 Found 299 hits with Last Name = 'hori' and Initial = 'n'
Found 299 hits with Last Name = 'hori' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370676

(CHEMBL607907)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)O[C@H]2O[C@@H](COCCOCCOCCOCc3ccc4ccccc4c3)[C@H](O)[C@H](O)[C@@H]2O)OC([C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C32H44N2O20P2/c35-24-7-8-34(32(41)33-24)30-28(39)26(37)23(51-30)18-50-55(42,43)54-56(44,45)53-31-29(40)27(38)25(36)22(52-31)17-49-14-12-47-10-9-46-11-13-48-16-19-5-6-20-3-1-2-4-21(20)15-19/h1-8,15,22-23,25-31,36-40H,9-14,16-18H2,(H,42,43)(H,44,45)(H,33,35,41)/t22-,23+,25-,26+,27-,28+,29-,30?,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human galactosyltransferase using UDP-Gal |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370674
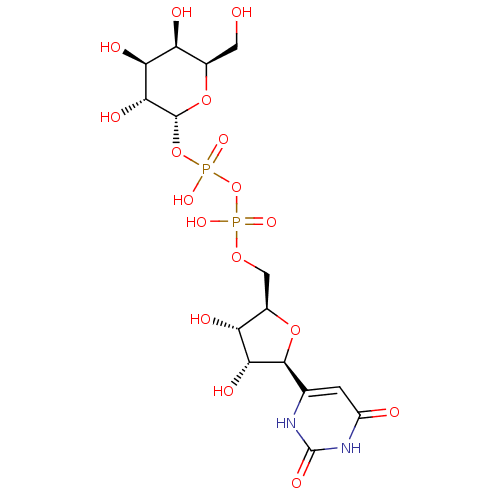
(UDP-GALACTOSE)Show SMILES OC[C@H]1O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)c2cc(=O)[nH]c(=O)[nH]2)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H24N2O17P2/c18-2-5-8(20)10(22)12(24)14(32-5)33-36(28,29)34-35(26,27)30-3-6-9(21)11(23)13(31-6)4-1-7(19)17-15(25)16-4/h1,5-6,8-14,18,20-24H,2-3H2,(H,26,27)(H,28,29)(H2,16,17,19,25)/t5-,6-,8+,9-,10+,11-,12-,13+,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370675
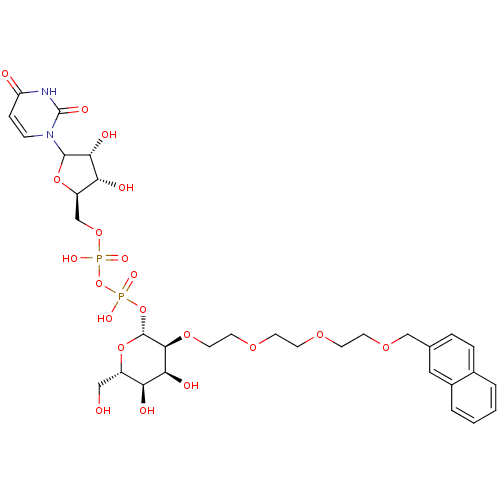
(CHEMBL607908)Show SMILES OC[C@@H]1O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@@H](OCCOCCOCCOCc2ccc3ccccc3c2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C32H44N2O20P2/c35-16-22-25(37)27(39)29(49-14-13-47-10-9-46-11-12-48-17-19-5-6-20-3-1-2-4-21(20)15-19)31(52-22)53-56(44,45)54-55(42,43)50-18-23-26(38)28(40)30(51-23)34-8-7-24(36)33-32(34)41/h1-8,15,22-23,25-31,35,37-40H,9-14,16-18H2,(H,42,43)(H,44,45)(H,33,36,41)/t22-,23+,25-,26+,27-,28+,29-,30?,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370678

(CHEMBL611116)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)O[C@H]2O[C@@H](CNNC(=O)COCCOCCOCc3ccc4ccccc4c3)[C@H](O)[C@H](O)[C@@H]2O)OC([C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C32H44N4O20P2/c37-23-7-8-36(32(44)34-23)30-28(42)26(40)22(53-30)16-52-57(45,46)56-58(47,48)55-31-29(43)27(41)25(39)21(54-31)14-33-35-24(38)17-51-12-10-49-9-11-50-15-18-5-6-19-3-1-2-4-20(19)13-18/h1-8,13,21-22,25-31,33,39-43H,9-12,14-17H2,(H,35,38)(H,45,46)(H,47,48)(H,34,37,44)/t21-,22+,25-,26+,27-,28+,29-,30?,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370677

(CHEMBL609634)Show SMILES Cc1ccc2ccc(COC(=O)COCCOCC(=O)NC[C@@H]3O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]4OC([C@H](O)[C@@H]4O)n4ccc(=O)[nH]c4=O)[C@@H](O)[C@@H](O)[C@H]3O)cc2c1 |r| Show InChI InChI=1S/C33H43N3O21P2/c1-17-2-4-19-5-3-18(11-20(19)10-17)13-52-25(39)16-51-9-8-50-15-24(38)34-12-21-26(40)28(42)30(44)32(55-21)56-59(48,49)57-58(46,47)53-14-22-27(41)29(43)31(54-22)36-7-6-23(37)35-33(36)45/h2-7,10-11,21-22,26-32,40-44H,8-9,12-16H2,1H3,(H,34,38)(H,46,47)(H,48,49)(H,35,37,45)/t21-,22+,26-,27+,28-,29+,30-,31?,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50173721
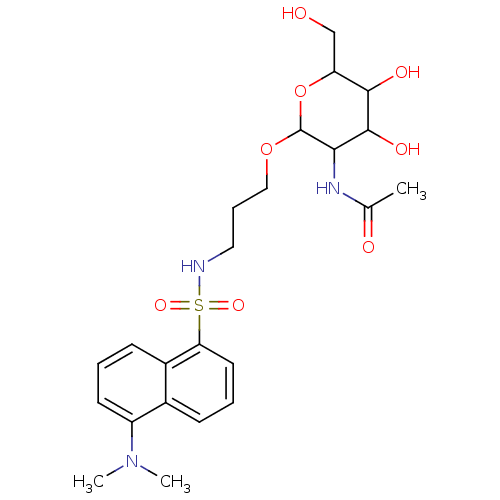
(CHEMBL196432 | Uridine-5'-diphosphogalactose deriv...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCOC1OC(CO)C(O)C(O)C1NC(C)=O Show InChI InChI=1S/C23H33N3O8S/c1-14(28)25-20-22(30)21(29)18(13-27)34-23(20)33-12-6-11-24-35(31,32)19-10-5-7-15-16(19)8-4-9-17(15)26(2)3/h4-5,7-10,18,20-24,27,29-30H,6,11-13H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Beta-1,4-galactosyltransferase I using UDP-Gal (0-160 uM) |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370679

(CHEMBL611112)Show SMILES COCCOCCOCCOC[C@@H]1O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H38N2O20P2/c1-36-4-5-37-6-7-38-8-9-39-10-12-15(26)17(28)19(30)21(42-12)43-46(34,35)44-45(32,33)40-11-13-16(27)18(29)20(41-13)24-3-2-14(25)23-22(24)31/h2-3,12-13,15-21,26-30H,4-11H2,1H3,(H,32,33)(H,34,35)(H,23,25,31)/t12-,13+,15-,16+,17-,18+,19-,20?,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264847
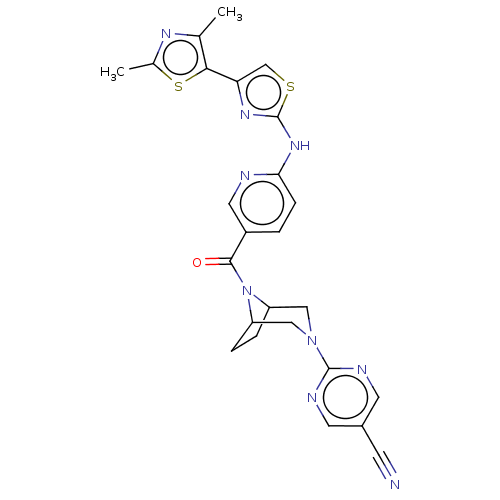
(CHEMBL4096902)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(cn2)C#N)n1 Show InChI InChI=1S/C25H23N9OS2/c1-14-22(37-15(2)30-14)20-13-36-25(31-20)32-21-6-3-17(10-27-21)23(35)34-18-4-5-19(34)12-33(11-18)24-28-8-16(7-26)9-29-24/h3,6,8-10,13,18-19H,4-5,11-12H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50256209
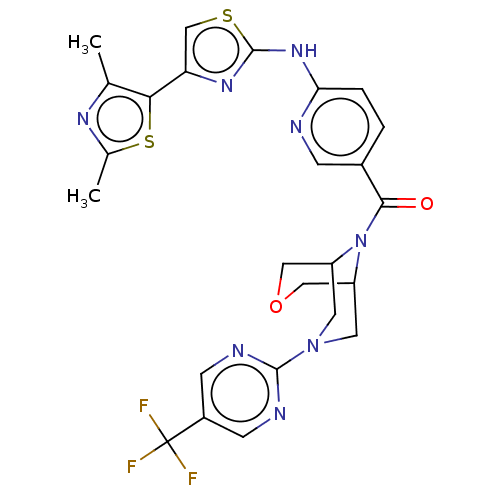
(CHEMBL4101768)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3COCC2CN(C3)c2ncc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H23F3N8O2S2/c1-13-21(40-14(2)32-13)19-12-39-24(33-19)34-20-4-3-15(5-29-20)22(37)36-17-8-35(9-18(36)11-38-10-17)23-30-6-16(7-31-23)25(26,27)28/h3-7,12,17-18H,8-11H2,1-2H3,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063912
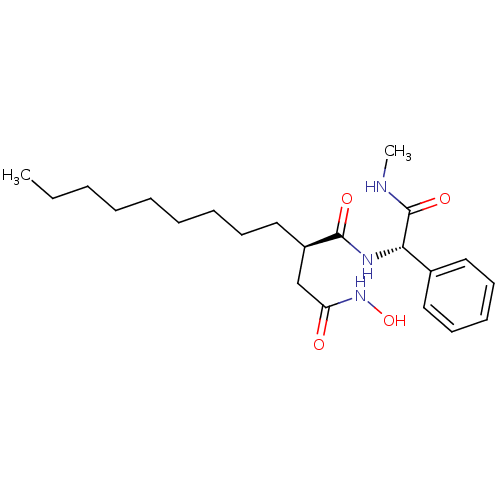
((R)-N*4*-Hydroxy-N*1*-((S)-methylcarbamoyl-phenyl-...)Show SMILES CCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)c1ccccc1 Show InChI InChI=1S/C22H35N3O4/c1-3-4-5-6-7-8-10-15-18(16-19(26)25-29)21(27)24-20(22(28)23-2)17-13-11-9-12-14-17/h9,11-14,18,20,29H,3-8,10,15-16H2,1-2H3,(H,23,28)(H,24,27)(H,25,26)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264846
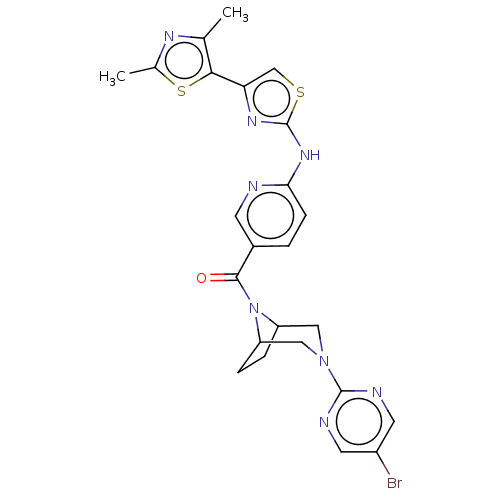
(CHEMBL4066531)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(Br)cn2)n1 Show InChI InChI=1S/C24H23BrN8OS2/c1-13-21(36-14(2)29-13)19-12-35-24(30-19)31-20-6-3-15(7-26-20)22(34)33-17-4-5-18(33)11-32(10-17)23-27-8-16(25)9-28-23/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192809
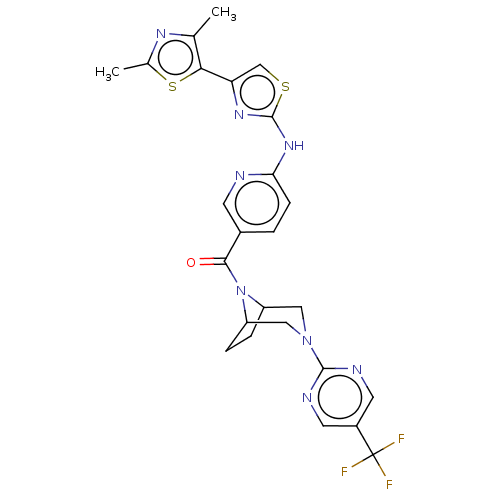
(CHEMBL3941914)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H23F3N8OS2/c1-13-21(39-14(2)32-13)19-12-38-24(33-19)34-20-6-3-15(7-29-20)22(37)36-17-4-5-18(36)11-35(10-17)23-30-8-16(9-31-23)25(26,27)28/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192809

(CHEMBL3941914)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H23F3N8OS2/c1-13-21(39-14(2)32-13)19-12-38-24(33-19)34-20-6-3-15(7-29-20)22(37)36-17-4-5-18(36)11-35(10-17)23-30-8-16(9-31-23)25(26,27)28/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50256228
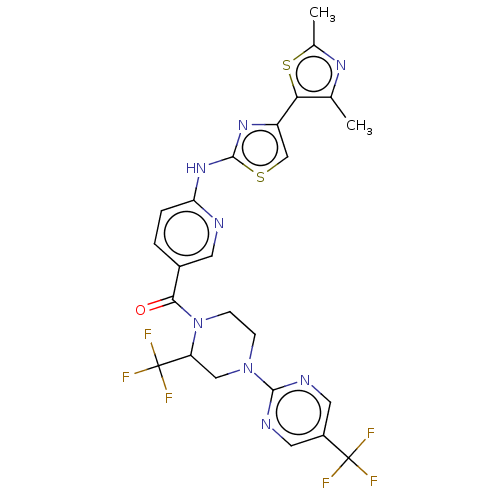
(CHEMBL4099293)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2CCN(CC2C(F)(F)F)c2ncc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C24H20F6N8OS2/c1-12-19(41-13(2)34-12)16-11-40-22(35-16)36-18-4-3-14(7-31-18)20(39)38-6-5-37(10-17(38)24(28,29)30)21-32-8-15(9-33-21)23(25,26)27/h3-4,7-9,11,17H,5-6,10H2,1-2H3,(H,31,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-2 (MMP-2). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192811
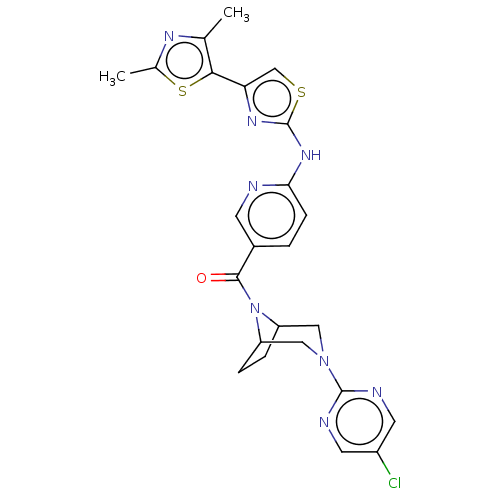
(CHEMBL3971502)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(Cl)cn2)n1 Show InChI InChI=1S/C24H23ClN8OS2/c1-13-21(36-14(2)29-13)19-12-35-24(30-19)31-20-6-3-15(7-26-20)22(34)33-17-4-5-18(33)11-32(10-17)23-27-8-16(25)9-28-23/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-1 (MMP-1). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192811

(CHEMBL3971502)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(Cl)cn2)n1 Show InChI InChI=1S/C24H23ClN8OS2/c1-13-21(36-14(2)29-13)19-12-35-24(30-19)31-20-6-3-15(7-26-20)22(34)33-17-4-5-18(33)11-32(10-17)23-27-8-16(25)9-28-23/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264858
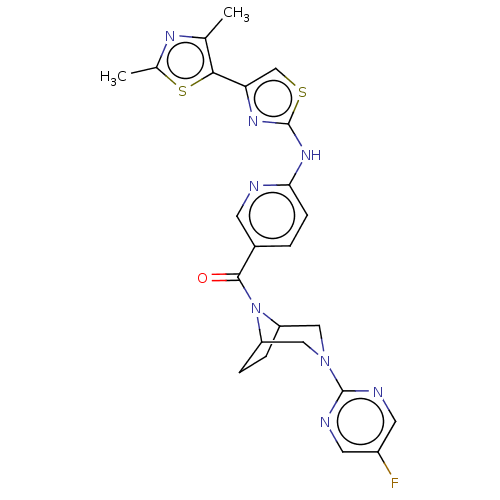
(CHEMBL4077638)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(F)cn2)n1 Show InChI InChI=1S/C24H23FN8OS2/c1-13-21(36-14(2)29-13)19-12-35-24(30-19)31-20-6-3-15(7-26-20)22(34)33-17-4-5-18(33)11-32(10-17)23-27-8-16(25)9-28-23/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192807
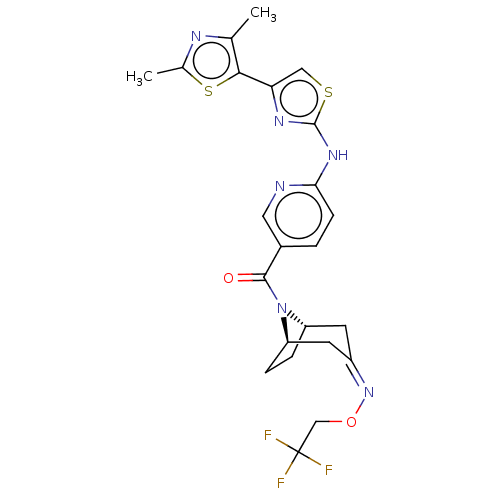
(CHEMBL3984947)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/OCC(F)(F)F)N2C(=O)c1ccc(Nc2nc(cs2)-c2sc(C)nc2C)nc1 |r| Show InChI InChI=1S/C23H23F3N6O2S2/c1-12-20(36-13(2)28-12)18-10-35-22(29-18)30-19-6-3-14(9-27-19)21(33)32-16-4-5-17(32)8-15(7-16)31-34-11-23(24,25)26/h3,6,9-10,16-17H,4-5,7-8,11H2,1-2H3,(H,27,29,30)/b31-15-/t16-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264859
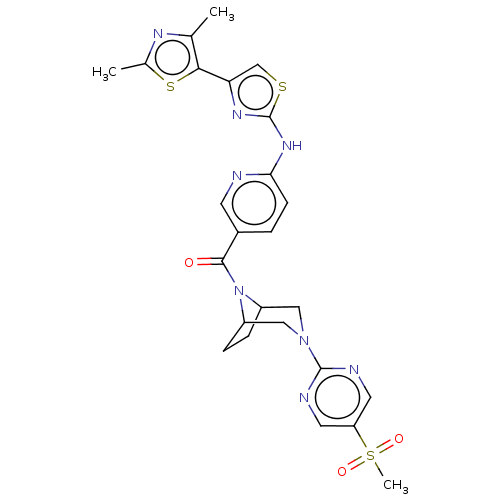
(CHEMBL4078117)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(cn2)S(C)(=O)=O)n1 Show InChI InChI=1S/C25H26N8O3S3/c1-14-22(38-15(2)29-14)20-13-37-25(30-20)31-21-7-4-16(8-26-21)23(34)33-17-5-6-18(33)12-32(11-17)24-27-9-19(10-28-24)39(3,35)36/h4,7-10,13,17-18H,5-6,11-12H2,1-3H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192809

(CHEMBL3941914)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H23F3N8OS2/c1-13-21(39-14(2)32-13)19-12-38-24(33-19)34-20-6-3-15(7-29-20)22(37)36-17-4-5-18(36)11-35(10-17)23-30-8-16(9-31-23)25(26,27)28/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of hypotonicity-induced activation pretreated for 5 mi... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192809

(CHEMBL3941914)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H23F3N8OS2/c1-13-21(39-14(2)32-13)19-12-38-24(33-19)34-20-6-3-15(7-29-20)22(37)36-17-4-5-18(36)11-35(10-17)23-30-8-16(9-31-23)25(26,27)28/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of hypotonicity-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-1 (MMP-1). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264845
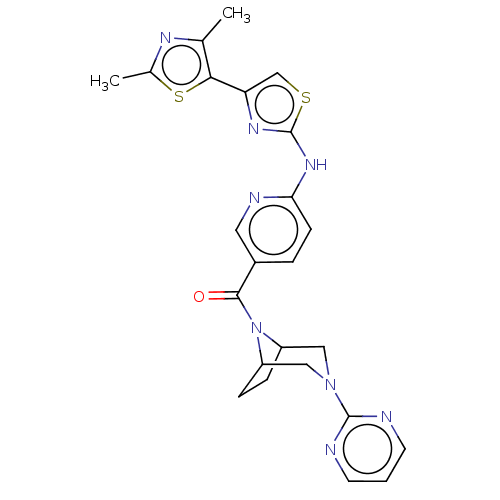
(CHEMBL4095552)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncccn2)n1 Show InChI InChI=1S/C24H24N8OS2/c1-14-21(35-15(2)28-14)19-13-34-24(29-19)30-20-7-4-16(10-27-20)22(33)32-17-5-6-18(32)12-31(11-17)23-25-8-3-9-26-23/h3-4,7-10,13,17-18H,5-6,11-12H2,1-2H3,(H,27,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192804
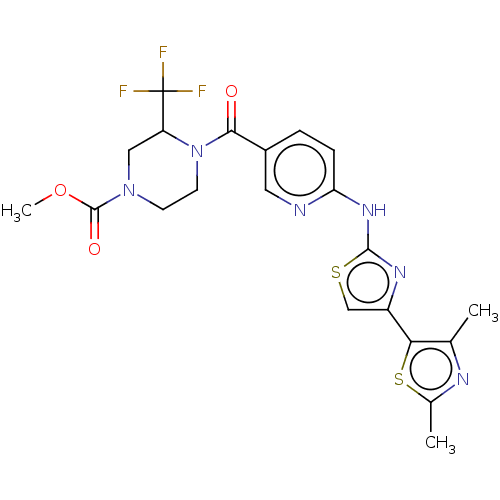
(CHEMBL3933401)Show SMILES COC(=O)N1CCN(C(C1)C(F)(F)F)C(=O)c1ccc(Nc2nc(cs2)-c2sc(C)nc2C)nc1 Show InChI InChI=1S/C21H21F3N6O3S2/c1-11-17(35-12(2)26-11)14-10-34-19(27-14)28-16-5-4-13(8-25-16)18(31)30-7-6-29(20(32)33-3)9-15(30)21(22,23)24/h4-5,8,10,15H,6-7,9H2,1-3H3,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-3 (MMP-3). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50256212
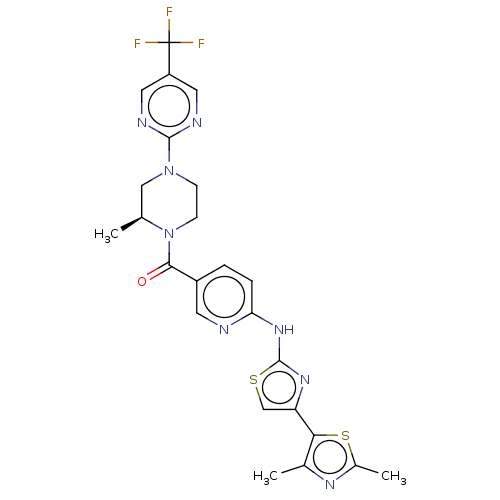
(CHEMBL4060956)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc(Nc2nc(cs2)-c2sc(C)nc2C)nc1)c1ncc(cn1)C(F)(F)F |r| Show InChI InChI=1S/C24H23F3N8OS2/c1-13-11-34(22-29-9-17(10-30-22)24(25,26)27)6-7-35(13)21(36)16-4-5-19(28-8-16)33-23-32-18(12-37-23)20-14(2)31-15(3)38-20/h4-5,8-10,12-13H,6-7,11H2,1-3H3,(H,28,32,33)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264848
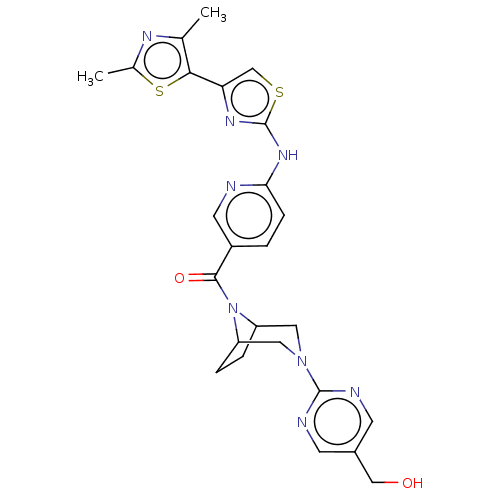
(CHEMBL4062935)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(CO)cn2)n1 Show InChI InChI=1S/C25H26N8O2S2/c1-14-22(37-15(2)29-14)20-13-36-25(30-20)31-21-6-3-17(9-26-21)23(35)33-18-4-5-19(33)11-32(10-18)24-27-7-16(12-34)8-28-24/h3,6-9,13,18-19,34H,4-5,10-12H2,1-2H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-3 (MMP-3). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264857
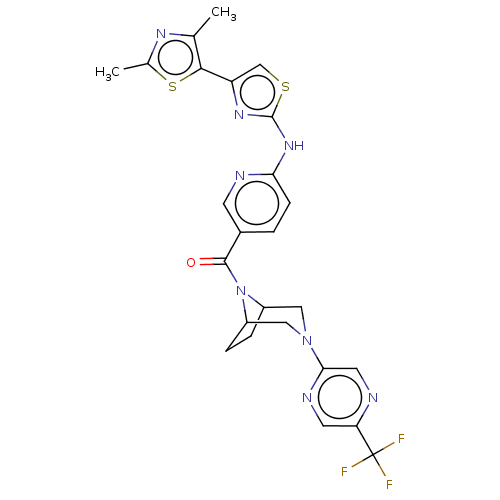
(CHEMBL4090714)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2cnc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H23F3N8OS2/c1-13-22(39-14(2)32-13)18-12-38-24(33-18)34-20-6-3-15(7-30-20)23(37)36-16-4-5-17(36)11-35(10-16)21-9-29-19(8-31-21)25(26,27)28/h3,6-9,12,16-17H,4-5,10-11H2,1-2H3,(H,30,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of hypotonicity-induced activation pretreated for 5 mi... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264856
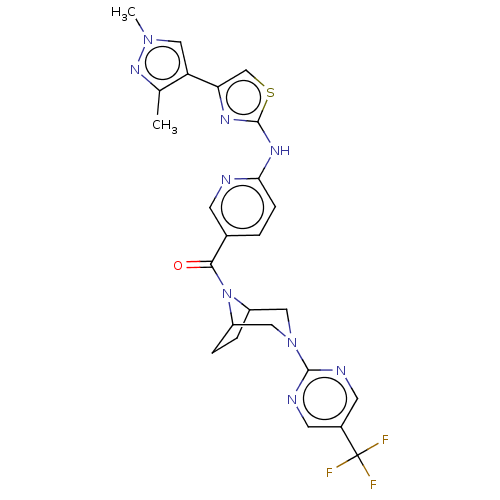
(CHEMBL4084992)Show SMILES Cc1nn(C)cc1-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ncc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H24F3N9OS/c1-14-19(12-35(2)34-14)20-13-39-24(32-20)33-21-6-3-15(7-29-21)22(38)37-17-4-5-18(37)11-36(10-17)23-30-8-16(9-31-23)25(26,27)28/h3,6-9,12-13,17-18H,4-5,10-11H2,1-2H3,(H,29,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192820
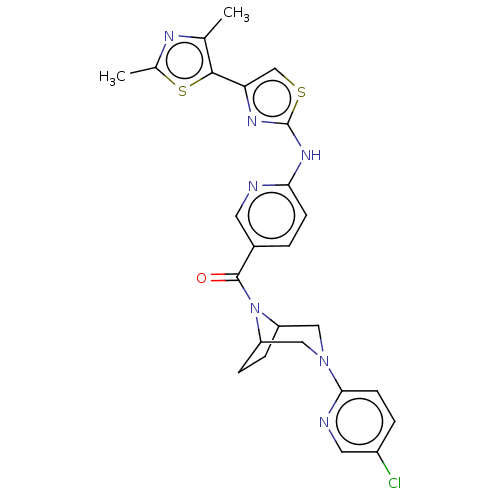
(CHEMBL3950646)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ccc(Cl)cn2)n1 Show InChI InChI=1S/C25H24ClN7OS2/c1-14-23(36-15(2)29-14)20-13-35-25(30-20)31-21-7-3-16(9-27-21)24(34)33-18-5-6-19(33)12-32(11-18)22-8-4-17(26)10-28-22/h3-4,7-10,13,18-19H,5-6,11-12H2,1-2H3,(H,27,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063921
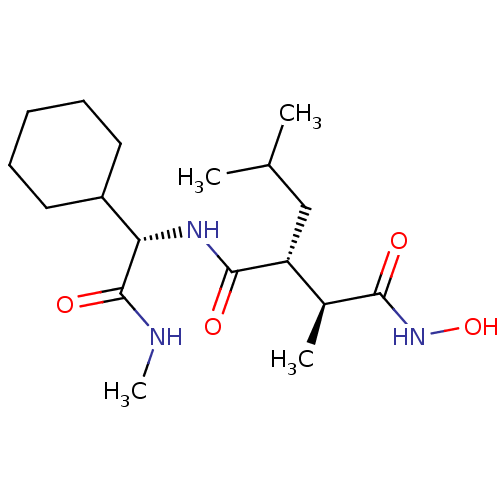
((2R,3S)-N*1*-((S)-Cyclohexyl-methylcarbamoyl-methy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO)C1CCCCC1 Show InChI InChI=1S/C18H33N3O4/c1-11(2)10-14(12(3)16(22)21-25)17(23)20-15(18(24)19-4)13-8-6-5-7-9-13/h11-15,25H,5-10H2,1-4H3,(H,19,24)(H,20,23)(H,21,22)/t12-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50256230
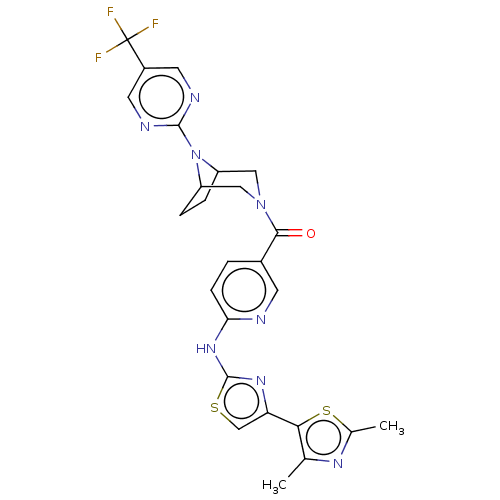
(CHEMBL4083772)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2CC3CCC(C2)N3c2ncc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H23F3N8OS2/c1-13-21(39-14(2)32-13)19-12-38-24(33-19)34-20-6-3-15(7-29-20)22(37)35-10-17-4-5-18(11-35)36(17)23-30-8-16(9-31-23)25(26,27)28/h3,6-9,12,17-18H,4-5,10-11H2,1-2H3,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264855
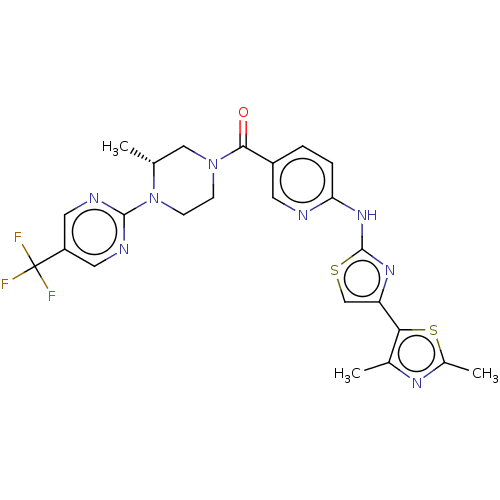
(CHEMBL4091558)Show SMILES C[C@@H]1CN(CCN1c1ncc(cn1)C(F)(F)F)C(=O)c1ccc(Nc2nc(cs2)-c2sc(C)nc2C)nc1 |r| Show InChI InChI=1S/C24H23F3N8OS2/c1-13-11-34(6-7-35(13)22-29-9-17(10-30-22)24(25,26)27)21(36)16-4-5-19(28-8-16)33-23-32-18(12-37-23)20-14(2)31-15(3)38-20/h4-5,8-10,12-13H,6-7,11H2,1-3H3,(H,28,32,33)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192808
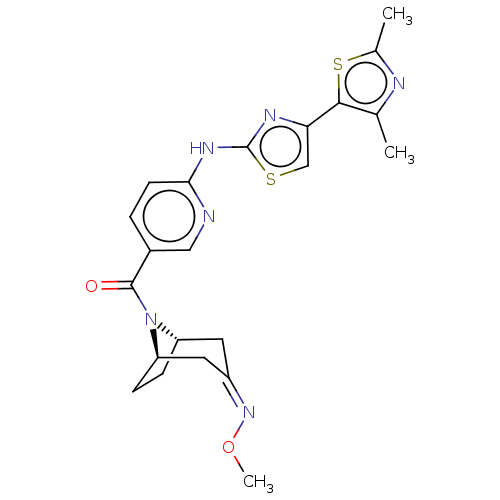
(CHEMBL3915150)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/OC)N2C(=O)c1ccc(Nc2nc(cs2)-c2sc(C)nc2C)nc1 |r| Show InChI InChI=1S/C22H24N6O2S2/c1-12-20(32-13(2)24-12)18-11-31-22(25-18)26-19-7-4-14(10-23-19)21(29)28-16-5-6-17(28)9-15(8-16)27-30-3/h4,7,10-11,16-17H,5-6,8-9H2,1-3H3,(H,23,25,26)/b27-15-/t16-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-2 (MMP-2). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-2 (MMP-2). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192779
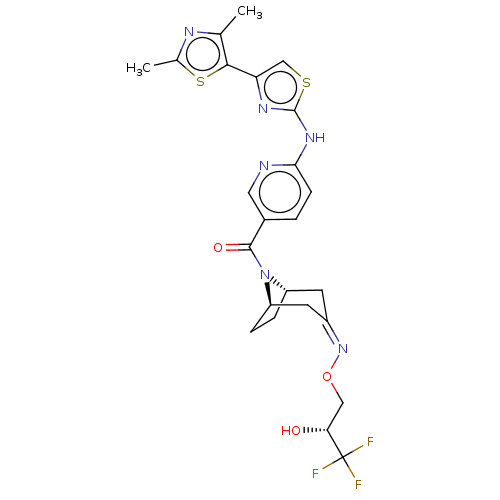
(CHEMBL3957347)Show SMILES [H][C@]12CC[C@]([H])(C\C(C1)=N/OC[C@@H](O)C(F)(F)F)N2C(=O)c1ccc(Nc2nc(cs2)-c2sc(C)nc2C)nc1 |r| Show InChI InChI=1S/C24H25F3N6O3S2/c1-12-21(38-13(2)29-12)18-11-37-23(30-18)31-20-6-3-14(9-28-20)22(35)33-16-4-5-17(33)8-15(7-16)32-36-10-19(34)24(25,26)27/h3,6,9,11,16-17,19,34H,4-5,7-8,10H2,1-2H3,(H,28,30,31)/b32-15-/t16-,17+,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50256210

(CHEMBL4080527)Show SMILES Cc1cc(-c2csc(Nc3ccc(cn3)C(=O)N3C4CCC3CN(C4)c3ncc(cn3)C(F)(F)F)n2)n(C)n1 Show InChI InChI=1S/C25H24F3N9OS/c1-14-7-20(35(2)34-14)19-13-39-24(32-19)33-21-6-3-15(8-29-21)22(38)37-17-4-5-18(37)12-36(11-17)23-30-9-16(10-31-23)25(26,27)28/h3,6-10,13,17-18H,4-5,11-12H2,1-2H3,(H,29,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of 4alphaPDD-induced activation pretreated for 5 mins ... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50264844
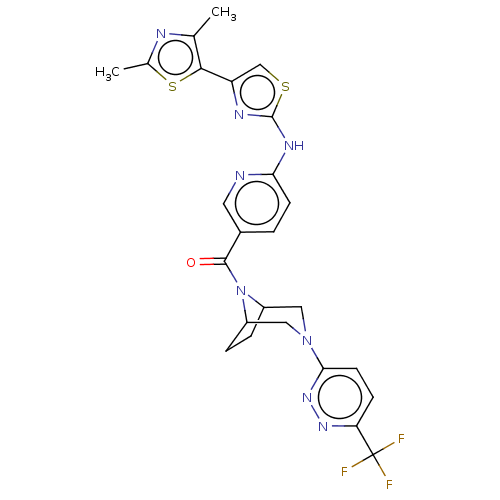
(CHEMBL4069724)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ccc(nn2)C(F)(F)F)n1 Show InChI InChI=1S/C25H23F3N8OS2/c1-13-22(39-14(2)30-13)18-12-38-24(31-18)32-20-7-3-15(9-29-20)23(37)36-16-4-5-17(36)11-35(10-16)21-8-6-19(33-34-21)25(26,27)28/h3,6-9,12,16-17H,4-5,10-11H2,1-2H3,(H,29,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of hypotonicity-induced activation pretreated for 5 mi... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063910
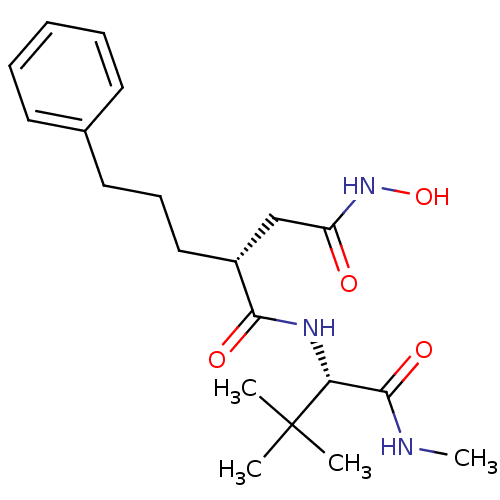
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CCCc1ccccc1)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C20H31N3O4/c1-20(2,3)17(19(26)21-4)22-18(25)15(13-16(24)23-27)12-8-11-14-9-6-5-7-10-14/h5-7,9-10,15,17,27H,8,11-13H2,1-4H3,(H,21,26)(H,22,25)(H,23,24)/t15-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-9 (MMP-9). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50263440
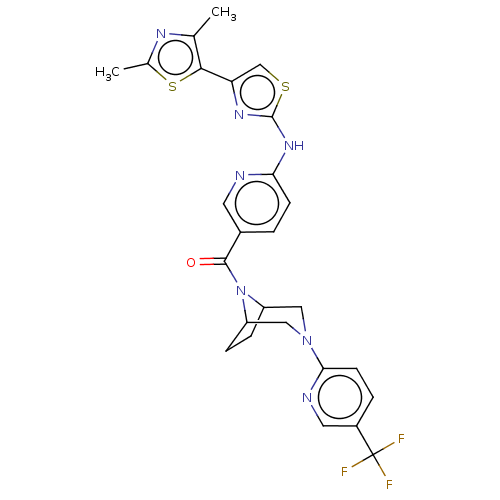
(CHEMBL4083195)Show SMILES Cc1nc(C)c(s1)-c1csc(Nc2ccc(cn2)C(=O)N2C3CCC2CN(C3)c2ccc(cn2)C(F)(F)F)n1 Show InChI InChI=1S/C26H24F3N7OS2/c1-14-23(39-15(2)32-14)20-13-38-25(33-20)34-21-7-3-16(9-30-21)24(37)36-18-5-6-19(36)12-35(11-18)22-8-4-17(10-31-22)26(27,28)29/h3-4,7-10,13,18-19H,5-6,11-12H2,1-2H3,(H,30,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV4 expressed in CHOK1 cells assessed as inhibition of hypotonicity-induced activation pretreated for 5 mi... |
Bioorg Med Chem 25: 2177-2190 (2017)
Article DOI: 10.1016/j.bmc.2017.02.047
BindingDB Entry DOI: 10.7270/Q2N58PT9 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-1 (MMP-1). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063912

((R)-N*4*-Hydroxy-N*1*-((S)-methylcarbamoyl-phenyl-...)Show SMILES CCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)c1ccccc1 Show InChI InChI=1S/C22H35N3O4/c1-3-4-5-6-7-8-10-15-18(16-19(26)25-29)21(27)24-20(22(28)23-2)17-13-11-9-12-14-17/h9,11-14,18,20,29H,3-8,10,15-16H2,1-2H3,(H,23,28)(H,24,27)(H,25,26)/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-2 (MMP-2). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 4
(Homo sapiens (Human)) | BDBM50192817
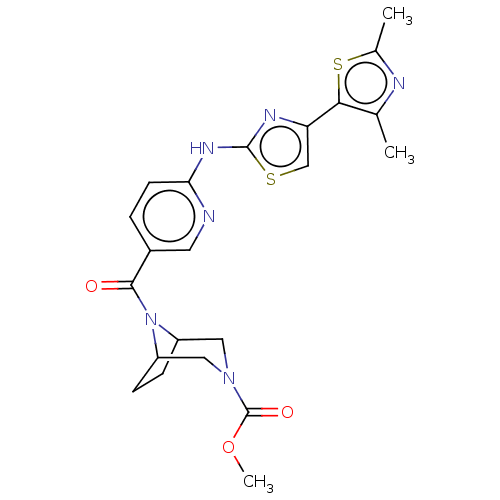
(CHEMBL3924485)Show SMILES COC(=O)N1CC2CCC(C1)N2C(=O)c1ccc(Nc2nc(cs2)-c2sc(C)nc2C)nc1 Show InChI InChI=1S/C22H24N6O3S2/c1-12-19(33-13(2)24-12)17-11-32-21(25-17)26-18-7-4-14(8-23-18)20(29)28-15-5-6-16(28)10-27(9-15)22(30)31-3/h4,7-8,11,15-16H,5-6,9-10H2,1-3H3,(H,23,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV4 assessed as inhibition of 4alpha-PDD-induced activation |
Bioorg Med Chem Lett 26: 4936-4941 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.014
BindingDB Entry DOI: 10.7270/Q2154K06 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data