES COc1ccc(cc1)S(=O)(=O)C=C[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:11.11,c:36| Show InChI InChI=1S/C44H38ClF3N4O7S/c1-58-33-19-21-34(22-20-33)60(56,57)25-24-32(18-16-29-10-4-2-5-11-29)49-40(53)27-52-39-15-9-8-14-35(39)41(30-12-6-3-7-13-30)50-38(42(52)54)28-59-43(55)51-37-23-17-31(45)26-36(37)44(46,47)48/h2-15,17,19-26,32,38H,16,18,27-28H2,1H3,(H,49,53)(H,51,55)/t32-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50371567

(CHEMBL270278)Show SMILES CCS(=O)(=O)C=CCNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:6.6,c:20| Show InChI InChI=1S/C31H28ClF3N4O6S/c1-2-46(43,44)16-8-15-36-27(40)18-39-26-12-7-6-11-22(26)28(20-9-4-3-5-10-20)37-25(29(39)41)19-45-30(42)38-24-14-13-21(32)17-23(24)31(33,34)35/h3-14,16-17,25H,2,15,18-19H2,1H3,(H,36,40)(H,38,42)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50371564

(CHEMBL403991)Show SMILES CCS(=O)(=O)C=C[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:5.4,c:29| Show InChI InChI=1S/C39H36ClF3N4O6S/c1-2-54(51,52)22-21-29(19-17-26-11-5-3-6-12-26)44-35(48)24-47-34-16-10-9-15-30(34)36(27-13-7-4-8-14-27)45-33(37(47)49)25-53-38(50)46-32-20-18-28(40)23-31(32)39(41,42)43/h3-16,18,20-23,29,33H,2,17,19,24-25H2,1H3,(H,44,48)(H,46,50)/t29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50371568

(CHEMBL408071)Show SMILES FC(F)(F)c1cc(Cl)ccc1NC(=O)OC[C@H]1N=C(c2ccccc2)c2ccccc2N(CC(=O)NCC=CS(=O)(=O)c2ccccc2)C1=O |w:37.39,t:18| Show InChI InChI=1S/C35H28ClF3N4O6S/c36-24-16-17-28(27(20-24)35(37,38)39)42-34(46)49-22-29-33(45)43(21-31(44)40-18-9-19-50(47,48)25-12-5-2-6-13-25)30-15-8-7-14-26(30)32(41-29)23-10-3-1-4-11-23/h1-17,19-20,29H,18,21-22H2,(H,40,44)(H,42,46)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50257016
((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES CC(=O)CC=CNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,w:5.5,c:18| Show InChI InChI=1S/C31H26ClF3N4O5/c1-19(40)8-7-15-36-27(41)17-39-26-12-6-5-11-22(26)28(20-9-3-2-4-10-20)37-25(29(39)42)18-44-30(43)38-24-14-13-21(32)16-23(24)31(33,34)35/h2-7,9-16,25H,8,17-18H2,1H3,(H,36,41)(H,38,43)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50371562

(CHEMBL271992)Show SMILES COc1ccc(cc1)S(=O)(=O)C=CCNC(=O)CN1c2ccccc2C(=NC(COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:12.13,c:27| Show InChI InChI=1S/C36H30ClF3N4O7S/c1-50-25-13-15-26(16-14-25)52(48,49)19-7-18-41-32(45)21-44-31-11-6-5-10-27(31)33(23-8-3-2-4-9-23)42-30(34(44)46)22-51-35(47)43-29-17-12-24(37)20-28(29)36(38,39)40/h2-17,19-20,30H,18,21-22H2,1H3,(H,41,45)(H,43,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50257016

((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES CC(=O)CC=CNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,w:5.5,c:18| Show InChI InChI=1S/C31H26ClF3N4O5/c1-19(40)8-7-15-36-27(41)17-39-26-12-6-5-11-22(26)28(20-9-3-2-4-10-20)37-25(29(39)42)18-44-30(43)38-24-14-13-21(32)16-23(24)31(33,34)35/h2-7,9-16,25H,8,17-18H2,1H3,(H,36,41)(H,38,43)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50257014
((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES CN(C)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,c:29| Show InChI InChI=1S/C40H37ClF3N5O5/c1-48(2)36(51)22-20-29(19-17-26-11-5-3-6-12-26)45-35(50)24-49-34-16-10-9-15-30(34)37(27-13-7-4-8-14-27)46-33(38(49)52)25-54-39(53)47-32-21-18-28(41)23-31(32)40(42,43)44/h3-16,18,20-23,29,33H,17,19,24-25H2,1-2H3,(H,45,50)(H,47,53)/b22-20+/t29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50371565

(CHEMBL402455)Show SMILES FC(F)(F)c1cc(Cl)ccc1NC(=O)OC[C@H]1N=C(c2ccccc2)c2ccccc2N(CC(=O)N[C@@H](CCc2ccccc2)C=CS(=O)(=O)c2ccccc2)C1=O |w:46.50,t:18| Show InChI InChI=1S/C43H36ClF3N4O6S/c44-31-21-23-36(35(26-31)43(45,46)47)50-42(54)57-28-37-41(53)51(38-19-11-10-18-34(38)40(49-37)30-14-6-2-7-15-30)27-39(52)48-32(22-20-29-12-4-1-5-13-29)24-25-58(55,56)33-16-8-3-9-17-33/h1-19,21,23-26,32,37H,20,22,27-28H2,(H,48,52)(H,50,54)/t32-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50257018

((E)-methyl4-(2-((R,Z)-3-((4-chloro-2-(trifluoromet...)Show SMILES COC(=O)\C=C\CNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,c:19| Show InChI InChI=1S/C31H26ClF3N4O6/c1-44-27(41)12-7-15-36-26(40)17-39-25-11-6-5-10-21(25)28(19-8-3-2-4-9-19)37-24(29(39)42)18-45-30(43)38-23-14-13-20(32)16-22(23)31(33,34)35/h2-14,16,24H,15,17-18H2,1H3,(H,36,40)(H,38,43)/b12-7+/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50257014

((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES CN(C)C(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,c:29| Show InChI InChI=1S/C40H37ClF3N5O5/c1-48(2)36(51)22-20-29(19-17-26-11-5-3-6-12-26)45-35(50)24-49-34-16-10-9-15-30(34)37(27-13-7-4-8-14-27)46-33(38(49)52)25-54-39(53)47-32-21-18-28(41)23-31(32)40(42,43)44/h3-16,18,20-23,29,33H,17,19,24-25H2,1-2H3,(H,45,50)(H,47,53)/b22-20+/t29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50257013
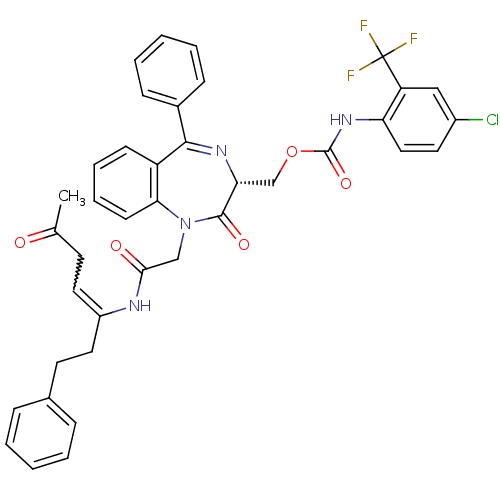
((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES CC(=O)CC=C(CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,w:4.3,c:27| Show InChI InChI=1S/C39H34ClF3N4O5/c1-25(48)16-19-29(20-17-26-10-4-2-5-11-26)44-35(49)23-47-34-15-9-8-14-30(34)36(27-12-6-3-7-13-27)45-33(37(47)50)24-52-38(51)46-32-21-18-28(40)22-31(32)39(41,42)43/h2-15,18-19,21-22,33H,16-17,20,23-24H2,1H3,(H,44,49)(H,46,51)/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50257013

((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES CC(=O)CC=C(CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,w:4.3,c:27| Show InChI InChI=1S/C39H34ClF3N4O5/c1-25(48)16-19-29(20-17-26-10-4-2-5-11-26)44-35(49)23-47-34-15-9-8-14-30(34)36(27-12-6-3-7-13-27)45-33(37(47)50)24-52-38(51)46-32-21-18-28(40)22-31(32)39(41,42)43/h2-15,18-19,21-22,33H,16-17,20,23-24H2,1H3,(H,44,49)(H,46,51)/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50257018

((E)-methyl4-(2-((R,Z)-3-((4-chloro-2-(trifluoromet...)Show SMILES COC(=O)\C=C\CNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,c:19| Show InChI InChI=1S/C31H26ClF3N4O6/c1-44-27(41)12-7-15-36-26(40)17-39-25-11-6-5-10-21(25)28(19-8-3-2-4-9-19)37-24(29(39)42)18-45-30(43)38-23-14-13-20(32)16-22(23)31(33,34)35/h2-14,16,24H,15,17-18H2,1H3,(H,36,40)(H,38,43)/b12-7+/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50257019

((4-Chloro-2-trifluoromethyl-phenyl)-carbamic acid ...)Show SMILES FC(F)(F)c1cc(Cl)ccc1NC(=O)OC[C@H]1N=C(c2ccccc2)c2ccccc2N(CC(=O)NC\C=C\C#N)C1=O |r,t:18| Show InChI InChI=1S/C30H23ClF3N5O4/c31-20-12-13-23(22(16-20)30(32,33)34)38-29(42)43-18-24-28(41)39(17-26(40)36-15-7-6-14-35)25-11-5-4-10-21(25)27(37-24)19-8-2-1-3-9-19/h1-13,16,24H,15,17-18H2,(H,36,40)(H,38,42)/b7-6+/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50371566

(CHEMBL407354)Show SMILES CS(=O)(=O)C=CCNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:5.5,c:19| Show InChI InChI=1S/C30H26ClF3N4O6S/c1-45(42,43)15-7-14-35-26(39)17-38-25-11-6-5-10-21(25)27(19-8-3-2-4-9-19)36-24(28(38)40)18-44-29(41)37-23-13-12-20(31)16-22(23)30(32,33)34/h2-13,15-16,24H,14,17-18H2,1H3,(H,35,39)(H,37,41)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50371564

(CHEMBL403991)Show SMILES CCS(=O)(=O)C=C[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:5.4,c:29| Show InChI InChI=1S/C39H36ClF3N4O6S/c1-2-54(51,52)22-21-29(19-17-26-11-5-3-6-12-26)44-35(48)24-47-34-16-10-9-15-30(34)36(27-13-7-4-8-14-27)45-33(37(47)49)25-53-38(50)46-32-20-18-28(40)23-31(32)39(41,42)43/h3-16,18,20-23,29,33H,2,17,19,24-25H2,1H3,(H,44,48)(H,46,50)/t29-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50257015

((4S)-4-{2-[3-(4-Chloro-2-trifluoromethyl-phenylcar...)Show SMILES COC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |r,c:28| Show InChI InChI=1S/C39H34ClF3N4O6/c1-52-35(49)21-19-28(18-16-25-10-4-2-5-11-25)44-34(48)23-47-33-15-9-8-14-29(33)36(26-12-6-3-7-13-26)45-32(37(47)50)24-53-38(51)46-31-20-17-27(40)22-30(31)39(41,42)43/h2-15,17,19-22,28,32H,16,18,23-24H2,1H3,(H,44,48)(H,46,51)/b21-19+/t28-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B (unknown origin) |
J Med Chem 52: 2157-60 (2009)
Article DOI: 10.1021/jm900047j
BindingDB Entry DOI: 10.7270/Q2BG2NX8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50371562

(CHEMBL271992)Show SMILES COc1ccc(cc1)S(=O)(=O)C=CCNC(=O)CN1c2ccccc2C(=NC(COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:12.13,c:27| Show InChI InChI=1S/C36H30ClF3N4O7S/c1-50-25-13-15-26(16-14-25)52(48,49)19-7-18-41-32(45)21-44-31-11-6-5-10-27(31)33(23-8-3-2-4-9-23)42-30(34(44)46)22-51-35(47)43-29-17-12-24(37)20-28(29)36(38,39)40/h2-17,19-20,30H,18,21-22H2,1H3,(H,41,45)(H,43,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50371563

(CHEMBL272015)Show SMILES CS(=O)(=O)C=C[C@H](CCc1ccccc1)NC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)Nc2ccc(Cl)cc2C(F)(F)F)C1=O)c1ccccc1 |w:4.3,c:28| Show InChI InChI=1S/C38H34ClF3N4O6S/c1-53(50,51)21-20-28(18-16-25-10-4-2-5-11-25)43-34(47)23-46-33-15-9-8-14-29(33)35(26-12-6-3-7-13-26)44-32(36(46)48)24-52-37(49)45-31-19-17-27(39)22-30(31)38(40,41)42/h2-15,17,19-22,28,32H,16,18,23-24H2,1H3,(H,43,47)(H,45,49)/t28-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50371568

(CHEMBL408071)Show SMILES FC(F)(F)c1cc(Cl)ccc1NC(=O)OC[C@H]1N=C(c2ccccc2)c2ccccc2N(CC(=O)NCC=CS(=O)(=O)c2ccccc2)C1=O |w:37.39,t:18| Show InChI InChI=1S/C35H28ClF3N4O6S/c36-24-16-17-28(27(20-24)35(37,38)39)42-34(46)49-22-29-33(45)43(21-31(44)40-18-9-19-50(47,48)25-12-5-2-6-13-25)30-15-8-7-14-26(30)32(41-29)23-10-3-1-4-11-23/h1-17,19-20,29H,18,21-22H2,(H,40,44)(H,42,46)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 51: 988-96 (2008)
Article DOI: 10.1021/jm701141u
BindingDB Entry DOI: 10.7270/Q2SX6F2M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM301997
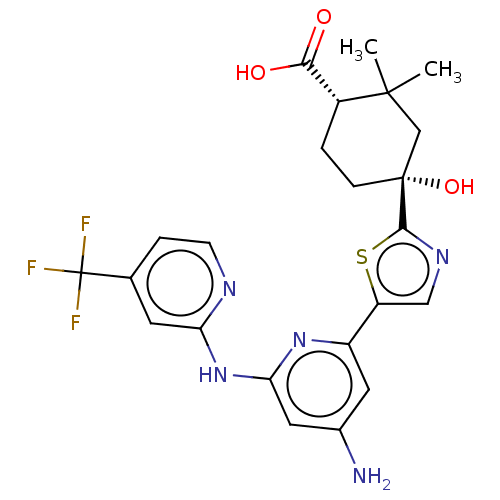
(US9598405, 1.5 | US9598405, 1.6)Show SMILES CC1(C)C[C@](O)(CC[C@@H]1C(O)=O)c1ncc(s1)-c1cc(N)cc(Nc2cc(ccn2)C(F)(F)F)n1 Show InChI InChI=1S/C23H24F3N5O3S/c1-21(2)11-22(34,5-3-14(21)19(32)33)20-29-10-16(35-20)15-8-13(27)9-18(30-15)31-17-7-12(4-6-28-17)23(24,25)26/h4,6-10,14,34H,3,5,11H2,1-2H3,(H,32,33)(H3,27,28,30,31)/t14-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302002

(US9598405, 2.1)Show SMILES Cc1cc(Nc2cc(ccn2)-c2c[nH]nn2)nc(c1)-c1cnc(s1)C1(O)CCC(CC1)C(O)=O Show InChI InChI=1S/C23H23N7O3S/c1-13-8-16(18-12-25-22(34-18)23(33)5-2-14(3-6-23)21(31)32)27-20(9-13)28-19-10-15(4-7-24-19)17-11-26-30-29-17/h4,7-12,14,33H,2-3,5-6H2,1H3,(H,31,32)(H,24,27,28)(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302016
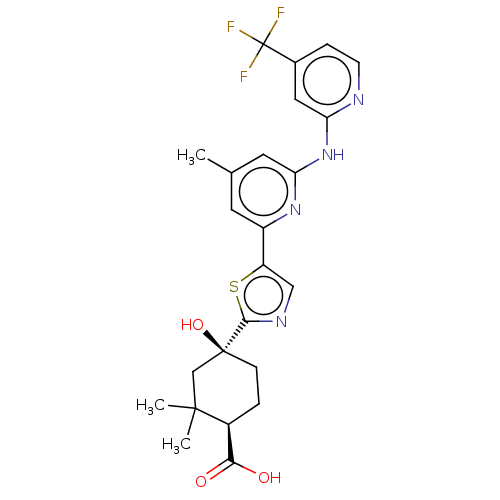
(US9598405, 3.1)Show SMILES Cc1cc(Nc2cc(ccn2)C(F)(F)F)nc(c1)-c1cnc(s1)[C@]1(O)CC[C@@H](C(O)=O)C(C)(C)C1 Show InChI InChI=1S/C24H25F3N4O3S/c1-13-8-16(30-19(9-13)31-18-10-14(5-7-28-18)24(25,26)27)17-11-29-21(35-17)23(34)6-4-15(20(32)33)22(2,3)12-23/h5,7-11,15,34H,4,6,12H2,1-3H3,(H,32,33)(H,28,30,31)/t15-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302034

(BDBM302035 | US9598405, 4.1)Show SMILES Cc1cc(Nc2cc(ccn2)C(F)(F)F)nc(c1)-c1cnc(s1)C1(O)CC2(CCCCC2)C1C(O)=O Show InChI InChI=1S/C25H25F3N4O3S/c1-14-9-16(31-19(10-14)32-18-11-15(5-8-29-18)25(26,27)28)17-12-30-22(36-17)24(35)13-23(20(24)21(33)34)6-3-2-4-7-23/h5,8-12,20,35H,2-4,6-7,13H2,1H3,(H,33,34)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302018
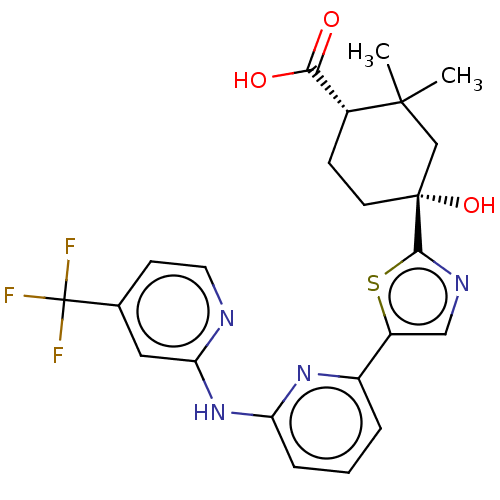
(US9598405, 3.3 | US9598405, 3.7)Show SMILES CC1(C)C[C@](O)(CC[C@@H]1C(O)=O)c1ncc(s1)-c1cccc(Nc2cc(ccn2)C(F)(F)F)n1 Show InChI InChI=1S/C23H23F3N4O3S/c1-21(2)12-22(33,8-6-14(21)19(31)32)20-28-11-16(34-20)15-4-3-5-17(29-15)30-18-10-13(7-9-27-18)23(24,25)26/h3-5,7,9-11,14,33H,6,8,12H2,1-2H3,(H,31,32)(H,27,29,30)/t14-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302028
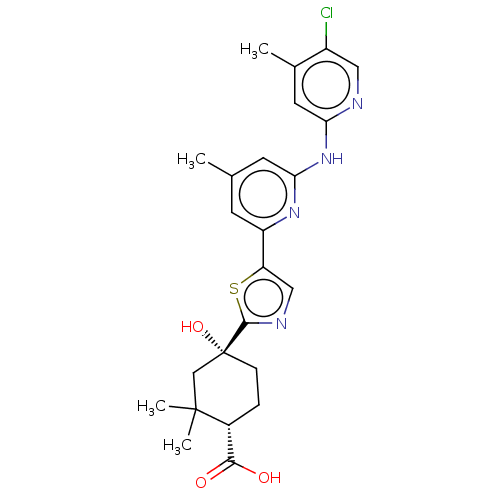
(US9598405, 3.13)Show SMILES Cc1cc(Nc2cc(C)c(Cl)cn2)nc(c1)-c1cnc(s1)[C@@]1(O)CC[C@H](C(O)=O)C(C)(C)C1 Show InChI InChI=1S/C24H27ClN4O3S/c1-13-7-17(28-20(8-13)29-19-9-14(2)16(25)10-26-19)18-11-27-22(33-18)24(32)6-5-15(21(30)31)23(3,4)12-24/h7-11,15,32H,5-6,12H2,1-4H3,(H,30,31)(H,26,28,29)/t15-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM177763
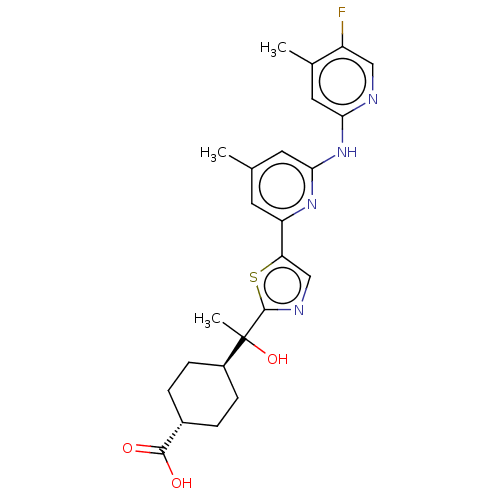
(US9120785, Example 10 | US9120785, Example 11)Show SMILES Cc1cc(Nc2cc(C)c(F)cn2)nc(c1)-c1cnc(s1)C(C)(O)[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:27.33,wD:24.26,(4.68,-7.21,;3.34,-6.44,;2.01,-7.21,;.68,-6.44,;-.66,-7.21,;-1.99,-6.44,;-1.99,-4.9,;-3.33,-4.13,;-3.33,-2.59,;-4.66,-4.9,;-5.99,-4.13,;-4.66,-6.44,;-3.33,-7.21,;.68,-4.9,;2.01,-4.13,;3.34,-4.9,;2.01,-2.59,;3.25,-1.68,;2.78,-.22,;1.24,-.22,;.76,-1.68,;.47,1.12,;-.86,1.89,;-.86,.35,;1.24,2.45,;2.73,2.05,;3.82,3.14,;3.42,4.63,;1.93,5.03,;.84,3.94,;4.51,5.72,;5.99,5.32,;4.11,7.21,)| Show InChI InChI=1S/C24H27FN4O3S/c1-13-8-18(28-21(9-13)29-20-10-14(2)17(25)11-26-20)19-12-27-23(33-19)24(3,32)16-6-4-15(5-7-16)22(30)31/h8-12,15-16,32H,4-7H2,1-3H3,(H,30,31)(H,26,28,29)/t15-,16-,24? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Homogeneous Time-Resolved Fluorescence (HTRF) assay for the recombinant human Syk enzyme: A recombinant GST-hSyk fusion protein was used to measure p... |
US Patent US9120785 (2015)
BindingDB Entry DOI: 10.7270/Q2959G9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM301993
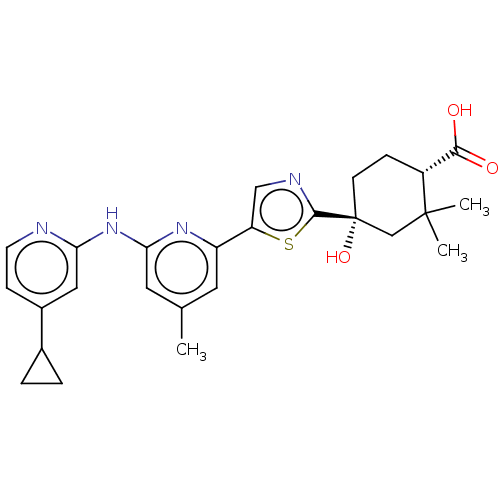
(BDBM301994 | US9598405, 1.1)Show SMILES Cc1cc(Nc2cc(ccn2)C2CC2)nc(c1)-c1cnc(s1)[C@@]1(O)CC[C@H](C(O)=O)C(C)(C)C1 Show InChI InChI=1S/C26H30N4O3S/c1-15-10-19(29-22(11-15)30-21-12-17(7-9-27-21)16-4-5-16)20-13-28-24(34-20)26(33)8-6-18(23(31)32)25(2,3)14-26/h7,9-13,16,18,33H,4-6,8,14H2,1-3H3,(H,31,32)(H,27,29,30)/t18-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302029
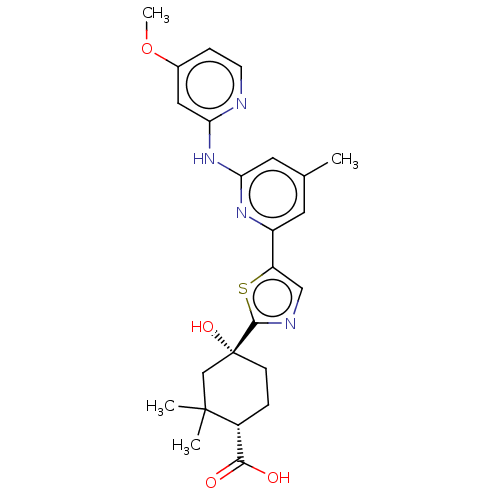
(US9598405, 3.14)Show SMILES COc1ccnc(Nc2cc(C)cc(n2)-c2cnc(s2)[C@@]2(O)CC[C@H](C(O)=O)C(C)(C)C2)c1 Show InChI InChI=1S/C24H28N4O4S/c1-14-9-17(27-20(10-14)28-19-11-15(32-4)6-8-25-19)18-12-26-22(33-18)24(31)7-5-16(21(29)30)23(2,3)13-24/h6,8-12,16,31H,5,7,13H2,1-4H3,(H,29,30)(H,25,27,28)/t16-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.104 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
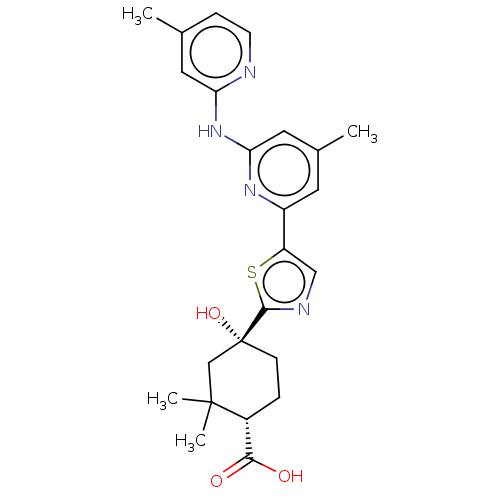
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302032
(US9598405, 3.17)Show SMILES Cc1ccnc(Nc2cc(C)cc(n2)-c2cnc(s2)[C@@]2(O)CC[C@H](C(O)=O)C(C)(C)C2)c1 Show InChI InChI=1S/C24H28N4O3S/c1-14-6-8-25-19(10-14)28-20-11-15(2)9-17(27-20)18-12-26-22(32-18)24(31)7-5-16(21(29)30)23(3,4)13-24/h6,8-12,16,31H,5,7,13H2,1-4H3,(H,29,30)(H,25,27,28)/t16-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302000
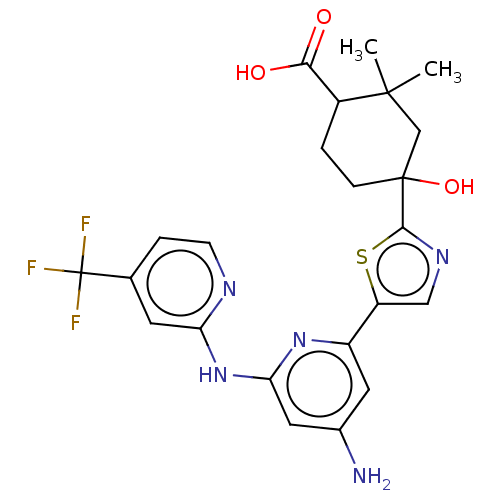
(US9598405, 1.8)Show SMILES CC1(C)CC(O)(CCC1C(O)=O)c1ncc(s1)-c1cc(N)cc(Nc2cc(ccn2)C(F)(F)F)n1 Show InChI InChI=1S/C23H24F3N5O3S/c1-21(2)11-22(34,5-3-14(21)19(32)33)20-29-10-16(35-20)15-8-13(27)9-18(30-15)31-17-7-12(4-6-28-17)23(24,25)26/h4,6-10,14,34H,3,5,11H2,1-2H3,(H,32,33)(H3,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.119 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302030
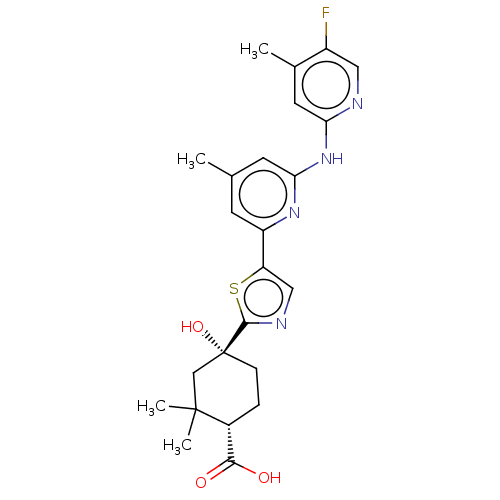
(US9598405, 3.15)Show SMILES Cc1cc(Nc2cc(C)c(F)cn2)nc(c1)-c1cnc(s1)[C@@]1(O)CC[C@H](C(O)=O)C(C)(C)C1 Show InChI InChI=1S/C24H27FN4O3S/c1-13-7-17(28-20(8-13)29-19-9-14(2)16(25)10-26-19)18-11-27-22(33-18)24(32)6-5-15(21(30)31)23(3,4)12-24/h7-11,15,32H,5-6,12H2,1-4H3,(H,30,31)(H,26,28,29)/t15-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.127 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302013
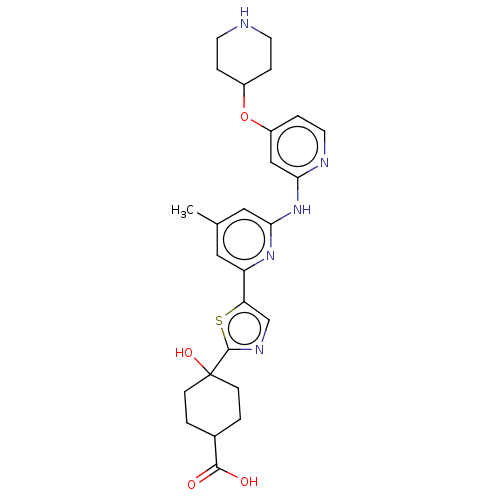
(US9598405, 2.12)Show SMILES Cc1cc(Nc2cc(OC3CCNCC3)ccn2)nc(c1)-c1cnc(s1)C1(O)CCC(CC1)C(O)=O |(10.66,-14.61,;11.29,-13.2,;12.82,-13.04,;13.45,-11.63,;14.98,-11.47,;15.61,-10.06,;17.14,-9.9,;17.76,-8.5,;19.3,-8.33,;20.2,-9.58,;21.73,-9.42,;22.64,-10.66,;22.01,-12.07,;20.48,-12.23,;19.57,-10.99,;16.86,-7.25,;15.33,-7.41,;14.7,-8.82,;12.54,-10.38,;11.01,-10.55,;10.38,-11.95,;10.11,-9.3,;8.57,-9.3,;8.09,-7.84,;9.34,-6.93,;10.58,-7.84,;9.34,-5.39,;10.85,-5.66,;7.82,-5.66,;6.83,-4.48,;7.36,-3.03,;8.87,-2.76,;9.86,-3.94,;6.37,-1.85,;4.85,-2.12,;6.89,-.4,)| Show InChI InChI=1S/C26H31N5O4S/c1-16-12-20(21-15-29-25(36-21)26(34)7-2-17(3-8-26)24(32)33)30-23(13-16)31-22-14-19(6-11-28-22)35-18-4-9-27-10-5-18/h6,11-15,17-18,27,34H,2-5,7-10H2,1H3,(H,32,33)(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302017
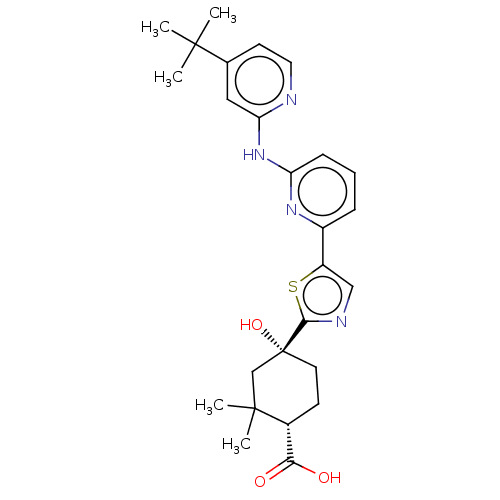
(US9598405, 3.2 | US9598405, 3.6)Show SMILES CC(C)(C)c1ccnc(Nc2cccc(n2)-c2cnc(s2)[C@@]2(O)CC[C@H](C(O)=O)C(C)(C)C2)c1 Show InChI InChI=1S/C26H32N4O3S/c1-24(2,3)16-10-12-27-21(13-16)30-20-8-6-7-18(29-20)19-14-28-23(34-19)26(33)11-9-17(22(31)32)25(4,5)15-26/h6-8,10,12-14,17,33H,9,11,15H2,1-5H3,(H,31,32)(H,27,29,30)/t17-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM302033
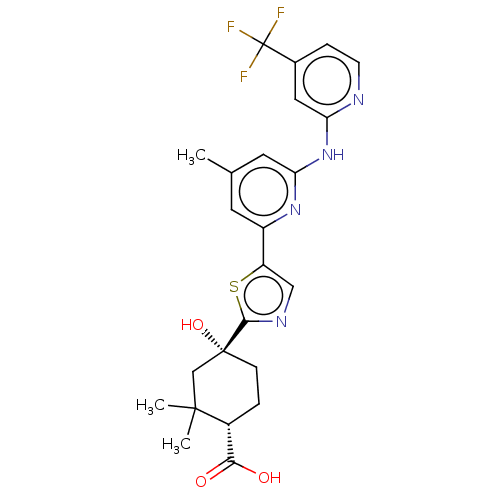
(US9598405, 3.18)Show SMILES Cc1cc(Nc2cc(ccn2)C(F)(F)F)nc(c1)-c1cnc(s1)[C@@]1(O)CC[C@H](C(O)=O)C(C)(C)C1 Show InChI InChI=1S/C24H25F3N4O3S/c1-13-8-16(30-19(9-13)31-18-10-14(5-7-28-18)24(25,26)27)17-11-29-21(35-17)23(34)6-4-15(20(32)33)22(2,3)12-23/h5,7-11,15,34H,4,6,12H2,1-3H3,(H,32,33)(H,28,30,31)/t15-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A recombinant GST-hSYK fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-SYK (Carna Bi... |
US Patent US9598405 (2017)
BindingDB Entry DOI: 10.7270/Q2BR8V7T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data