 Found 430 hits with Last Name = 'boyce' and Initial = 's'
Found 430 hits with Last Name = 'boyce' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM86054
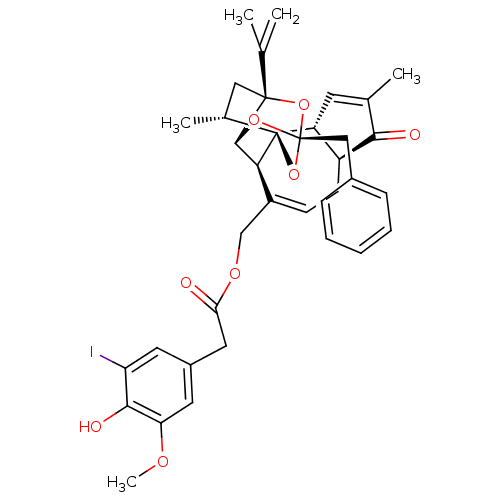
(5'-IODORESINIFERATOXIN | I-RTX)Show SMILES COc1cc(CC(=O)OCC2=CC[C@H]3[C@@H](C=C(C)C3=O)[C@]34O[C@]5(Cc6ccccc6)O[C@H]([C@H]23)[C@](C[C@H]4C)(O5)C(C)=C)cc(I)c1O |t:10,15,THB:37:22:32:35.34.33,23:22:32:35.34.33| Show InChI InChI=1S/C37H39IO8/c1-20(2)35-17-22(4)37-27-13-21(3)32(40)26(27)12-11-25(19-43-30(39)16-24-14-28(38)33(41)29(15-24)42-5)31(37)34(35)44-36(45-35,46-37)18-23-9-7-6-8-10-23/h6-11,13-15,22,26-27,31,34,41H,1,12,16-19H2,2-5H3/t22-,26+,27-,31+,34-,35-,36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1052-60 (2002)
Article DOI: 10.1124/jpet.102.040394
BindingDB Entry DOI: 10.7270/Q279437S |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157515
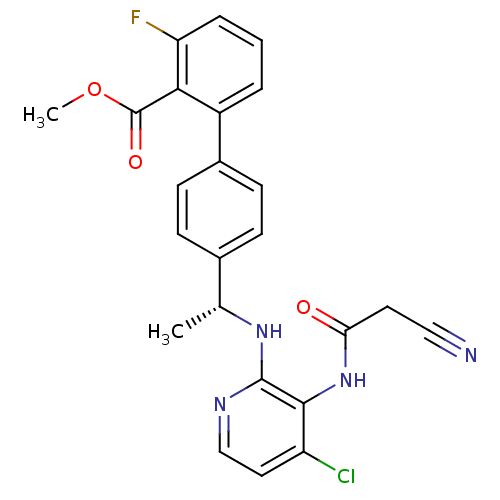
(4'-{(R)-1-[4-chloro-3-(2-cyano-acetylamino)-pyridi...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc(cc1)[C@@H](C)Nc1nccc(Cl)c1NC(=O)CC#N |r| Show InChI InChI=1S/C24H20ClFN4O3/c1-14(29-23-22(18(25)11-13-28-23)30-20(31)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)24(32)33-2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,31)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157509
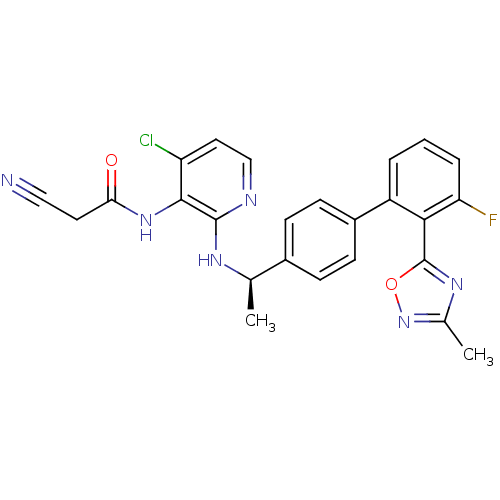
(CHEMBL222038 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1nc(C)no1 |r| Show InChI InChI=1S/C25H20ClFN6O2/c1-14(30-24-23(19(26)11-13-29-24)32-21(34)10-12-28)16-6-8-17(9-7-16)18-4-3-5-20(27)22(18)25-31-15(2)33-35-25/h3-9,11,13-14H,10H2,1-2H3,(H,29,30)(H,32,34)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157511
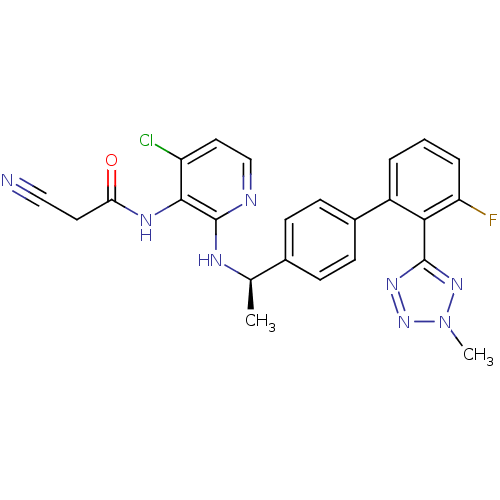
(CHEMBL387638 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1nnn(C)n1 |r| Show InChI InChI=1S/C24H20ClFN8O/c1-14(29-24-22(18(25)11-13-28-24)30-20(35)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)23-31-33-34(2)32-23/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,35)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Oryctolagus cuniculus) | BDBM50157515

(4'-{(R)-1-[4-chloro-3-(2-cyano-acetylamino)-pyridi...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc(cc1)[C@@H](C)Nc1nccc(Cl)c1NC(=O)CC#N |r| Show InChI InChI=1S/C24H20ClFN4O3/c1-14(29-23-22(18(25)11-13-28-23)30-20(31)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)24(32)33-2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,31)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit bradykinin B1 receptor |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157510
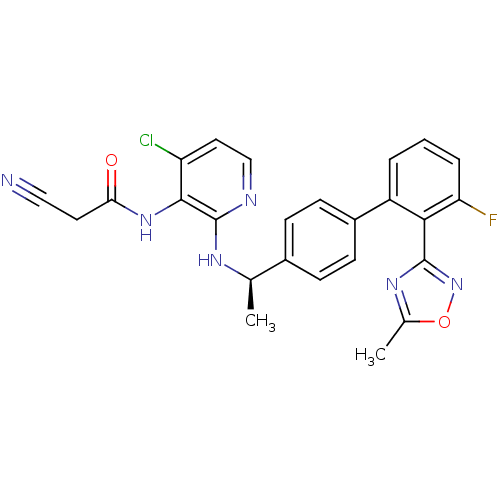
(CHEMBL225218 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1noc(C)n1 |r| Show InChI InChI=1S/C25H20ClFN6O2/c1-14(30-25-23(19(26)11-13-29-25)32-21(34)10-12-28)16-6-8-17(9-7-16)18-4-3-5-20(27)22(18)24-31-15(2)35-33-24/h3-9,11,13-14H,10H2,1-2H3,(H,29,30)(H,32,34)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157513
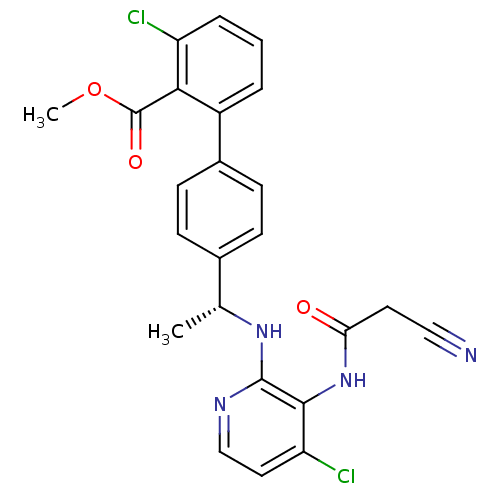
(3-chloro-4'-{(R)-1-[4-chloro-3-(2-cyano-acetylamin...)Show SMILES COC(=O)c1c(Cl)cccc1-c1ccc(cc1)[C@@H](C)Nc1nccc(Cl)c1NC(=O)CC#N |r| Show InChI InChI=1S/C24H20Cl2N4O3/c1-14(29-23-22(19(26)11-13-28-23)30-20(31)10-12-27)15-6-8-16(9-7-15)17-4-3-5-18(25)21(17)24(32)33-2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,31)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157518
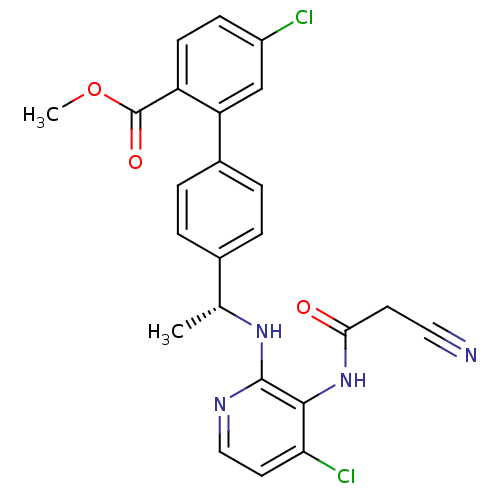
(5-chloro-4'-{(R)-1-[4-chloro-3-(2-cyano-acetylamin...)Show SMILES COC(=O)c1ccc(Cl)cc1-c1ccc(cc1)[C@@H](C)Nc1nccc(Cl)c1NC(=O)CC#N |r| Show InChI InChI=1S/C24H20Cl2N4O3/c1-14(29-23-22(20(26)10-12-28-23)30-21(31)9-11-27)15-3-5-16(6-4-15)19-13-17(25)7-8-18(19)24(32)33-2/h3-8,10,12-14H,9H2,1-2H3,(H,28,29)(H,30,31)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157517
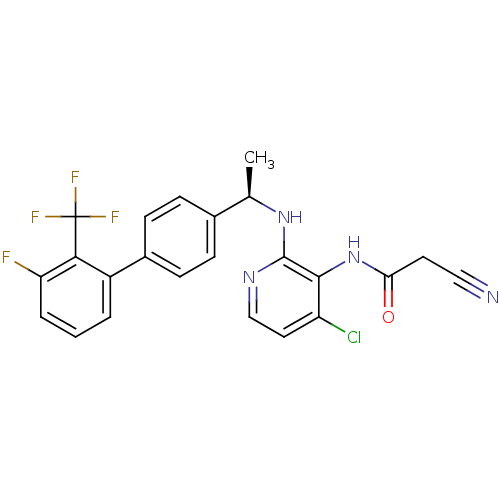
(CHEMBL225006 | N-{4-chloro-2-[(R)-1-(3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1C(F)(F)F |r| Show InChI InChI=1S/C23H17ClF4N4O/c1-13(31-22-21(17(24)10-12-30-22)32-19(33)9-11-29)14-5-7-15(8-6-14)16-3-2-4-18(25)20(16)23(26,27)28/h2-8,10,12-13H,9H2,1H3,(H,30,31)(H,32,33)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Oryctolagus cuniculus) | BDBM50157513

(3-chloro-4'-{(R)-1-[4-chloro-3-(2-cyano-acetylamin...)Show SMILES COC(=O)c1c(Cl)cccc1-c1ccc(cc1)[C@@H](C)Nc1nccc(Cl)c1NC(=O)CC#N |r| Show InChI InChI=1S/C24H20Cl2N4O3/c1-14(29-23-22(19(26)11-13-28-23)30-20(31)10-12-27)15-6-8-16(9-7-15)17-4-3-5-18(25)21(17)24(32)33-2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,31)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit bradykinin B1 receptor |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157516
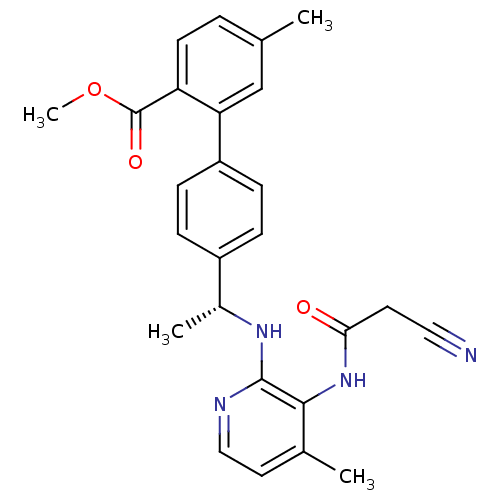
(4'-{(R)-1-[3-(2-cyano-acetylamino)-4-methyl-pyridi...)Show SMILES COC(=O)c1ccc(C)cc1-c1ccc(cc1)[C@@H](C)Nc1nccc(C)c1NC(=O)CC#N |r| Show InChI InChI=1S/C26H26N4O3/c1-16-5-10-21(26(32)33-4)22(15-16)20-8-6-19(7-9-20)18(3)29-25-24(17(2)12-14-28-25)30-23(31)11-13-27/h5-10,12,14-15,18H,11H2,1-4H3,(H,28,29)(H,30,31)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157512
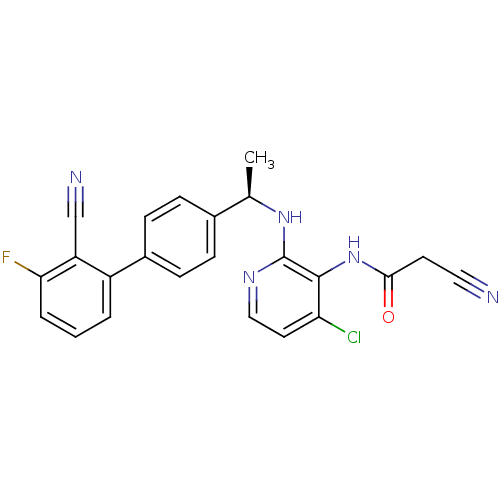
(CHEMBL374779 | N-{4-chloro-2-[(R)-1-(2'-cyano-3'-f...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1C#N |r| Show InChI InChI=1S/C23H17ClFN5O/c1-14(29-23-22(19(24)10-12-28-23)30-21(31)9-11-26)15-5-7-16(8-6-15)17-3-2-4-20(25)18(17)13-27/h2-8,10,12,14H,9H2,1H3,(H,28,29)(H,30,31)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Oryctolagus cuniculus) | BDBM50157509

(CHEMBL222038 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1nc(C)no1 |r| Show InChI InChI=1S/C25H20ClFN6O2/c1-14(30-24-23(19(26)11-13-29-24)32-21(34)10-12-28)16-6-8-17(9-7-16)18-4-3-5-20(27)22(18)25-31-15(2)33-35-25/h3-9,11,13-14H,10H2,1-2H3,(H,29,30)(H,32,34)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit bradykinin B1 receptor |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Oryctolagus cuniculus) | BDBM50157511

(CHEMBL387638 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1nnn(C)n1 |r| Show InChI InChI=1S/C24H20ClFN8O/c1-14(29-24-22(18(25)11-13-28-24)30-20(35)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)23-31-33-34(2)32-23/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,35)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit bradykinin B1 receptor |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50380719
(CHEMBL1682275)Show SMILES OCCNC(=O)C1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C25H32ClN5O4/c26-21-3-1-2-19-20(15-31(23(19)21)14-17-6-12-34-13-7-17)24-28-22(35-29-24)16-30-9-4-18(5-10-30)25(33)27-8-11-32/h1-3,15,17-18,32H,4-14,16H2,(H,27,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50157514
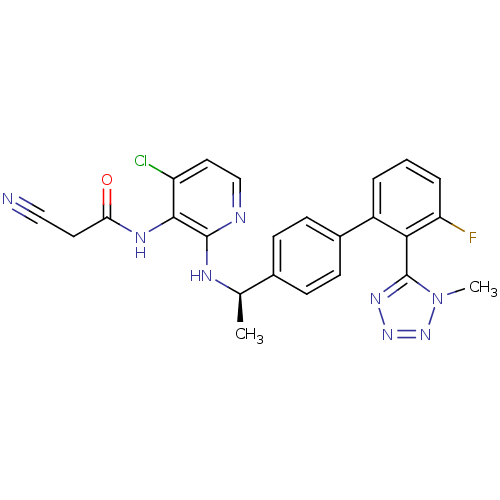
(CHEMBL221984 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1nnnn1C |r,wU:1.0,(12.95,-46.3,;14.28,-45.52,;14.28,-43.98,;12.94,-43.22,;11.6,-43.99,;10.27,-43.22,;10.27,-41.67,;11.6,-40.9,;11.6,-39.36,;12.94,-41.67,;14.27,-40.89,;15.6,-41.66,;15.61,-43.2,;16.93,-40.88,;18.27,-41.65,;19.6,-42.41,;15.61,-46.29,;15.61,-47.83,;16.94,-48.6,;18.27,-47.82,;18.27,-46.28,;16.93,-45.51,;19.61,-48.59,;19.61,-50.13,;20.94,-50.9,;22.27,-50.12,;22.27,-48.57,;23.59,-47.8,;20.93,-47.81,;20.92,-46.27,;19.66,-45.38,;20.12,-43.91,;21.67,-43.9,;22.15,-45.36,;23.62,-45.82,)| Show InChI InChI=1S/C24H20ClFN8O/c1-14(29-23-22(18(25)11-13-28-23)30-20(35)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)24-31-32-33-34(24)2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,35)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50380717

(CHEMBL2017678)Show SMILES COc1cccc2c(cn(CC3CCOCC3)c12)-c1nsc(CN2CCN(CC(N)=O)CC2)n1 Show InChI InChI=1S/C24H32N6O3S/c1-32-20-4-2-3-18-19(14-30(23(18)20)13-17-5-11-33-12-6-17)24-26-22(34-27-24)16-29-9-7-28(8-10-29)15-21(25)31/h2-4,14,17H,5-13,15-16H2,1H3,(H2,25,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM86054

(5'-IODORESINIFERATOXIN | I-RTX)Show SMILES COc1cc(CC(=O)OCC2=CC[C@H]3[C@@H](C=C(C)C3=O)[C@]34O[C@]5(Cc6ccccc6)O[C@H]([C@H]23)[C@](C[C@H]4C)(O5)C(C)=C)cc(I)c1O |t:10,15,THB:37:22:32:35.34.33,23:22:32:35.34.33| Show InChI InChI=1S/C37H39IO8/c1-20(2)35-17-22(4)37-27-13-21(3)32(40)26(27)12-11-25(19-43-30(39)16-24-14-28(38)33(41)29(15-24)42-5)31(37)34(35)44-36(45-35,46-37)18-23-9-7-6-8-10-23/h6-11,13-15,22,26-27,31,34,41H,1,12,16-19H2,2-5H3/t22-,26+,27-,31+,34-,35-,36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1052-60 (2002)
Article DOI: 10.1124/jpet.102.040394
BindingDB Entry DOI: 10.7270/Q279437S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50174201
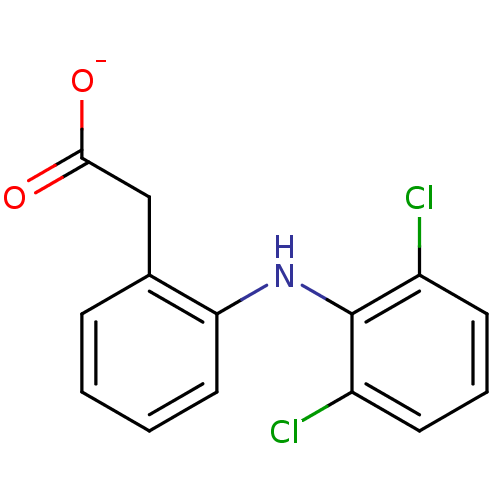
(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50157514

(CHEMBL221984 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1nnnn1C |r,wU:1.0,(12.95,-46.3,;14.28,-45.52,;14.28,-43.98,;12.94,-43.22,;11.6,-43.99,;10.27,-43.22,;10.27,-41.67,;11.6,-40.9,;11.6,-39.36,;12.94,-41.67,;14.27,-40.89,;15.6,-41.66,;15.61,-43.2,;16.93,-40.88,;18.27,-41.65,;19.6,-42.41,;15.61,-46.29,;15.61,-47.83,;16.94,-48.6,;18.27,-47.82,;18.27,-46.28,;16.93,-45.51,;19.61,-48.59,;19.61,-50.13,;20.94,-50.9,;22.27,-50.12,;22.27,-48.57,;23.59,-47.8,;20.93,-47.81,;20.92,-46.27,;19.66,-45.38,;20.12,-43.91,;21.67,-43.9,;22.15,-45.36,;23.62,-45.82,)| Show InChI InChI=1S/C24H20ClFN8O/c1-14(29-23-22(18(25)11-13-28-23)30-20(35)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)24-31-32-33-34(24)2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,35)/t14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10 kallidin from rat bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50380717

(CHEMBL2017678)Show SMILES COc1cccc2c(cn(CC3CCOCC3)c12)-c1nsc(CN2CCN(CC(N)=O)CC2)n1 Show InChI InChI=1S/C24H32N6O3S/c1-32-20-4-2-3-18-19(14-30(23(18)20)13-17-5-11-33-12-6-17)24-26-22(34-27-24)16-29-9-7-28(8-10-29)15-21(25)31/h2-4,14,17H,5-13,15-16H2,1H3,(H2,25,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
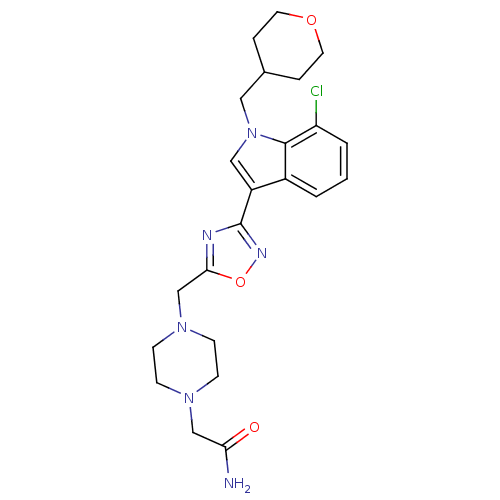
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50380718
(CHEMBL2017684)Show SMILES NC(=O)CN1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C23H29ClN6O3/c24-19-3-1-2-17-18(13-30(22(17)19)12-16-4-10-32-11-5-16)23-26-21(33-27-23)15-29-8-6-28(7-9-29)14-20(25)31/h1-3,13,16H,4-12,14-15H2,(H2,25,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50380719

(CHEMBL1682275)Show SMILES OCCNC(=O)C1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C25H32ClN5O4/c26-21-3-1-2-19-20(15-31(23(19)21)14-17-6-12-34-13-7-17)24-28-22(35-29-24)16-30-9-4-18(5-10-30)25(33)27-8-11-32/h1-3,15,17-18,32H,4-14,16H2,(H,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50380720
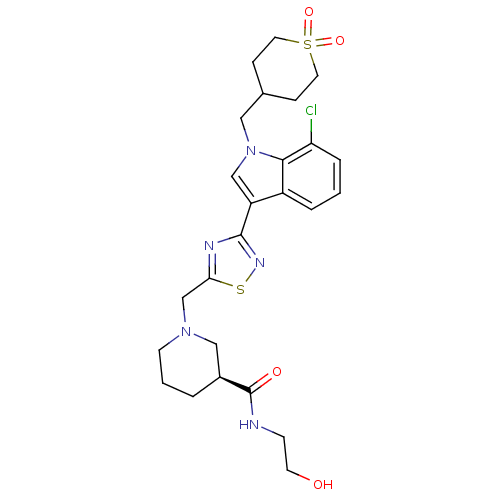
(CHEMBL2017682)Show SMILES OCCNC(=O)[C@H]1CCCN(Cc2nc(ns2)-c2cn(CC3CCS(=O)(=O)CC3)c3c(Cl)cccc23)C1 |r| Show InChI InChI=1S/C25H32ClN5O4S2/c26-21-5-1-4-19-20(15-31(23(19)21)13-17-6-11-37(34,35)12-7-17)24-28-22(36-29-24)16-30-9-2-3-18(14-30)25(33)27-8-10-32/h1,4-5,15,17-18,32H,2-3,6-14,16H2,(H,27,33)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50380720

(CHEMBL2017682)Show SMILES OCCNC(=O)[C@H]1CCCN(Cc2nc(ns2)-c2cn(CC3CCS(=O)(=O)CC3)c3c(Cl)cccc23)C1 |r| Show InChI InChI=1S/C25H32ClN5O4S2/c26-21-5-1-4-19-20(15-31(23(19)21)13-17-6-11-37(34,35)12-7-17)24-28-22(36-29-24)16-30-9-2-3-18(14-30)25(33)27-8-10-32/h1,4-5,15,17-18,32H,2-3,6-14,16H2,(H,27,33)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB1 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50157515

(4'-{(R)-1-[4-chloro-3-(2-cyano-acetylamino)-pyridi...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc(cc1)[C@@H](C)Nc1nccc(Cl)c1NC(=O)CC#N |r| Show InChI InChI=1S/C24H20ClFN4O3/c1-14(29-23-22(18(25)11-13-28-23)30-20(31)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)24(32)33-2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,31)/t14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10 kallidin from rat bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50157513

(3-chloro-4'-{(R)-1-[4-chloro-3-(2-cyano-acetylamin...)Show SMILES COC(=O)c1c(Cl)cccc1-c1ccc(cc1)[C@@H](C)Nc1nccc(Cl)c1NC(=O)CC#N |r| Show InChI InChI=1S/C24H20Cl2N4O3/c1-14(29-23-22(19(26)11-13-28-23)30-20(31)10-12-27)15-6-8-16(9-7-15)17-4-3-5-18(25)21(17)24(32)33-2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,31)/t14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10 kallidin from rat bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Oryctolagus cuniculus) | BDBM50157514

(CHEMBL221984 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1nnnn1C |r,wU:1.0,(12.95,-46.3,;14.28,-45.52,;14.28,-43.98,;12.94,-43.22,;11.6,-43.99,;10.27,-43.22,;10.27,-41.67,;11.6,-40.9,;11.6,-39.36,;12.94,-41.67,;14.27,-40.89,;15.6,-41.66,;15.61,-43.2,;16.93,-40.88,;18.27,-41.65,;19.6,-42.41,;15.61,-46.29,;15.61,-47.83,;16.94,-48.6,;18.27,-47.82,;18.27,-46.28,;16.93,-45.51,;19.61,-48.59,;19.61,-50.13,;20.94,-50.9,;22.27,-50.12,;22.27,-48.57,;23.59,-47.8,;20.93,-47.81,;20.92,-46.27,;19.66,-45.38,;20.12,-43.91,;21.67,-43.9,;22.15,-45.36,;23.62,-45.82,)| Show InChI InChI=1S/C24H20ClFN8O/c1-14(29-23-22(18(25)11-13-28-23)30-20(35)10-12-27)15-6-8-16(9-7-15)17-4-3-5-19(26)21(17)24-31-32-33-34(24)2/h3-9,11,13-14H,10H2,1-2H3,(H,28,29)(H,30,35)/t14-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit bradykinin B1 receptor |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50174201

(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50174201

(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50380718

(CHEMBL2017684)Show SMILES NC(=O)CN1CCN(Cc2nc(no2)-c2cn(CC3CCOCC3)c3c(Cl)cccc23)CC1 Show InChI InChI=1S/C23H29ClN6O3/c24-19-3-1-2-17-18(13-30(22(17)19)12-16-4-10-32-11-5-16)23-26-21(33-27-23)15-29-8-6-28(7-9-29)14-20(25)31/h1-3,13,16H,4-12,14-15H2,(H2,25,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP 55,940 from human CB2 receptor expressed in insect sf9 membranes |
Bioorg Med Chem Lett 22: 2932-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.048
BindingDB Entry DOI: 10.7270/Q2PV6MDJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50157509

(CHEMBL222038 | N-(4-chloro-2-{(R)-1-[3'-fluoro-2'-...)Show SMILES C[C@@H](Nc1nccc(Cl)c1NC(=O)CC#N)c1ccc(cc1)-c1cccc(F)c1-c1nc(C)no1 |r| Show InChI InChI=1S/C25H20ClFN6O2/c1-14(30-24-23(19(26)11-13-29-24)32-21(34)10-12-28)16-6-8-17(9-7-16)18-4-3-5-20(27)22(18)25-31-15(2)33-35-25/h3-9,11,13-14H,10H2,1-2H3,(H,29,30)(H,32,34)/t14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]des-arg10 kallidin from rat bradykinin B1 receptor expresed in CHO cells |
J Med Chem 47: 6439-42 (2004)
Article DOI: 10.1021/jm049394l
BindingDB Entry DOI: 10.7270/Q22B8XHM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data