 Found 1606 hits with Last Name = 'kamboj' and Initial = 'r'
Found 1606 hits with Last Name = 'kamboj' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM50060627
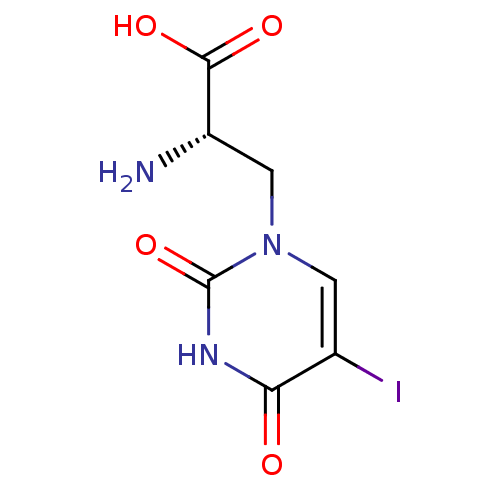
((S)-2-Amino-3-(5-iodo-2,4-dioxo-3,4-dihydro-2H-pyr...)Show InChI InChI=1S/C7H8IN3O4/c8-3-1-11(2-4(9)6(13)14)7(15)10-5(3)12/h1,4H,2,9H2,(H,13,14)(H,10,12,15)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Displacement of [3H]kainate from human Ionotropic glutamate receptor ionotropic kainate 1 expressed in HEK293 cells |
J Med Chem 40: 3645-50 (1997)
Article DOI: 10.1021/jm9702387
BindingDB Entry DOI: 10.7270/Q28G8MCB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50090516
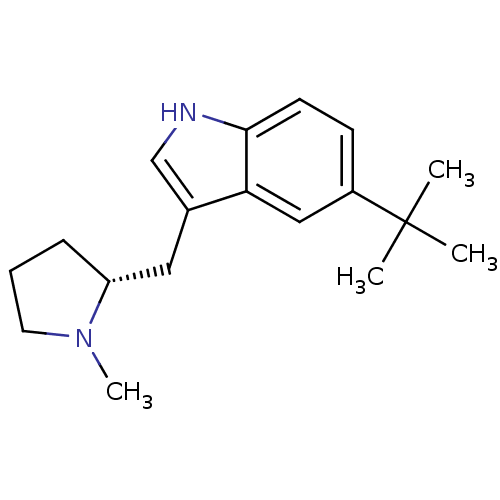
(5-tert-Butyl-3-((R)-1-methyl-pyrrolidin-2-ylmethyl...)Show InChI InChI=1S/C18H26N2/c1-18(2,3)14-7-8-17-16(11-14)13(12-19-17)10-15-6-5-9-20(15)4/h7-8,11-12,15,19H,5-6,9-10H2,1-4H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 1707-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0SRT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50090517
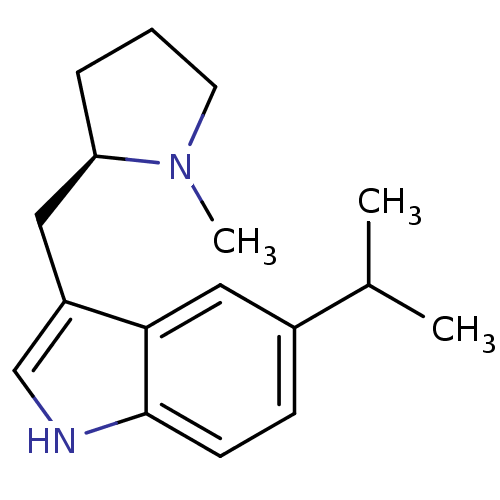
(5-Isopropyl-3-((R)-1-methyl-pyrrolidin-2-ylmethyl)...)Show InChI InChI=1S/C17H24N2/c1-12(2)13-6-7-17-16(10-13)14(11-18-17)9-15-5-4-8-19(15)3/h6-7,10-12,15,18H,4-5,8-9H2,1-3H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 1707-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0SRT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM30712
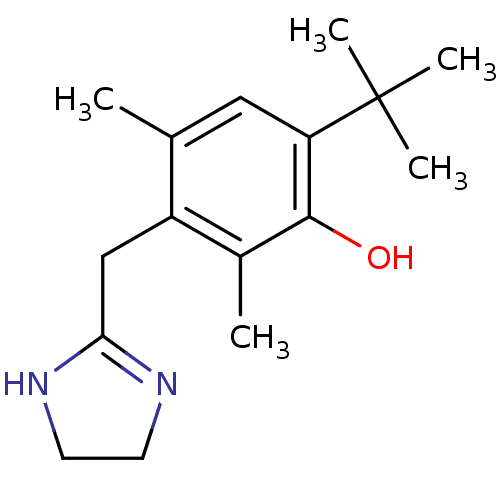
(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-hydroxytryptamine 1B receptor using [3H]-5-HT trifluoroacetate as radioligand |
J Med Chem 41: 2243-51 (1998)
Article DOI: 10.1021/jm970513p
BindingDB Entry DOI: 10.7270/Q28K7872 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50090519

(CHEMBL300519 | [2-(5-Isopropyl-1H-indol-3-yl)-ethy...)Show InChI InChI=1S/C15H22N2/c1-11(2)12-5-6-15-14(9-12)13(10-16-15)7-8-17(3)4/h5-6,9-11,16H,7-8H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 1707-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0SRT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM30712

(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-hydroxytryptamine 1D receptor using [3H]-5-HT trifluoroacetate as radioligand |
J Med Chem 41: 2243-51 (1998)
Article DOI: 10.1021/jm970513p
BindingDB Entry DOI: 10.7270/Q28K7872 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50090523

(2-(5-tert-Butyl-1-methyl-1H-indol-3-yl)-ethylamine...)Show InChI InChI=1S/C15H22N2/c1-15(2,3)12-5-6-14-13(9-12)11(7-8-16)10-17(14)4/h5-6,9-10H,7-8,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 1707-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0SRT |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84440
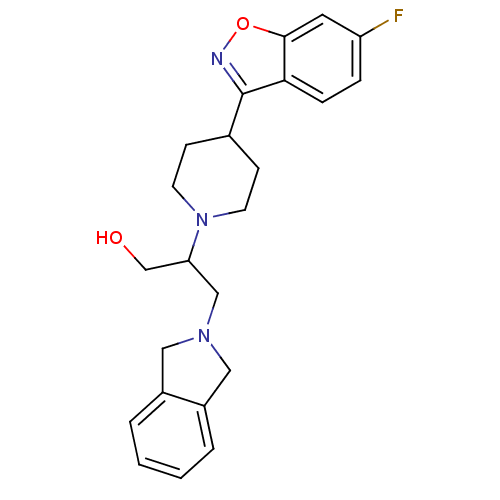
(Isoindoline, 7 | Isoindoline, 8 | Isoindoline, 9)Show SMILES OCC(CN1Cc2ccccc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H26FN3O2/c24-19-5-6-21-22(11-19)29-25-23(21)16-7-9-27(10-8-16)20(15-28)14-26-12-17-3-1-2-4-18(17)13-26/h1-6,11,16,20,28H,7-10,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84445
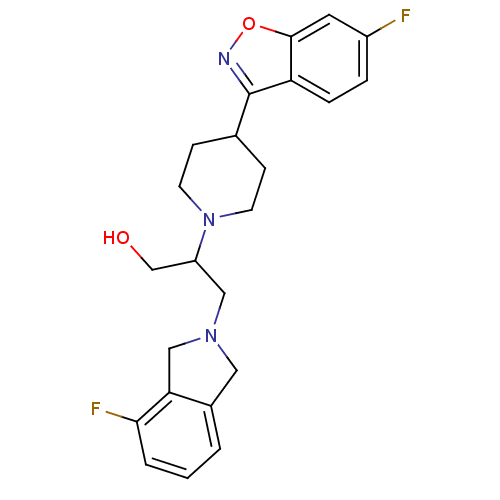
(Isoindoline, 12)Show SMILES OCC(CN1Cc2cccc(F)c2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H25F2N3O2/c24-17-4-5-19-22(10-17)30-26-23(19)15-6-8-28(9-7-15)18(14-29)12-27-11-16-2-1-3-21(25)20(16)13-27/h1-5,10,15,18,29H,6-9,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50088870

(CHEMBL171129 | Dimethyl-[2-(5-thiophen-3-yl-1H-ind...)Show InChI InChI=1S/C16H18N2S/c1-18(2)7-5-13-10-17-16-4-3-12(9-15(13)16)14-6-8-19-11-14/h3-4,6,8-11,17H,5,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 903-5 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PZJ |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84451
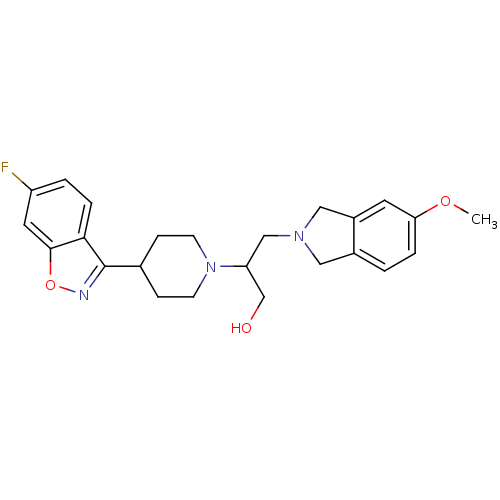
(Isoindoline, 18 | Isoindoline, 19)Show SMILES COc1ccc2CN(CC(CO)N3CCC(CC3)c3noc4cc(F)ccc34)Cc2c1 Show InChI InChI=1S/C24H28FN3O3/c1-30-21-4-2-17-12-27(13-18(17)10-21)14-20(15-29)28-8-6-16(7-9-28)24-22-5-3-19(25)11-23(22)31-26-24/h2-5,10-11,16,20,29H,6-9,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM30703
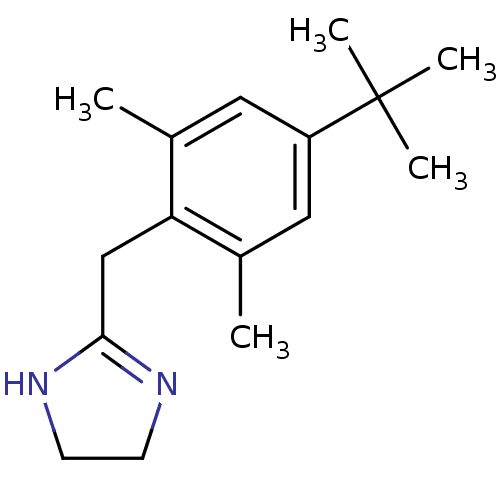
(2-(4-tert-butyl-2,6-dimethyl-benzyl)-2-imidazoline...)Show InChI InChI=1S/C16H24N2/c1-11-8-13(16(3,4)5)9-12(2)14(11)10-15-17-6-7-18-15/h8-9H,6-7,10H2,1-5H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-hydroxytryptamine 1D receptor using [3H]-5-HT trifluoroacetate as radioligand |
J Med Chem 41: 2243-51 (1998)
Article DOI: 10.1021/jm970513p
BindingDB Entry DOI: 10.7270/Q28K7872 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50088867
(CHEMBL170192 | Dimethyl-[2-(5-thiophen-2-yl-1H-ind...)Show InChI InChI=1S/C16H18N2S/c1-18(2)8-7-13-11-17-15-6-5-12(10-14(13)15)16-4-3-9-19-16/h3-6,9-11,17H,7-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 903-5 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PZJ |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115819
BindingDB Entry DOI: 10.7270/Q21N84R1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50090522
(5-Ethyl-3-((R)-1-methyl-pyrrolidin-2-ylmethyl)-1H-...)Show InChI InChI=1S/C16H22N2/c1-3-12-6-7-16-15(9-12)13(11-17-16)10-14-5-4-8-18(14)2/h6-7,9,11,14,17H,3-5,8,10H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 1707-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0SRT |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84440

(Isoindoline, 7 | Isoindoline, 8 | Isoindoline, 9)Show SMILES OCC(CN1Cc2ccccc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H26FN3O2/c24-19-5-6-21-22(11-19)29-25-23(21)16-7-9-27(10-8-16)20(15-28)14-26-12-17-3-1-2-4-18(17)13-26/h1-6,11,16,20,28H,7-10,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50049091
(2-(5-Heptyloxy-1H-indol-3-yl)-ethylamine | CHEMBL3...)Show InChI InChI=1S/C17H26N2O/c1-2-3-4-5-6-11-20-15-7-8-17-16(12-15)14(9-10-18)13-19-17/h7-8,12-13,19H,2-6,9-11,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50049093
(CHEMBL321190 | Nonanoic acid 3-(2-dimethylamino-et...)Show InChI InChI=1S/C21H32N2O2/c1-4-5-6-7-8-9-10-21(24)25-18-11-12-20-19(15-18)17(16-22-20)13-14-23(2)3/h11-12,15-16,22H,4-10,13-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50064806
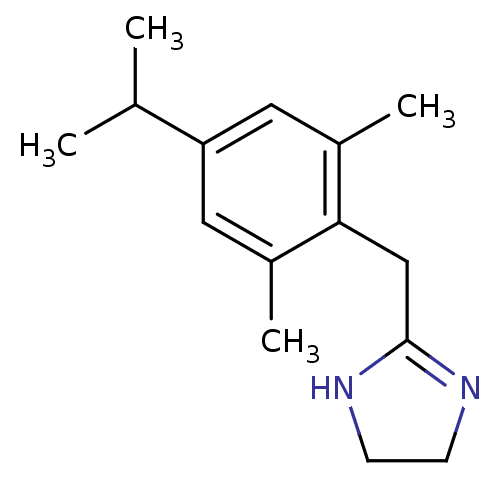
(2-(4-Isopropyl-2,6-dimethyl-benzyl)-4,5-dihydro-1H...)Show InChI InChI=1S/C15H22N2/c1-10(2)13-7-11(3)14(12(4)8-13)9-15-16-5-6-17-15/h7-8,10H,5-6,9H2,1-4H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-hydroxytryptamine 1D receptor using [3H]-5-HT trifluoroacetate as radioligand |
J Med Chem 41: 2243-51 (1998)
Article DOI: 10.1021/jm970513p
BindingDB Entry DOI: 10.7270/Q28K7872 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84443
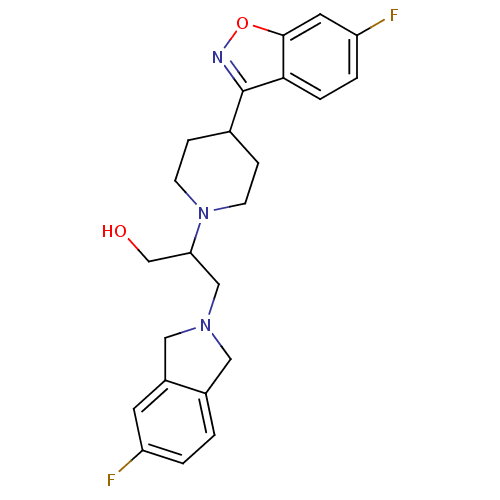
(Isoindoline, 10 | Isoindoline, 11)Show SMILES OCC(CN1Cc2ccc(F)cc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H25F2N3O2/c24-18-2-1-16-11-27(12-17(16)9-18)13-20(14-29)28-7-5-15(6-8-28)23-21-4-3-19(25)10-22(21)30-26-23/h1-4,9-10,15,20,29H,5-8,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84446
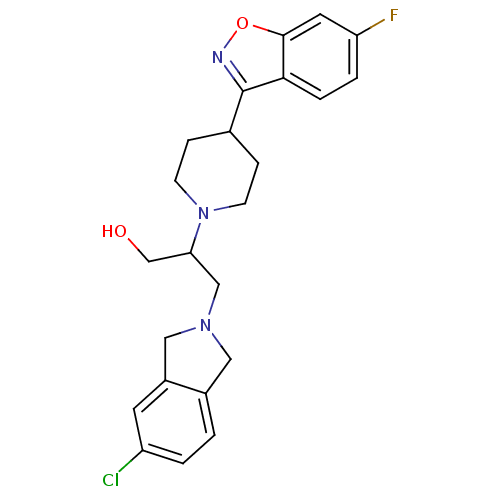
(Isoindoline, 13)Show SMILES OCC(CN1Cc2ccc(Cl)cc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H25ClFN3O2/c24-18-2-1-16-11-27(12-17(16)9-18)13-20(14-29)28-7-5-15(6-8-28)23-21-4-3-19(25)10-22(21)30-26-23/h1-4,9-10,15,20,29H,5-8,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50049085
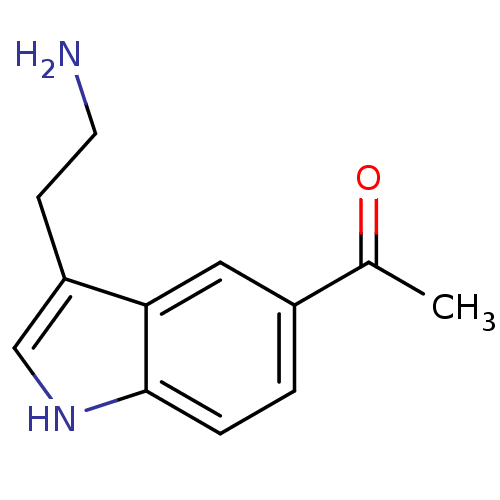
(1-[3-(2-Amino-ethyl)-1H-indol-5-yl]-ethanone | Ace...)Show InChI InChI=1S/C12H14N2O/c1-8(15)9-2-3-12-11(6-9)10(4-5-13)7-14-12/h2-3,6-7,14H,4-5,13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
In vitro affinity at human cloned 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84453
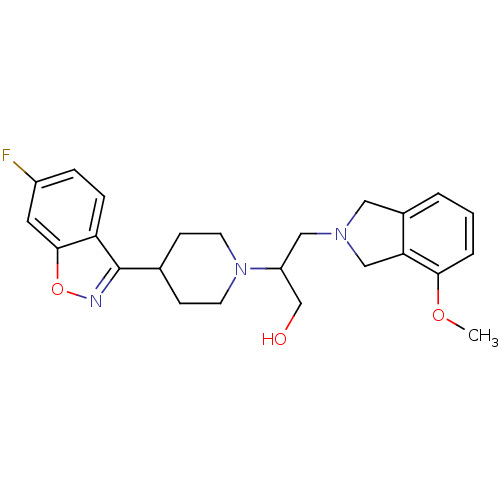
(Isoindoline, 20)Show SMILES COc1cccc2CN(CC(CO)N3CCC(CC3)c3noc4cc(F)ccc34)Cc12 Show InChI InChI=1S/C24H28FN3O3/c1-30-22-4-2-3-17-12-27(14-21(17)22)13-19(15-29)28-9-7-16(8-10-28)24-20-6-5-18(25)11-23(20)31-26-24/h2-6,11,16,19,29H,7-10,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115819
BindingDB Entry DOI: 10.7270/Q21N84R1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50049077
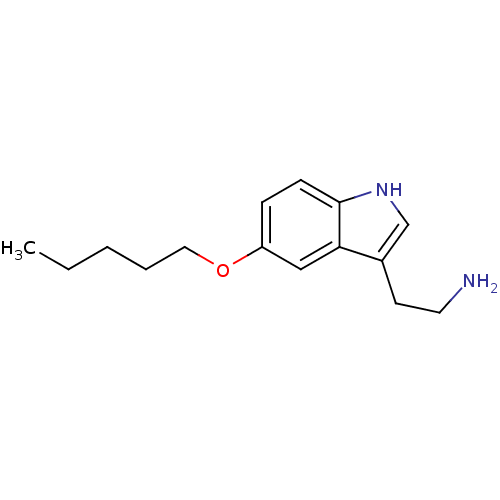
(2-(5-Pentyloxy-1H-indol-3-yl)-ethylamine | CHEMBL1...)Show InChI InChI=1S/C15H22N2O/c1-2-3-4-9-18-13-5-6-15-14(10-13)12(7-8-16)11-17-15/h5-6,10-11,17H,2-4,7-9,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50049077

(2-(5-Pentyloxy-1H-indol-3-yl)-ethylamine | CHEMBL1...)Show InChI InChI=1S/C15H22N2O/c1-2-3-4-9-18-13-5-6-15-14(10-13)12(7-8-16)11-17-15/h5-6,10-11,17H,2-4,7-9,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053067
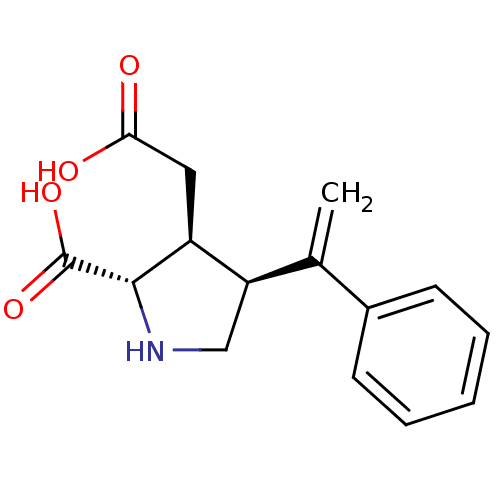
((2S,3S,4S)-3-Carboxymethyl-4-(1-phenyl-vinyl)-pyrr...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccccc1 Show InChI InChI=1S/C15H17NO4/c1-9(10-5-3-2-4-6-10)12-8-16-14(15(19)20)11(12)7-13(17)18/h2-6,11-12,14,16H,1,7-8H2,(H,17,18)(H,19,20)/t11-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50049082
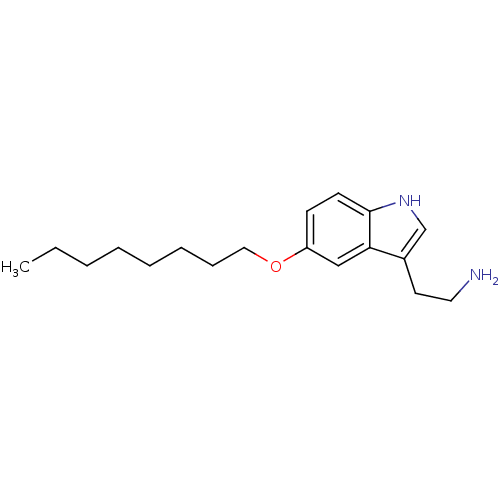
(2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...)Show InChI InChI=1S/C18H28N2O/c1-2-3-4-5-6-7-12-21-16-8-9-18-17(13-16)15(10-11-19)14-20-18/h8-9,13-14,20H,2-7,10-12,19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50088863

(2-(5-Thiophen-2-yl-1H-indol-3-yl)-ethylamine | CHE...)Show InChI InChI=1S/C14H14N2S/c15-6-5-11-9-16-13-4-3-10(8-12(11)13)14-2-1-7-17-14/h1-4,7-9,16H,5-6,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 903-5 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PZJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50088864
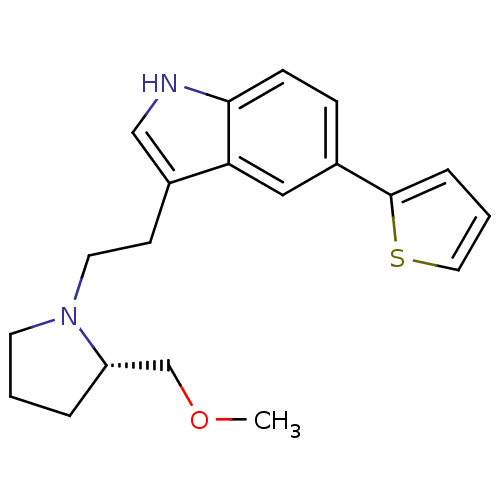
(3-[2-((S)-2-Methoxymethyl-pyrrolidin-1-yl)-ethyl]-...)Show SMILES COC[C@@H]1CCCN1CCc1c[nH]c2ccc(cc12)-c1cccs1 Show InChI InChI=1S/C20H24N2OS/c1-23-14-17-4-2-9-22(17)10-8-16-13-21-19-7-6-15(12-18(16)19)20-5-3-11-24-20/h3,5-7,11-13,17,21H,2,4,8-10,14H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 903-5 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PZJ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053071
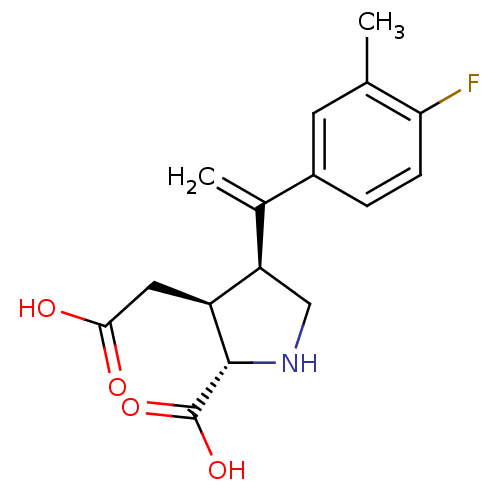
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-3-methyl...)Show SMILES Cc1cc(ccc1F)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H18FNO4/c1-8-5-10(3-4-13(8)17)9(2)12-7-18-15(16(21)22)11(12)6-14(19)20/h3-5,11-12,15,18H,2,6-7H2,1H3,(H,19,20)(H,21,22)/t11-,12+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50049081
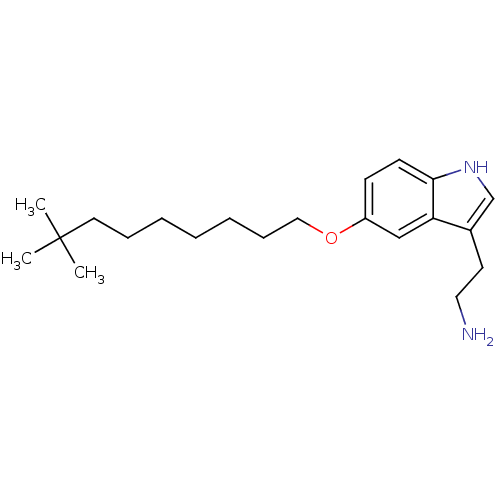
(2-[5-(8,8-Dimethyl-nonyloxy)-1H-indol-3-yl]-ethyla...)Show InChI InChI=1S/C21H34N2O/c1-21(2,3)12-7-5-4-6-8-14-24-18-9-10-20-19(15-18)17(11-13-22)16-23-20/h9-10,15-16,23H,4-8,11-14,22H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053075
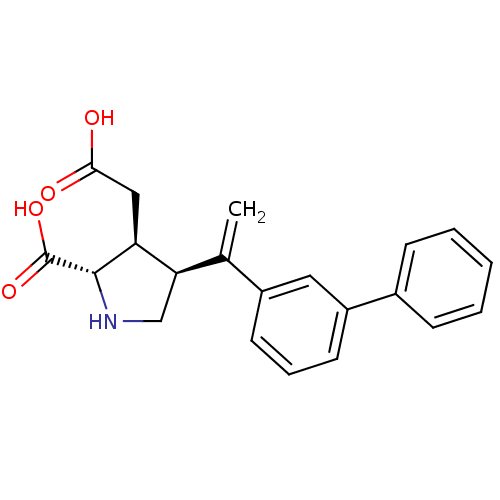
((2S,3S,4S)-4-(1-Biphenyl-3-yl-vinyl)-3-carboxymeth...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C21H21NO4/c1-13(18-12-22-20(21(25)26)17(18)11-19(23)24)15-8-5-9-16(10-15)14-6-3-2-4-7-14/h2-10,17-18,20,22H,1,11-12H2,(H,23,24)(H,25,26)/t17-,18+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053083
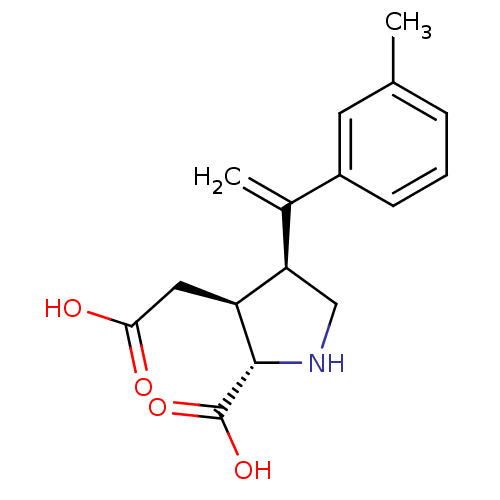
((2S,3S,4S)-3-Carboxymethyl-4-(1-m-tolyl-vinyl)-pyr...)Show SMILES Cc1cccc(c1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-4-3-5-11(6-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 4
(Homo sapiens (Human)) | BDBM50002369
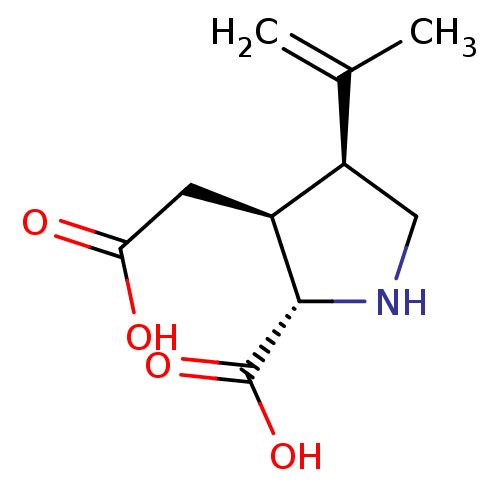
((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...)Show SMILES CC(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allelix Biopharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
J Neurochem 62: 1-9 (1994)
Article DOI: 10.1046/j.1471-4159.1994.62010001.x
BindingDB Entry DOI: 10.7270/Q2RJ4H0P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50049084
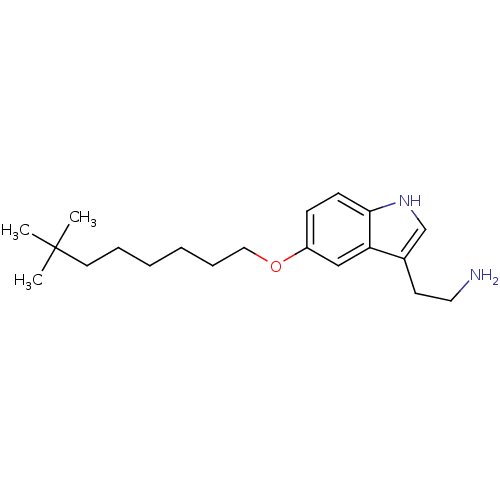
(2-[5-(7,7-Dimethyl-octyloxy)-1H-indol-3-yl]-ethyla...)Show InChI InChI=1S/C20H32N2O/c1-20(2,3)11-6-4-5-7-13-23-17-8-9-19-18(14-17)16(10-12-21)15-22-19/h8-9,14-15,22H,4-7,10-13,21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053080
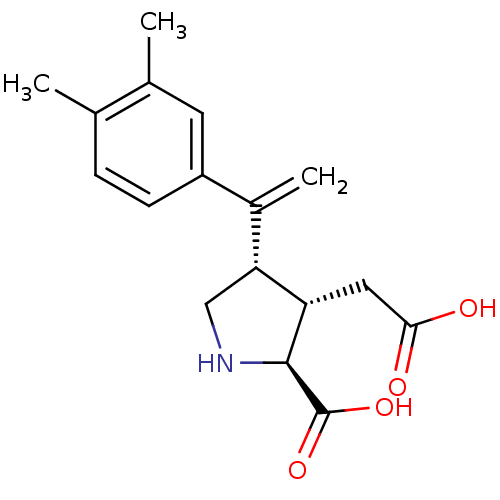
((2S,3S,4S)-3-Carboxymethyl-4-[1-(3,4-dimethyl-phen...)Show SMILES Cc1ccc(cc1C)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C17H21NO4/c1-9-4-5-12(6-10(9)2)11(3)14-8-18-16(17(21)22)13(14)7-15(19)20/h4-6,13-14,16,18H,3,7-8H2,1-2H3,(H,19,20)(H,21,22)/t13-,14+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50090521

(5-tert-Butyl-3-(2-pyrrolidin-1-yl-ethyl)-1H-indole...)Show InChI InChI=1S/C18H26N2/c1-18(2,3)15-6-7-17-16(12-15)14(13-19-17)8-11-20-9-4-5-10-20/h6-7,12-13,19H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 1707-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0SRT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50088869
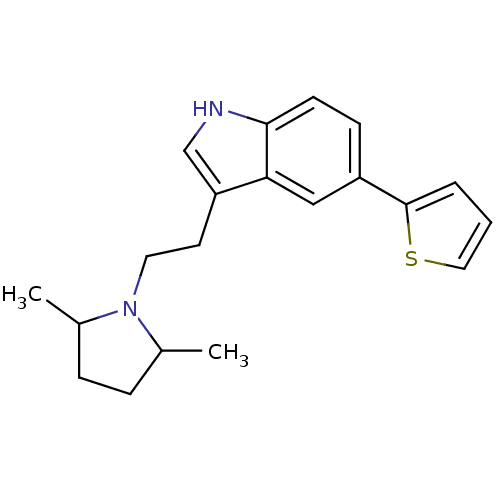
(3-[2-(2,5-Dimethyl-pyrrolidin-1-yl)-ethyl]-5-thiop...)Show InChI InChI=1S/C20H24N2S/c1-14-5-6-15(2)22(14)10-9-17-13-21-19-8-7-16(12-18(17)19)20-4-3-11-23-20/h3-4,7-8,11-15,21H,5-6,9-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 903-5 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PZJ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053088
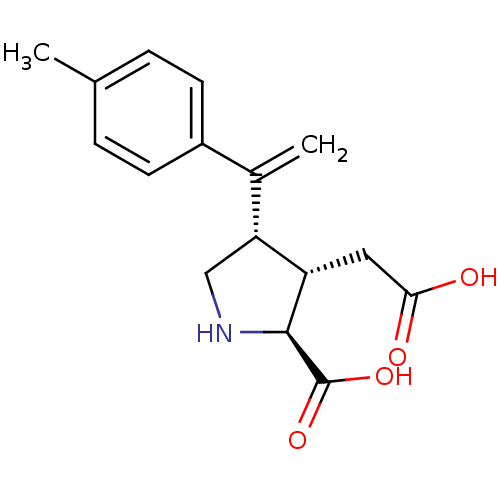
((2S,3S,4S)-3-Carboxymethyl-4-(1-p-tolyl-vinyl)-pyr...)Show SMILES Cc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C16H19NO4/c1-9-3-5-11(6-4-9)10(2)13-8-17-15(16(20)21)12(13)7-14(18)19/h3-6,12-13,15,17H,2,7-8H2,1H3,(H,18,19)(H,20,21)/t12-,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053087
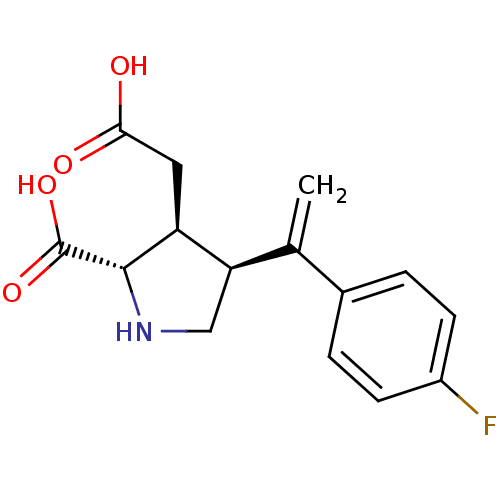
((2S,3S,4S)-3-Carboxymethyl-4-[1-(4-fluoro-phenyl)-...)Show SMILES OC(=O)C[C@H]1[C@H](CN[C@@H]1C(O)=O)C(=C)c1ccc(F)cc1 Show InChI InChI=1S/C15H16FNO4/c1-8(9-2-4-10(16)5-3-9)12-7-17-14(15(20)21)11(12)6-13(18)19/h2-5,11-12,14,17H,1,6-7H2,(H,18,19)(H,20,21)/t11-,12+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 2
(Rattus norvegicus) | BDBM50053064
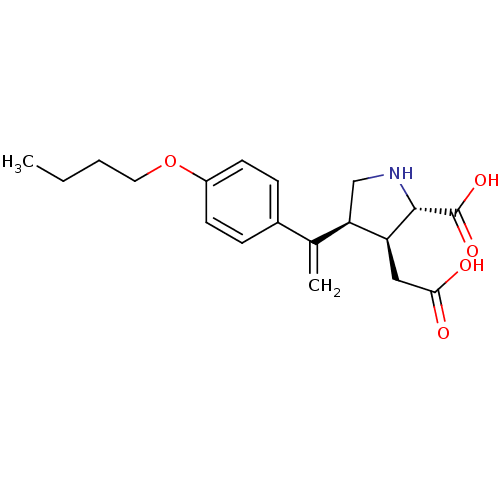
((2S,3S,4S)-4-[1-(4-Butoxy-phenyl)-vinyl]-3-carboxy...)Show SMILES CCCCOc1ccc(cc1)C(=C)[C@H]1CN[C@@H]([C@H]1CC(O)=O)C(O)=O Show InChI InChI=1S/C19H25NO5/c1-3-4-9-25-14-7-5-13(6-8-14)12(2)16-11-20-18(19(23)24)15(16)10-17(21)22/h5-8,15-16,18,20H,2-4,9-11H2,1H3,(H,21,22)(H,23,24)/t15-,16+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-kainate binding at rat forebrain ionotropic glutamate receptor kainate 2 |
J Med Chem 39: 3617-24 (1996)
Article DOI: 10.1021/jm960155a
BindingDB Entry DOI: 10.7270/Q2WW7GRZ |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM50060630
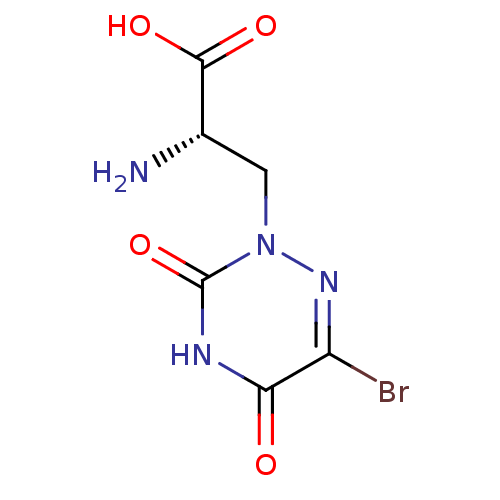
((S)-2-Amino-3-(6-bromo-3,5-dioxo-4,5-dihydro-3H-[1...)Show InChI InChI=1S/C6H7BrN4O4/c7-3-4(12)9-6(15)11(10-3)1-2(8)5(13)14/h2H,1,8H2,(H,13,14)(H,9,12,15)/t2-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol
Curated by ChEMBL
| Assay Description
Displacement of [3H]kainate from human Ionotropic glutamate receptor ionotropic kainate 1 expressed in HEK293 cells |
J Med Chem 40: 3645-50 (1997)
Article DOI: 10.1021/jm9702387
BindingDB Entry DOI: 10.7270/Q28G8MCB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50049082

(2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...)Show InChI InChI=1S/C18H28N2O/c1-2-3-4-5-6-7-12-21-16-8-9-18-17(13-16)15(10-11-19)14-20-18/h8-9,13-14,20H,2-7,10-12,19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50031704
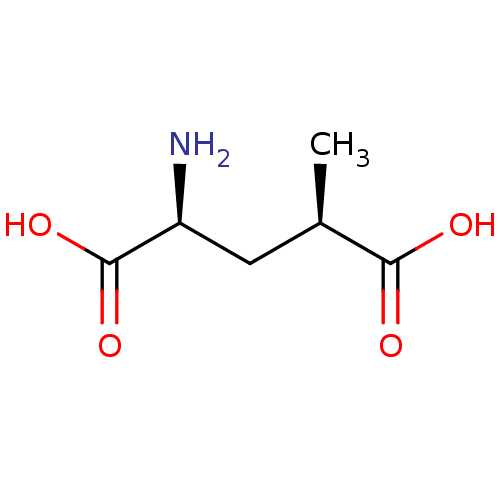
((2S,4R)-2-Amino-4-methyl-pentanedioic acid | (2S,4...)Show InChI InChI=1S/C6H11NO4/c1-3(5(8)9)2-4(7)6(10)11/h3-4H,2,7H2,1H3,(H,8,9)(H,10,11)/t3-,4+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly, S.A.
Curated by ChEMBL
| Assay Description
Binding affinity against human ionotropic glutamate receptor kainate 1 in HK293 cells using [3H]-kainate as radioligand |
J Med Chem 43: 1958-68 (2000)
BindingDB Entry DOI: 10.7270/Q2FX78QB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50049082

(2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...)Show InChI InChI=1S/C18H28N2O/c1-2-3-4-5-6-7-12-21-16-8-9-18-17(13-16)15(10-11-19)14-20-18/h8-9,13-14,20H,2-7,10-12,19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50088866

(CHEMBL174355 | Dimethyl-{2-[5-(5-methyl-thiophen-2...)Show InChI InChI=1S/C17H20N2S/c1-12-4-7-17(20-12)13-5-6-16-15(10-13)14(11-18-16)8-9-19(2)3/h4-7,10-11,18H,8-9H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-hydroxytryptamine 1D receptor |
Bioorg Med Chem Lett 10: 903-5 (2000)
BindingDB Entry DOI: 10.7270/Q24F1PZJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM82087
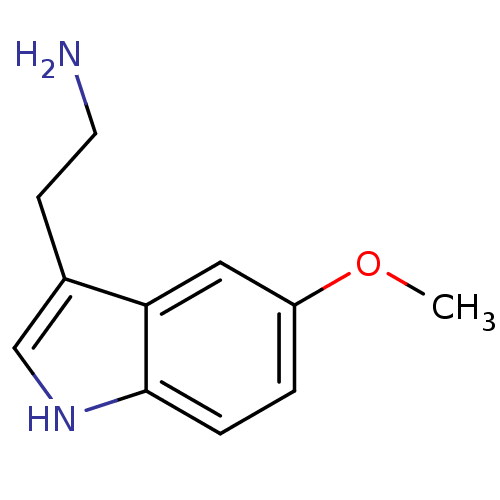
(2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...)Show InChI InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
In vitro affinity at human cloned 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM82087

(2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...)Show InChI InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand |
J Med Chem 39: 314-22 (1996)
Article DOI: 10.1021/jm950498t
BindingDB Entry DOI: 10.7270/Q2DJ5DRK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data