

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM14338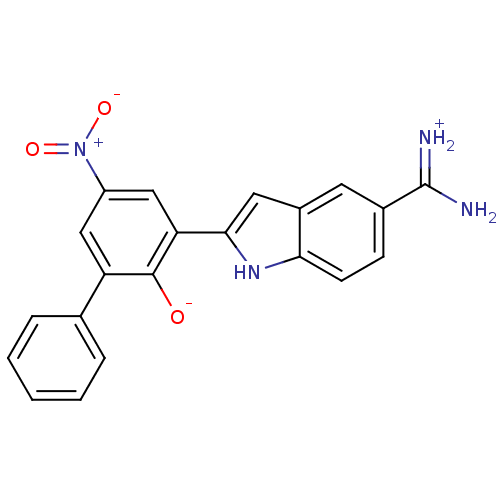 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14352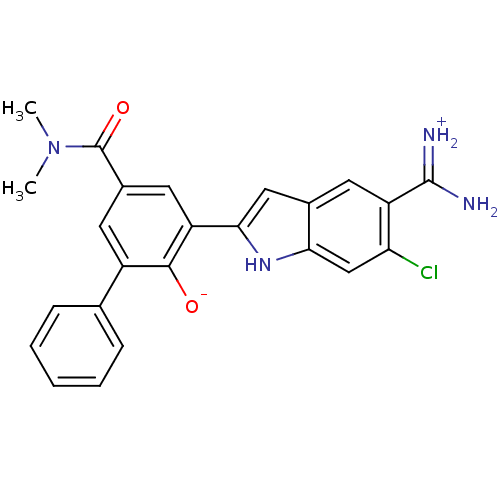 (2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14351 (2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14354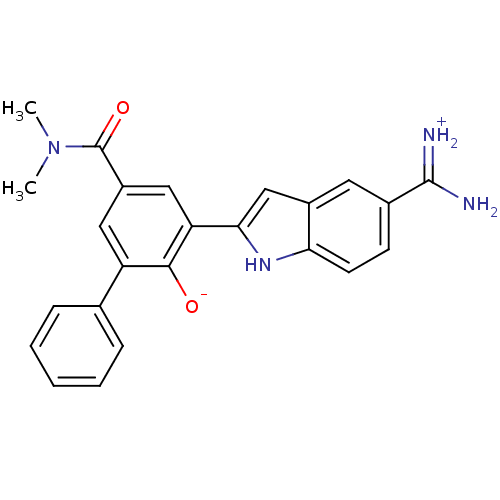 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-(di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277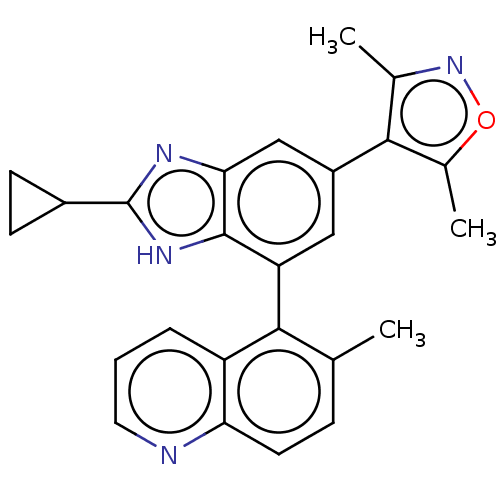 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230294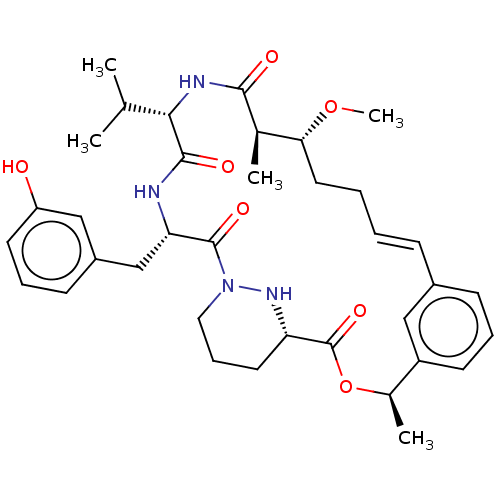 (CHEMBL4071740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230211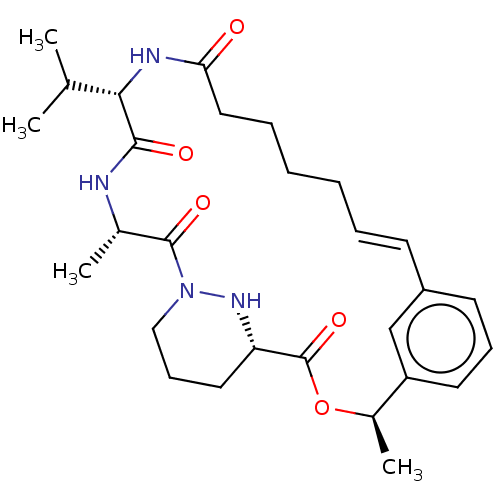 (CHEMBL4063126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815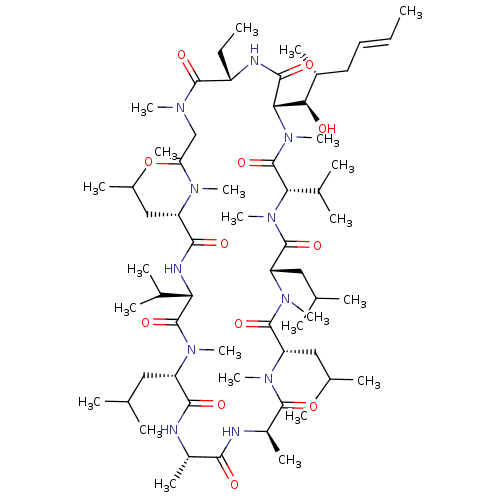 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230212 (CHEMBL4084776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50115874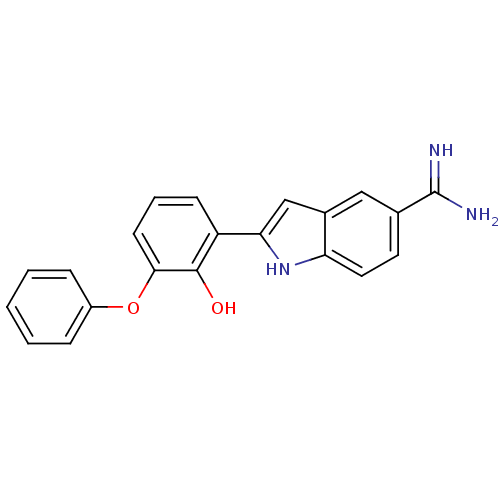 (2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102780 (2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14142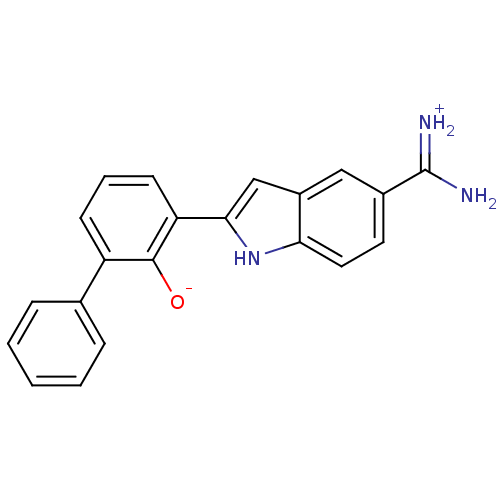 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13940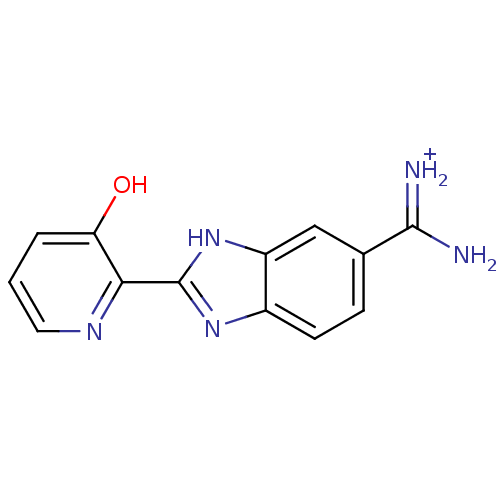 (2-(3-HYDROXY-PYRIDIN-2-YL)-1H-BENZOIMIDAZOLE-5-CAR...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Corporation | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 307: 1451-86 (2001) Article DOI: 10.1006/jmbi.2001.4516 BindingDB Entry DOI: 10.7270/Q2FX77PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14152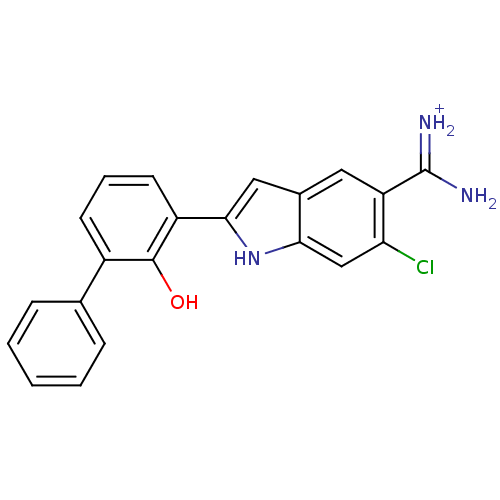 (6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50106240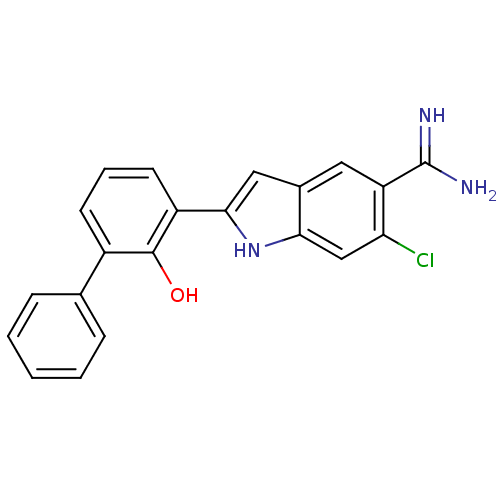 (6-Chloro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50115868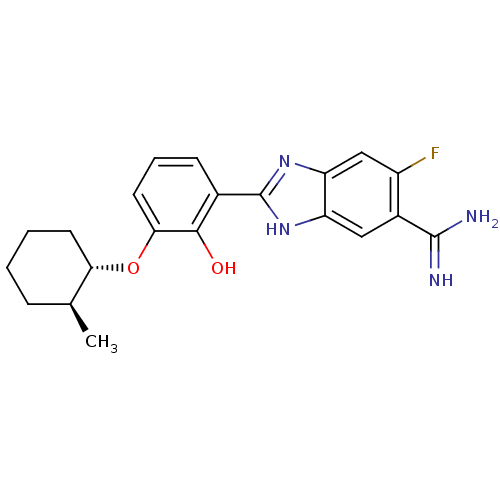 (2-{5-[AMINO(IMINIO)METHYL]-6-FLUORO-1H-BENZIMIDAZO...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14142 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.45 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14149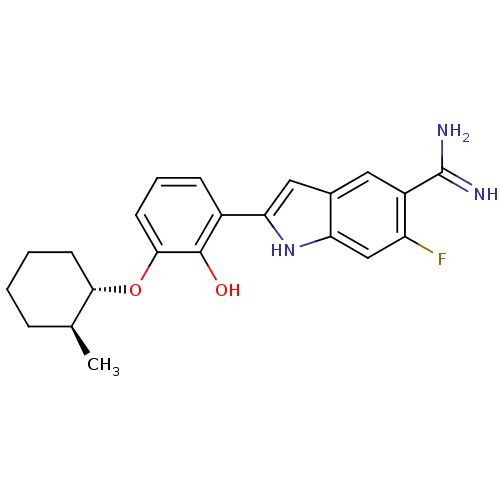 (6-fluoro-2-(2-hydroxy-3-{[(1S,2S)-2-methylcyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14152 (6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14335 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-chl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14338 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230213 (CHEMBL4092526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity against human coagulation factor X | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14337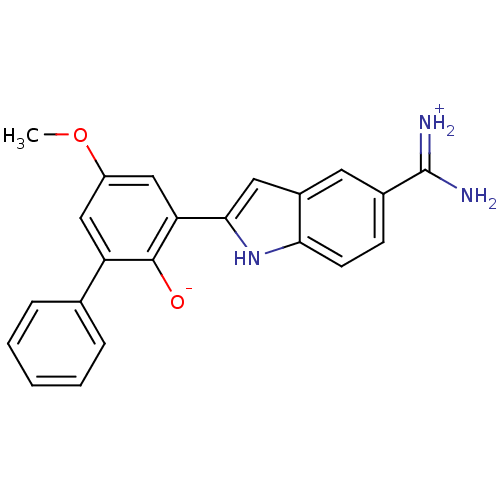 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14152 (6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14329 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.6 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50230210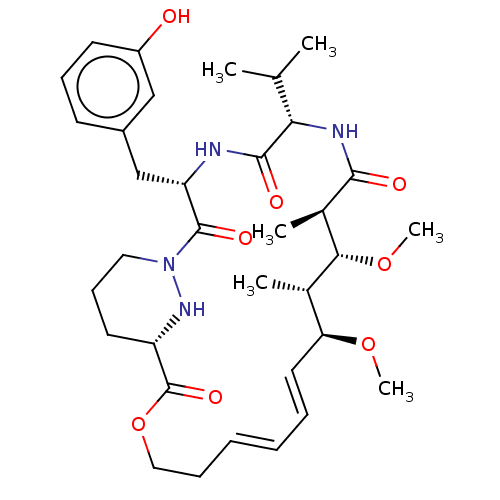 (CHEMBL4090107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selcia Ltd. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His8-tagged Cyclophilin A (1 to 169 residues) expressed in Escherichia coli BL21(DE3) using Su... | J Med Chem 60: 1000-1017 (2017) Article DOI: 10.1021/acs.jmedchem.6b01329 BindingDB Entry DOI: 10.7270/Q2JQ137K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14150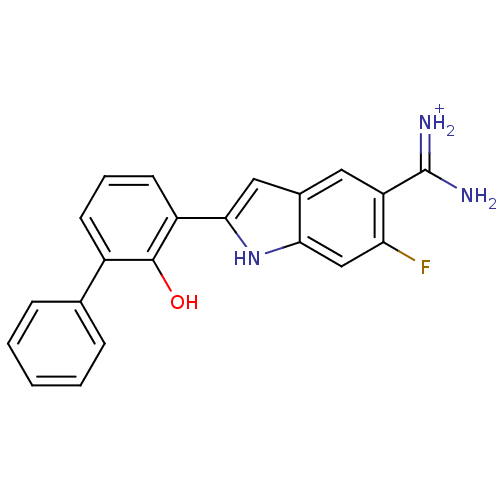 (APC-11417 | CA-12 | CRA-11417 | {amino[6-fluoro-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13940 (2-(3-HYDROXY-PYRIDIN-2-YL)-1H-BENZOIMIDAZOLE-5-CAR...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Corporation | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 307: 1451-86 (2001) Article DOI: 10.1006/jmbi.2001.4516 BindingDB Entry DOI: 10.7270/Q2FX77PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14481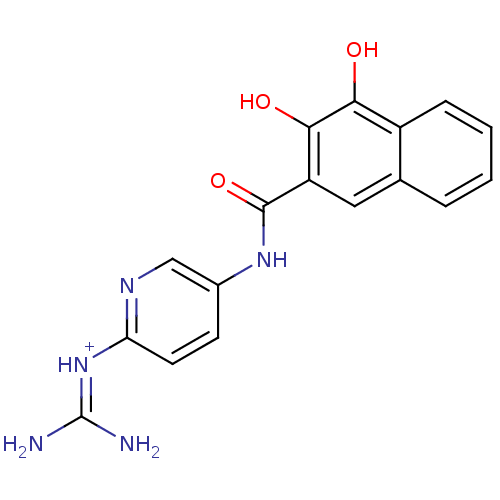 (CA-23 | [amino({5-[(3,4-dihydroxynaphthalene-2-)am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA). | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50106239 (6-Fluoro-2-(2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description The compound was tested for inhibition of human urokinase type plasminogen activator (microPa) | J Med Chem 44: 3856-71 (2001) BindingDB Entry DOI: 10.7270/Q22R3QXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14150 (APC-11417 | CA-12 | CRA-11417 | {amino[6-fluoro-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14150 (APC-11417 | CA-12 | CRA-11417 | {amino[6-fluoro-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14334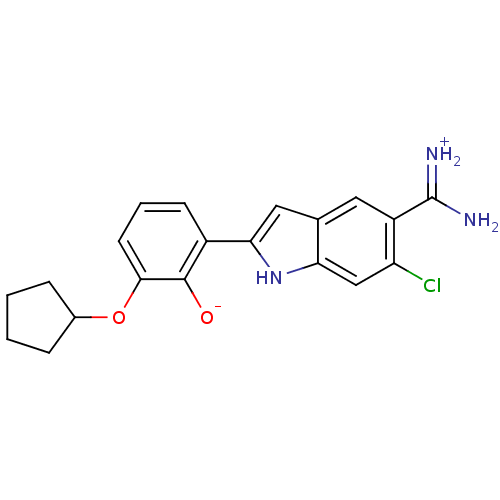 (2-{5-[amino(iminiumyl)methyl]-6-chloro-1H-indol-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50102790 (2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc. Curated by ChEMBL | Assay Description ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA). | J Med Chem 44: 2753-71 (2001) BindingDB Entry DOI: 10.7270/Q2RX9BC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14149 (6-fluoro-2-(2-hydroxy-3-{[(1S,2S)-2-methylcyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 25 | -43.0 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14338 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-nit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14145 (2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceutical | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Chem Biol 8: 1107-21 (2001) Article DOI: 10.1016/S1074-5521(01)00084-9 BindingDB Entry DOI: 10.7270/Q2S75DKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14353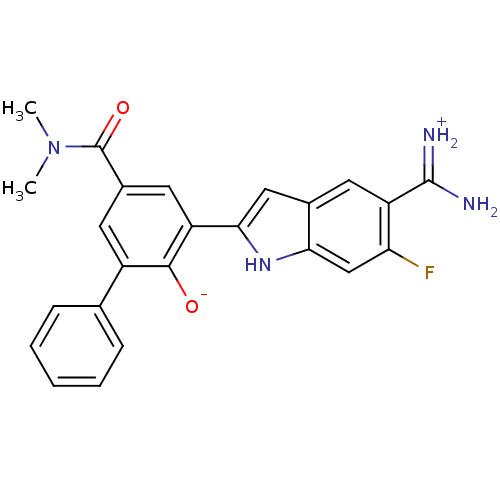 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-indol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14344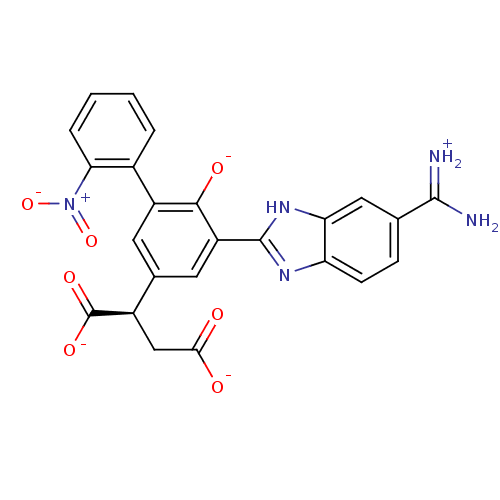 ((2R)-2-(3-{5-[amino(iminiumyl)methyl]-1H-1,3-benzo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14350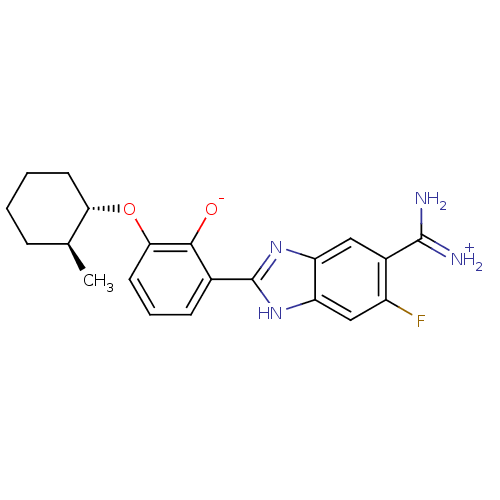 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14337 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50115869 (6-Fluoro-2-[2-hydroxy-3-((S)-2-methyl-cyclohexylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50115871 (6-Fluoro-2-[2-hydroxy-3-((S)-2-methyl-cyclopentylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Curated by ChEMBL | Assay Description Inhibition of urokinase-type plasminogen activator | Bioorg Med Chem Lett 12: 2019-22 (2002) BindingDB Entry DOI: 10.7270/Q237782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14142 (2-{5-[amino(iminiumyl)methyl]-1H-indol-2-yl}-6-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 344: 527-47 (2004) Article DOI: 10.1016/j.jmb.2004.09.032 BindingDB Entry DOI: 10.7270/Q2V40SF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM14350 (2-{5-[amino(iminiumyl)methyl]-6-fluoro-1H-1,3-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | J Mol Biol 329: 93-120 (2003) Article DOI: 10.1016/s0022-2836(03)00399-1 BindingDB Entry DOI: 10.7270/Q2R78CGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 2103 total ) | Next | Last >> |