

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121753 (1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C. Curated by ChEMBL | Assay Description Binding affinity towards recombinant thymidine phosphorylase TP | Bioorg Med Chem Lett 13: 107-10 (2002) BindingDB Entry DOI: 10.7270/Q2GM87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50133876 ((S)-3-{(S)-3-Methyl-2-[2-(naphthalen-1-yloxy)-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against caspase-3 (Csp-3) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Mus musculus) | BDBM50133876 ((S)-3-{(S)-3-Methyl-2-[2-(naphthalen-1-yloxy)-acet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against Murine caspase-1 (mCsp-1) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50133889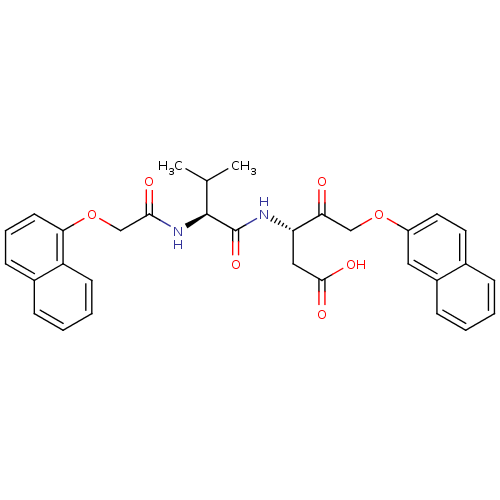 ((S)-3-{(S)-3-Methyl-2-[2-(naphthalen-1-yloxy)-acet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against caspase-3 (Csp-3) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50133879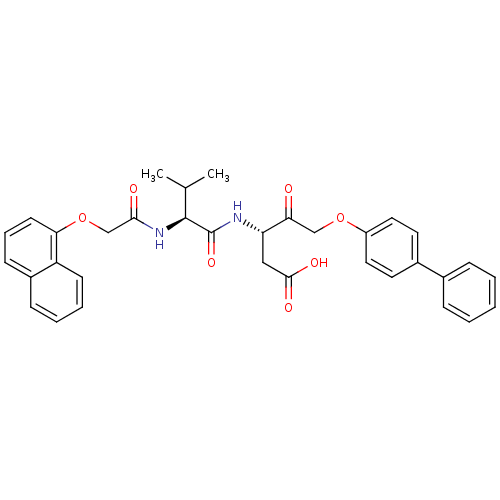 ((S)-5-(Biphenyl-4-yloxy)-3-{(S)-3-methyl-2-[2-(nap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against caspase-3 (Csp-3) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201010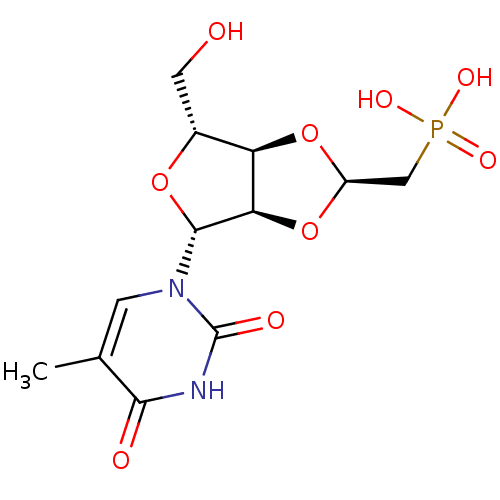 (((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201013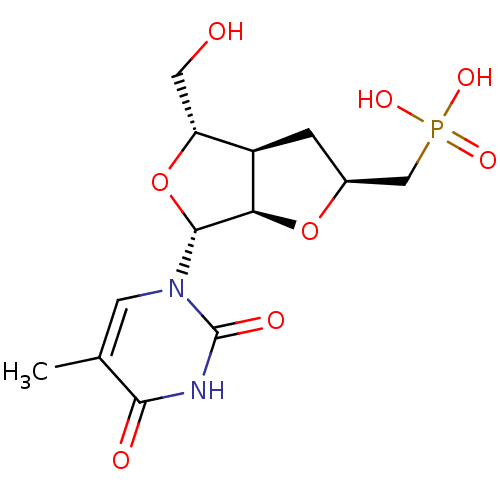 (((2S,3aR,4S,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Mus musculus) | BDBM50133879 ((S)-5-(Biphenyl-4-yloxy)-3-{(S)-3-methyl-2-[2-(nap...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against Murine caspase-1 (mCsp-1) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Mus musculus) | BDBM50133889 ((S)-3-{(S)-3-Methyl-2-[2-(naphthalen-1-yloxy)-acet...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound was tested against Murine caspase-1 (mCsp-1) | Bioorg Med Chem Lett 13: 3623-6 (2003) BindingDB Entry DOI: 10.7270/Q21835WJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201015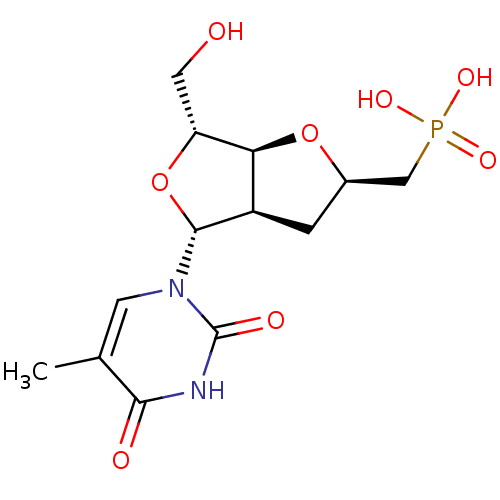 (((2R,3aR,4R,6R,6aS)-6-(hydroxymethyl)-4-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201012 (((2R,3aR,4S,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201011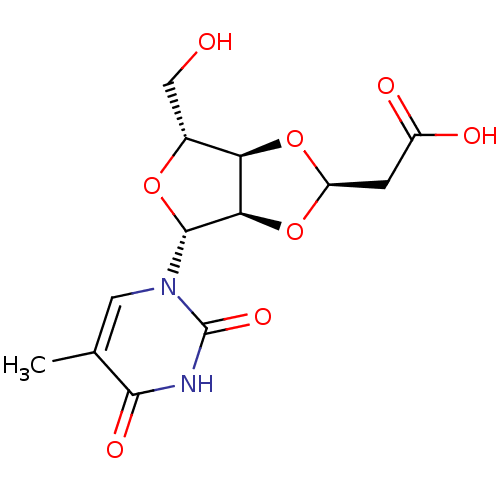 (2-((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121756 ((benzylamino)[(2,4-dioxo-1,2,3,4-tetrahydrothieno[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C. Curated by ChEMBL | Assay Description Binding affinity towards recombinant thymidine phosphorylase TP | Bioorg Med Chem Lett 13: 107-10 (2002) BindingDB Entry DOI: 10.7270/Q2GM87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121754 (CHEMBL12488 | amino[(2,4-dioxo-1,2,3,4-tetrahydrot...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C. Curated by ChEMBL | Assay Description Binding affinity towards recombinant thymidine phosphorylase TP | Bioorg Med Chem Lett 13: 107-10 (2002) BindingDB Entry DOI: 10.7270/Q2GM87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121757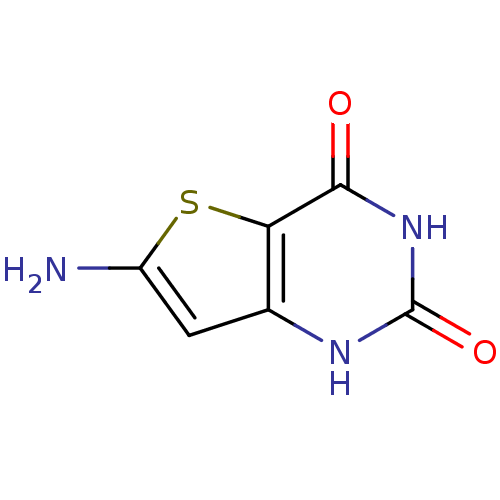 (6-Amino-1H-thieno[3,2-d]pyrimidine-2,4-dione | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C. Curated by ChEMBL | Assay Description Binding affinity towards recombinant thymidine phosphorylase TP | Bioorg Med Chem Lett 13: 107-10 (2002) BindingDB Entry DOI: 10.7270/Q2GM87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201014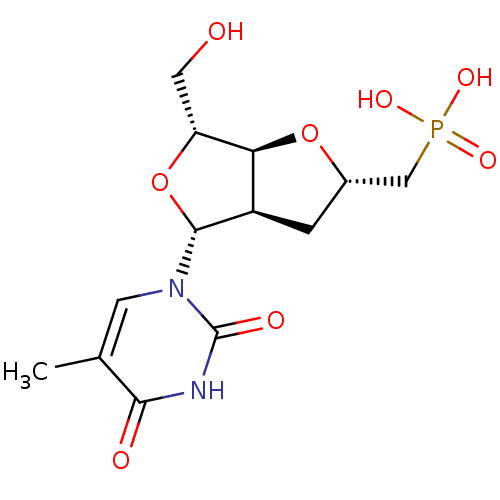 (((2S,3aR,4R,6R,6aS)-6-(hydroxymethyl)-4-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121755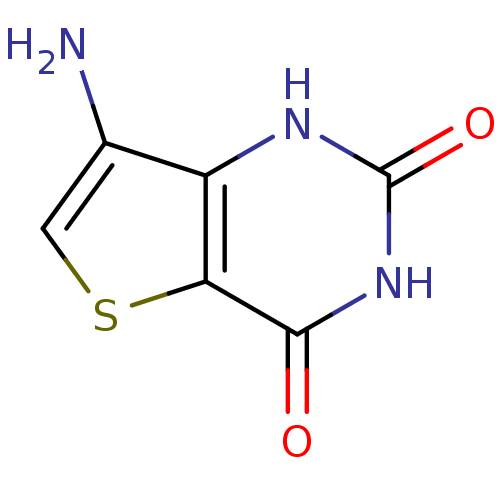 (7-Amino-1H-thieno[3,2-d]pyrimidine-2,4-dione | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C. Curated by ChEMBL | Assay Description Binding affinity towards recombinant thymidine phosphorylase TP | Bioorg Med Chem Lett 13: 107-10 (2002) BindingDB Entry DOI: 10.7270/Q2GM87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121758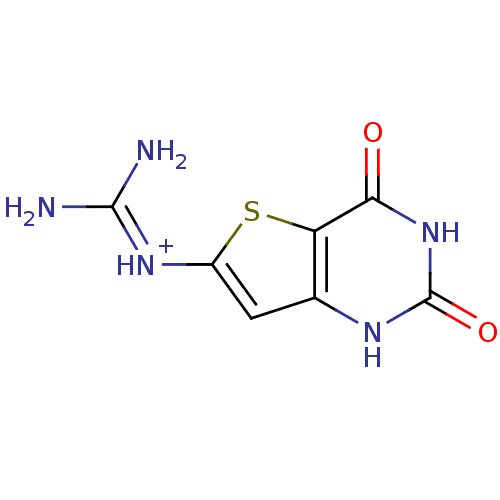 (CHEMBL274649 | amino[(2,4-dioxo-1,2,3,4-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C. Curated by ChEMBL | Assay Description Binding affinity towards recombinant thymidine phosphorylase TP | Bioorg Med Chem Lett 13: 107-10 (2002) BindingDB Entry DOI: 10.7270/Q2GM87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2156 (1-(5-chloropyridin-2-yl)-3-[2-(1,3-dioxo-2,3-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1890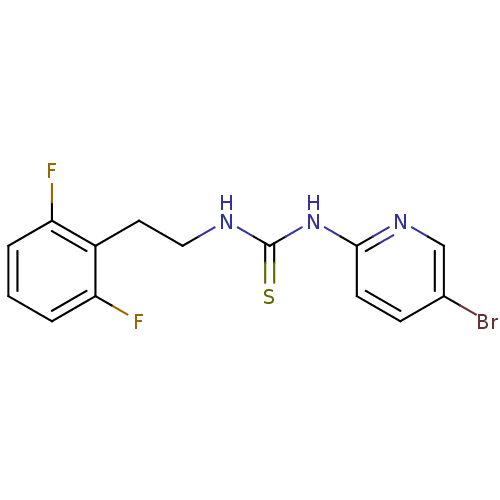 (3-(5-bromopyridin-2-yl)-1-[2-(2,6-difluorophenyl)e...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM572147 (US11447497, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Caspase-1 was diluted to 10 U/μl in assay buffer consisting of 50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 1 mM EDTA, 10% glycerol and 10 mM D... | Citation and Details BindingDB Entry DOI: 10.7270/Q23X89W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2139 (1-(5-chloropyridin-2-yl)-3-[2-(2-fluoro-3,6-dimeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2132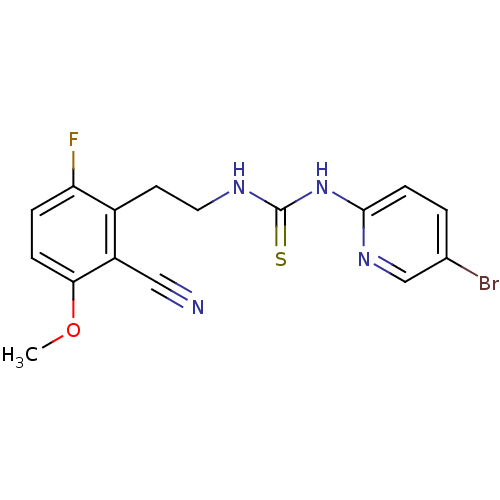 (1-(5-bromopyridin-2-yl)-3-[2-(2-cyano-6-fluoro-3-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2142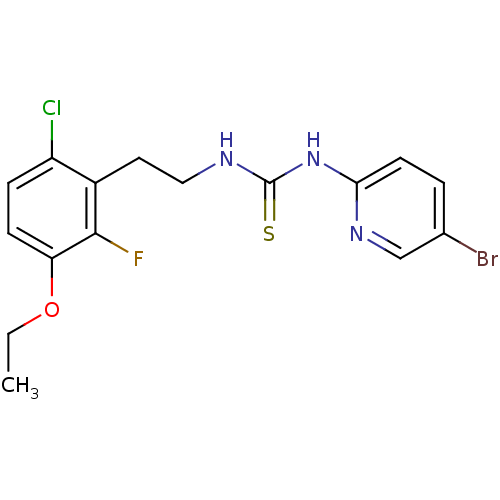 (1-(5-bromopyridin-2-yl)-3-[2-(6-chloro-3-ethoxy-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2131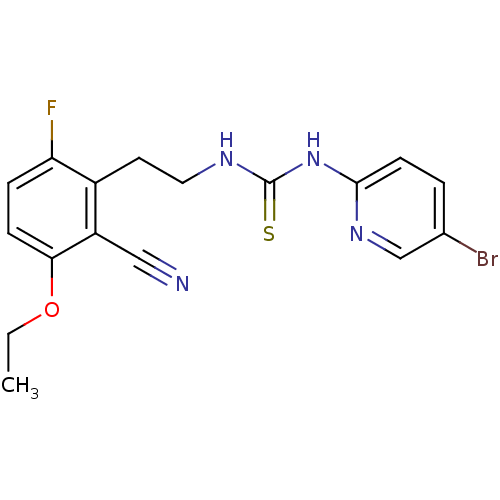 (1-(5-bromopyridin-2-yl)-3-[2-(2-cyano-3-ethoxy-6-f...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM572147 (US11447497, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Caspase-1 was diluted to 10 U/μl in assay buffer consisting of 50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 1 mM EDTA, 10% glycerol and 10 mM D... | Citation and Details BindingDB Entry DOI: 10.7270/Q23X89W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2143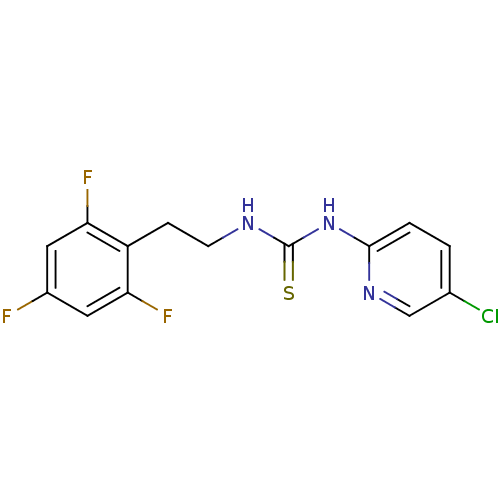 (1-(5-chloropyridin-2-yl)-3-[2-(2,4,6-trifluorophen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2159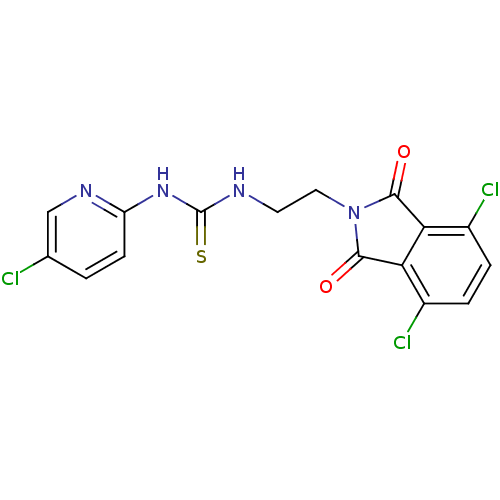 (1-(5-chloropyridin-2-yl)-3-[2-(4,7-dichloro-1,3-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50119218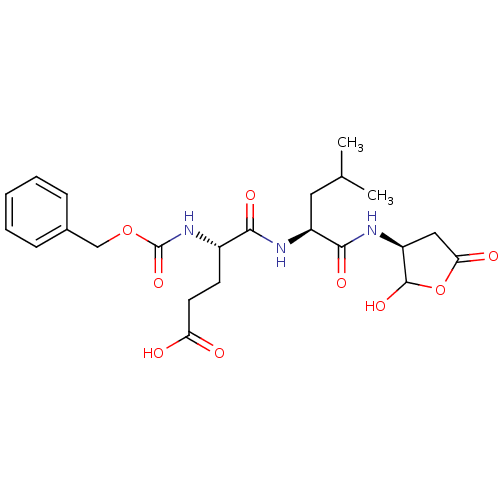 (4-Benzyloxycarbonylamino-4-[1-(2-hydroxy-5-oxo-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-3 compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2969-71 (2002) BindingDB Entry DOI: 10.7270/Q289157S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50119218 (4-Benzyloxycarbonylamino-4-[1-(2-hydroxy-5-oxo-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-3 enzyme compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2153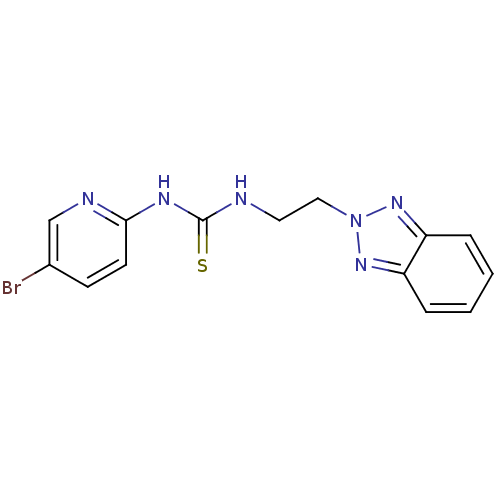 (3-[2-(2H-1,2,3-benzotriazol-2-yl)ethyl]-1-(5-bromo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2133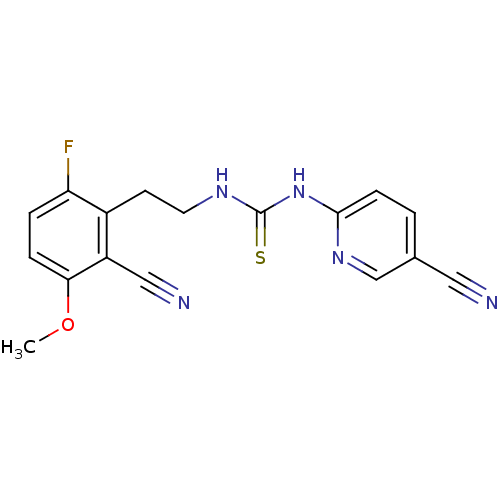 (3-[2-(2-cyano-6-fluoro-3-methoxyphenyl)ethyl]-1-(5...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2138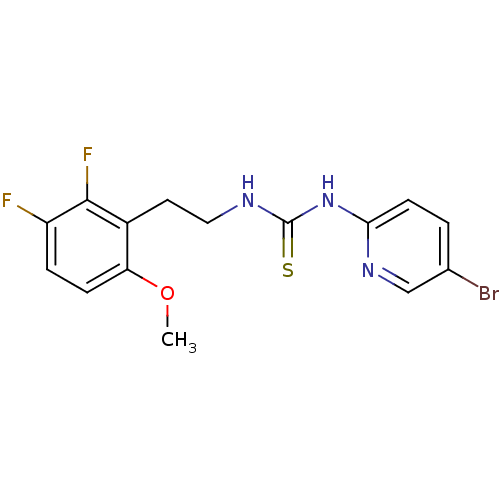 (1-(5-bromopyridin-2-yl)-3-[2-(2,3-difluoro-6-metho...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50119245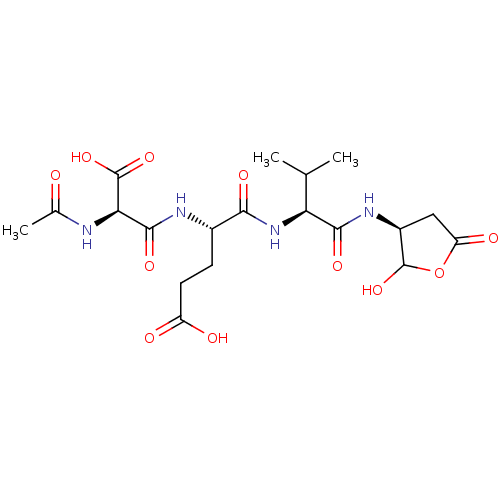 (4-(2-Acetylamino-2-carboxy-acetylamino)-4-[1-(2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-3 compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2969-71 (2002) BindingDB Entry DOI: 10.7270/Q289157S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50072047 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration of compound required against Caspase-3 enzyme compared to acylated dipeptides | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM50119267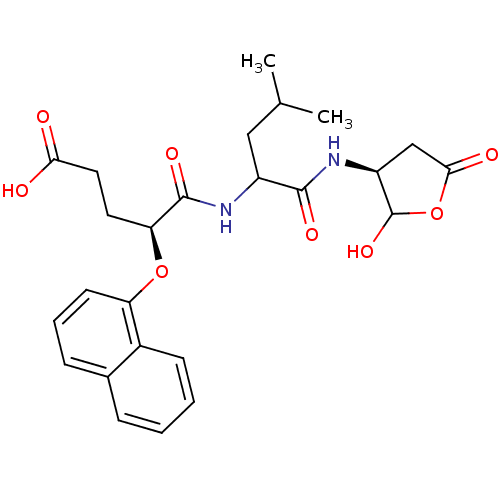 (4-[1-(2-Hydroxy-5-oxo-tetrahydro-furan-3-ylcarbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Caspase-6 | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM50119267 (4-[1-(2-Hydroxy-5-oxo-tetrahydro-furan-3-ylcarbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Idun Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Caspase-3 | Bioorg Med Chem Lett 12: 2973-5 (2002) BindingDB Entry DOI: 10.7270/Q24M93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2149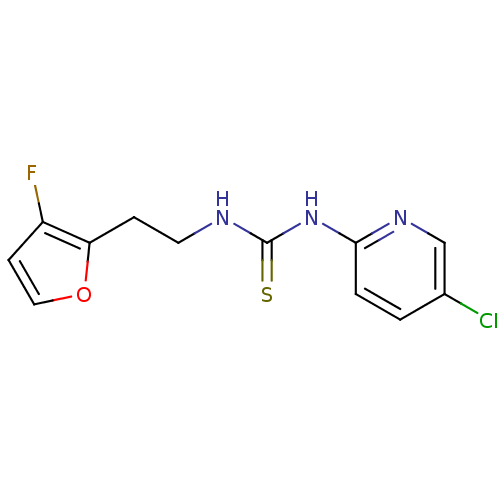 (1-(5-chloropyridin-2-yl)-3-[2-(3-fluorofuran-2-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2124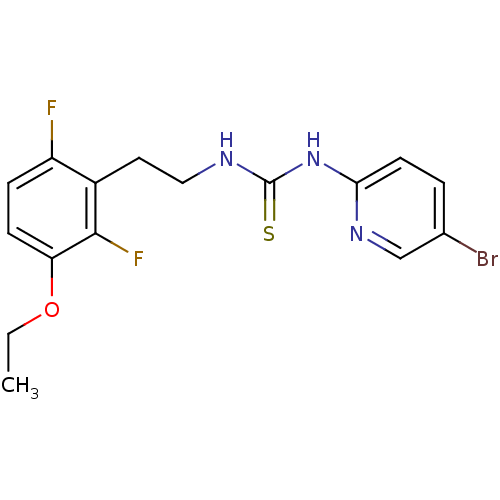 (1-(5-bromopyridin-2-yl)-3-[2-(3-ethoxy-2,6-difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM572145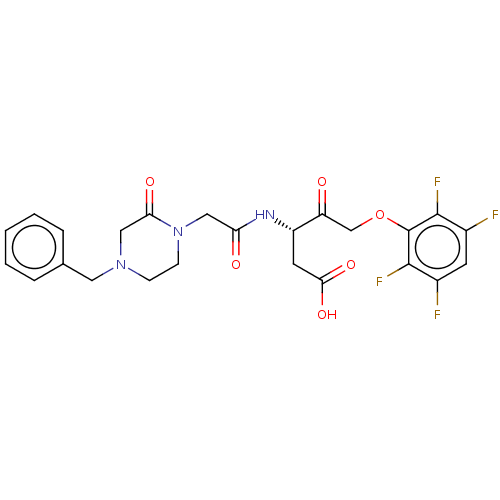 (US11447497, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Caspase-1 was diluted to 10 U/μl in assay buffer consisting of 50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 1 mM EDTA, 10% glycerol and 10 mM D... | Citation and Details BindingDB Entry DOI: 10.7270/Q23X89W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM2138 (1-(5-bromopyridin-2-yl)-3-[2-(2,3-difluoro-6-metho...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2125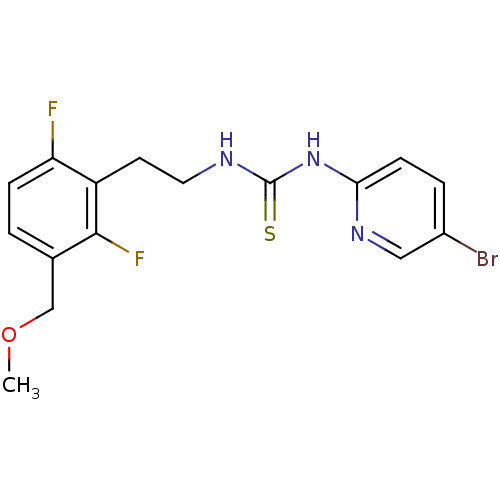 (1-(5-bromopyridin-2-yl)-3-{2-[2,6-difluoro-3-(meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2128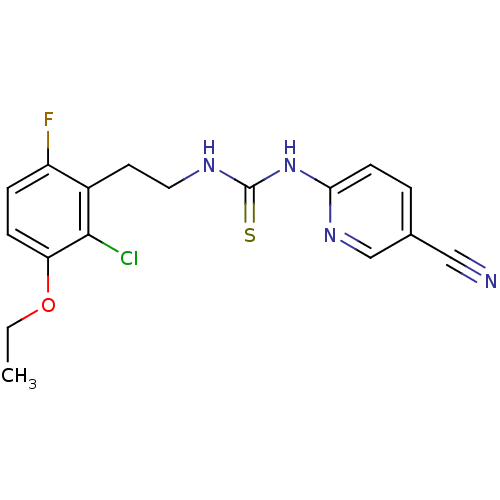 (3-[2-(2-chloro-3-ethoxy-6-fluorophenyl)ethyl]-1-(5...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2129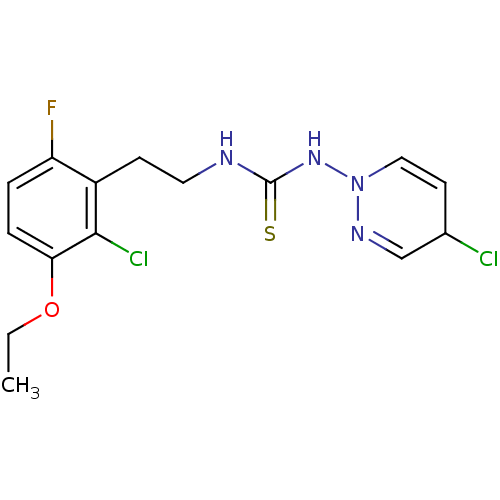 (1-(4-chloro-1,2-dihydropyridazin-1-yl)-3-[2-(2-chl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2140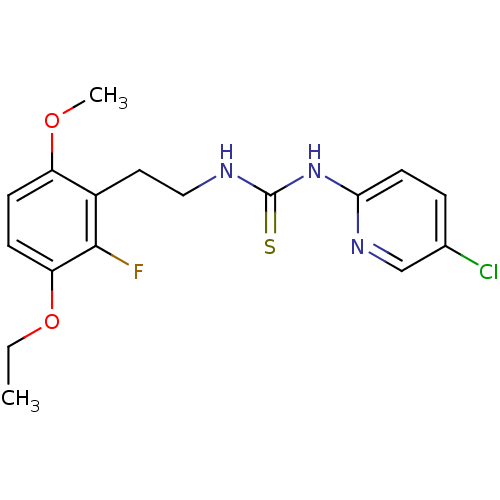 (1-(5-chloropyridin-2-yl)-3-[2-(3-ethoxy-2-fluoro-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2135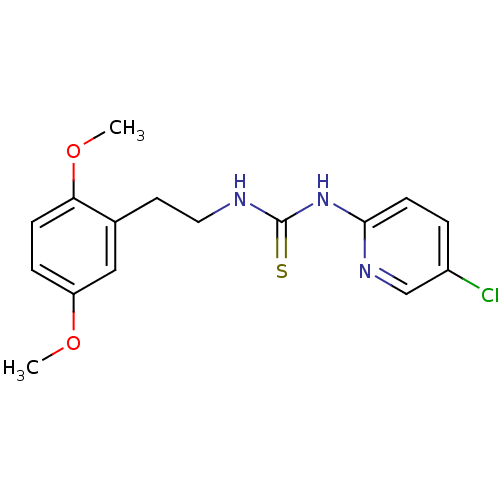 (1-(5-chloropyridin-2-yl)-3-[2-(2,5-dimethoxyphenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2137 (1-(5-bromopyridin-2-yl)-3-[2-(6-ethoxy-2,3-difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2134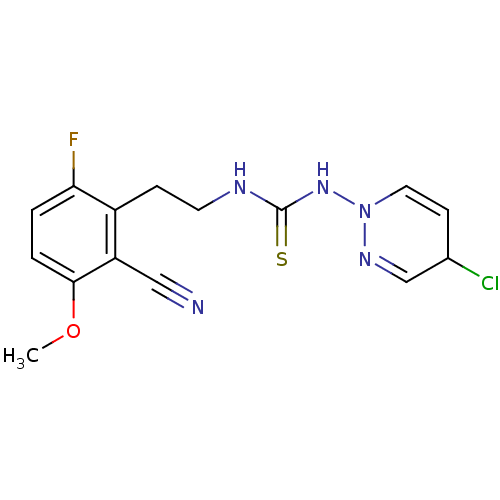 (1-(4-chloro-1,2-dihydropyridazin-1-yl)-3-[2-(2-cya...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2157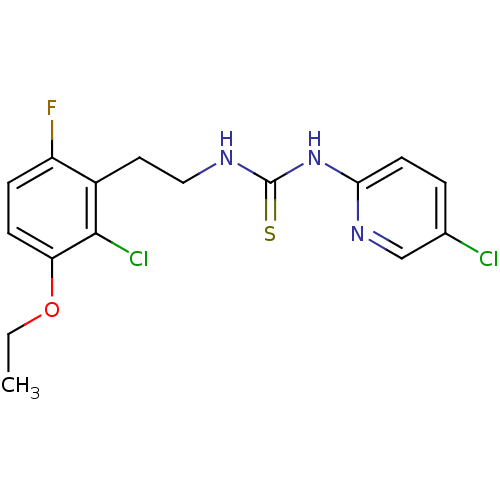 (3-[2-(2-chloro-3-ethoxy-6-fluorophenyl)ethyl]-1-(5...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1893 (3-(5-bromopyridin-2-yl)-1-[2-(2-ethoxy-6-fluorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 4261-74 (1996) Article DOI: 10.1021/jm950639r BindingDB Entry DOI: 10.7270/Q2542KSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 547 total ) | Next | Last >> |