 Found 189 hits with Last Name = 'claeysen' and Initial = 's'
Found 189 hits with Last Name = 'claeysen' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(RAT) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85027

(1-(7-Chloro-8-amino-1,4-benzodioxane-5-yl)-3-[1-[3...)Show SMILES COc1ccc(CCCN2CCC(CCC(=O)c3cc(Cl)c(N)c4OCCOc34)CC2)cc1OC Show InChI InChI=1S/C27H35ClN2O5/c1-32-23-8-6-19(16-24(23)33-2)4-3-11-30-12-9-18(10-13-30)5-7-22(31)20-17-21(28)25(29)27-26(20)34-14-15-35-27/h6,8,16-18H,3-5,7,9-15,29H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85020
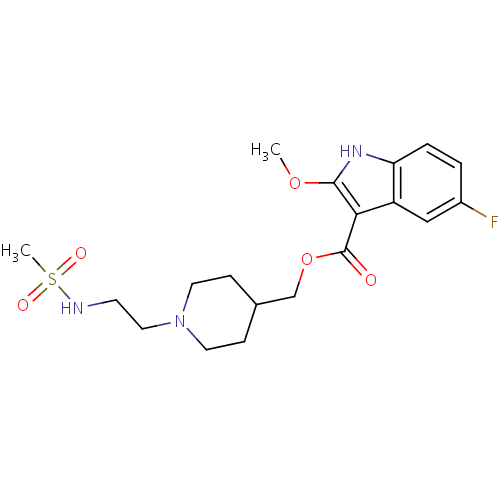
(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.000910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85026
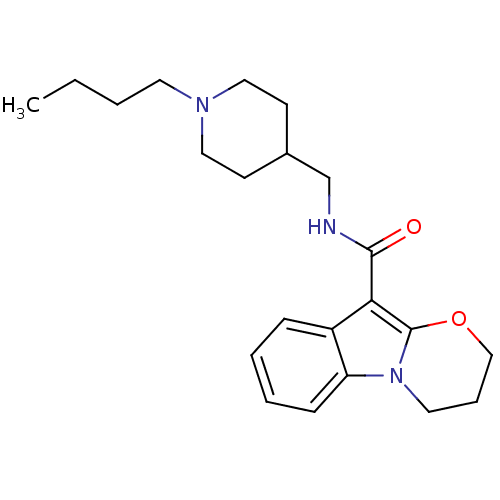
(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.00890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85020

(5-Fluoro-2-methoxy-1H-indole-3-carboxylic acid [1-...)Show SMILES COc1[nH]c2ccc(F)cc2c1C(=O)OCC1CCN(CCN[S](C)(=O)=O)CC1 Show InChI InChI=1S/C19H26FN3O5S/c1-27-18-17(15-11-14(20)3-4-16(15)22-18)19(24)28-12-13-5-8-23(9-6-13)10-7-21-29(2,25)26/h3-4,11,13,21-22H,5-10,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM29525

(3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...)Show SMILES Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c2ccccc12 Show InChI InChI=1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85026

(N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...)Show InChI InChI=1S/C22H31N3O2/c1-2-3-11-24-13-9-17(10-14-24)16-23-21(26)20-18-7-4-5-8-19(18)25-12-6-15-27-22(20)25/h4-5,7-8,17H,2-3,6,9-16H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(MOUSE) | BDBM86428
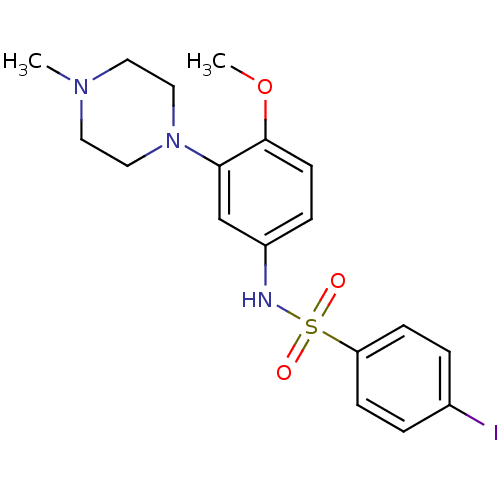
(SB-258585)Show SMILES COc1ccc(NS(=O)(=O)c2ccc(I)cc2)cc1N1CCN(C)CC1 Show InChI InChI=1S/C18H22IN3O3S/c1-21-9-11-22(12-10-21)17-13-15(5-8-18(17)25-2)20-26(23,24)16-6-3-14(19)4-7-16/h3-8,13,20H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse wild type 5HT6 receptor expressed in COS7 cells assessed as inhibition of seratonin-induced cAMP accumulation by HTRF as... |
J Med Chem 53: 1357-69 (2010)
Article DOI: 10.1021/jm901672k
BindingDB Entry DOI: 10.7270/Q2Z320KQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079375
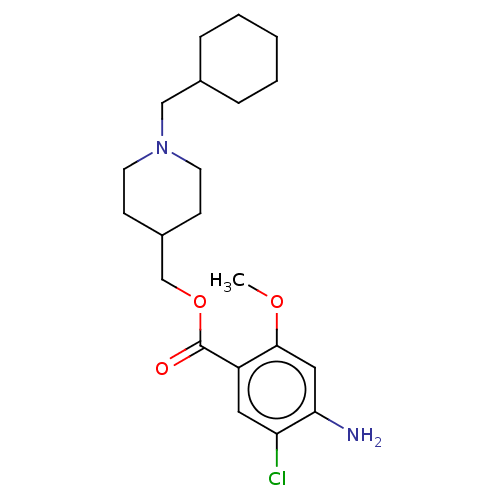
(CHEMBL3417008 | US9663465, 20)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OCC1CCN(CC2CCCCC2)CC1 Show InChI InChI=1S/C21H31ClN2O3/c1-26-20-12-19(23)18(22)11-17(20)21(25)27-14-16-7-9-24(10-8-16)13-15-5-3-2-4-6-15/h11-12,15-16H,2-10,13-14,23H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85019
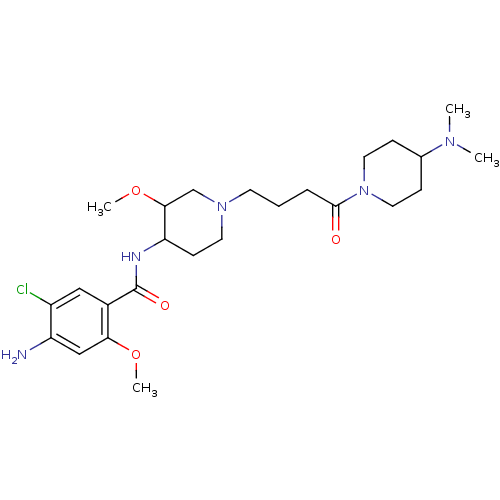
(4-Amino-5-chloro-N-(1-{4-[4-(dimethylamino)-1-pipe...)Show SMILES COC1CN(CCCC(=O)N2CCC(CC2)N(C)C)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C25H40ClN5O4/c1-29(2)17-7-12-31(13-8-17)24(32)6-5-10-30-11-9-21(23(16-30)35-4)28-25(33)18-14-19(26)20(27)15-22(18)34-3/h14-15,17,21,23H,5-13,16,27H2,1-4H3,(H,28,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
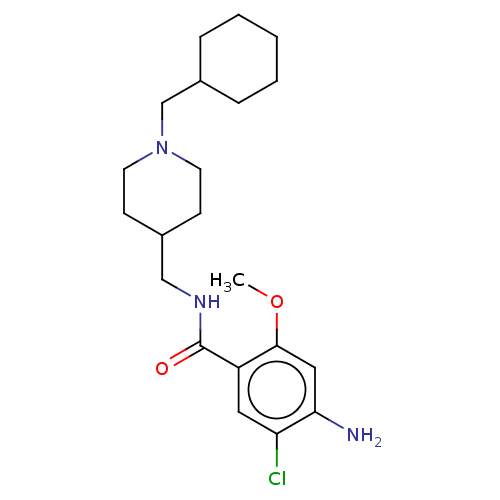
(GUINEA PIG) | BDBM50079377
(CHEMBL3417007)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NCC1CCN(CC2CCCCC2)CC1 Show InChI InChI=1S/C21H32ClN3O2/c1-27-20-12-19(23)18(22)11-17(20)21(26)24-13-15-7-9-25(10-8-15)14-16-5-3-2-4-6-16/h11-12,15-16H,2-10,13-14,23H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
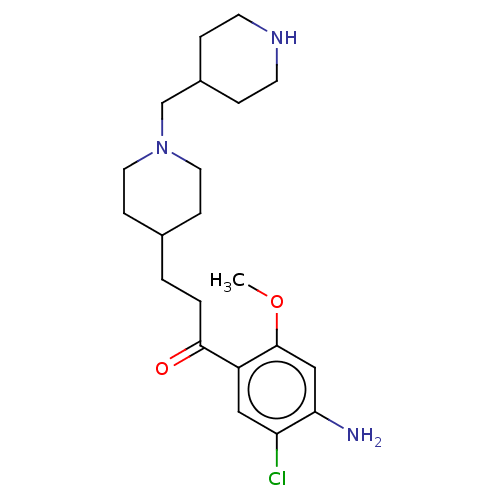
(GUINEA PIG) | BDBM50079367
(CHEMBL3416998)Show SMILES Cl.COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(CC2CCNCC2)CC1 Show InChI InChI=1S/C21H32ClN3O2/c1-27-21-13-19(23)18(22)12-17(21)20(26)3-2-15-6-10-25(11-7-15)14-16-4-8-24-9-5-16/h12-13,15-16,24H,2-11,14,23H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM85019

(4-Amino-5-chloro-N-(1-{4-[4-(dimethylamino)-1-pipe...)Show SMILES COC1CN(CCCC(=O)N2CCC(CC2)N(C)C)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C25H40ClN5O4/c1-29(2)17-7-12-31(13-8-17)24(32)6-5-10-30-11-9-21(23(16-30)35-4)28-25(33)18-14-19(26)20(27)15-22(18)34-3/h14-15,17,21,23H,5-13,16,27H2,1-4H3,(H,28,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
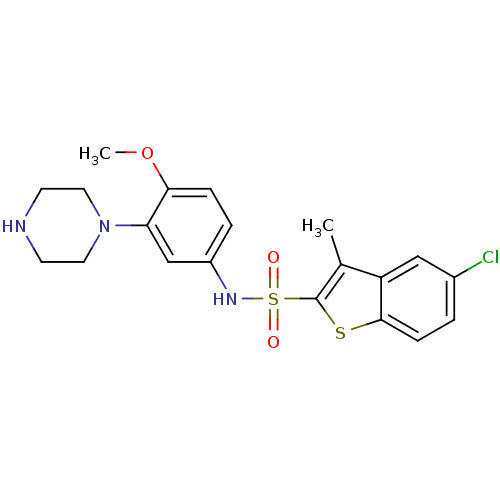
(Homo sapiens (Human)) | BDBM28583
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in BHK cell membrane measured after 60 mins by scintillation counter method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113059
BindingDB Entry DOI: 10.7270/Q2KP85V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079365
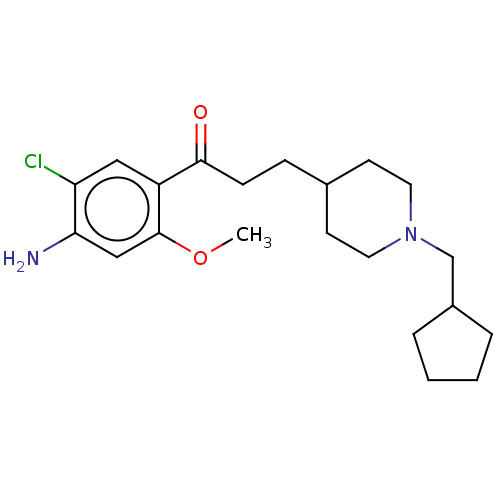
(CHEMBL3416996 | US9663465, 8)Show InChI InChI=1S/C21H31ClN2O2/c1-26-21-13-19(23)18(22)12-17(21)20(25)7-6-15-8-10-24(11-9-15)14-16-4-2-3-5-16/h12-13,15-16H,2-11,14,23H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079366
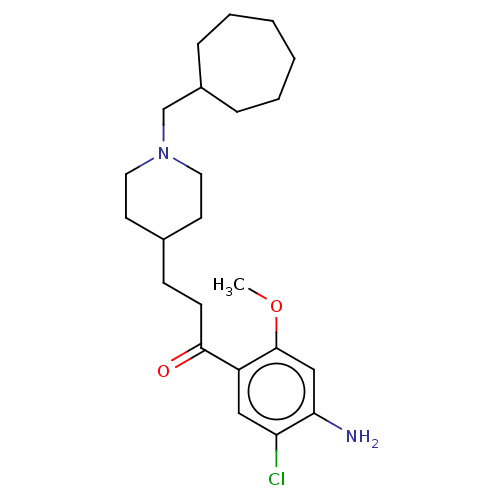
(CHEMBL3416997 | US9663465, 12)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(CC2CCCCCC2)CC1 Show InChI InChI=1S/C23H35ClN2O2/c1-28-23-15-21(25)20(24)14-19(23)22(27)9-8-17-10-12-26(13-11-17)16-18-6-4-2-3-5-7-18/h14-15,17-18H,2-13,16,25H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562651
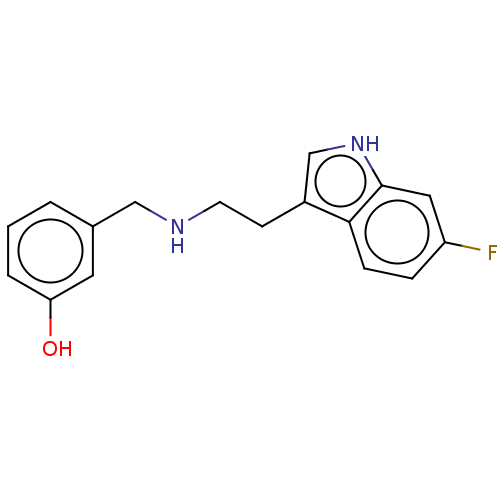
(CHEMBL4788250) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membrane measured after 60 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113059
BindingDB Entry DOI: 10.7270/Q2KP85V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079369

(CHEMBL3417000 | US9663465, 11)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(CC2CCCCC2C)CC1 Show InChI InChI=1S/C23H35ClN2O2/c1-16-5-3-4-6-18(16)15-26-11-9-17(10-12-26)7-8-22(27)19-13-20(24)21(25)14-23(19)28-2/h13-14,16-18H,3-12,15,25H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50304001

(CHEMBL584690 | [4-(4-Methylpiperazin-1-yl)-1H-benz...)Show SMILES CN1CCN(CC1)c1cccc2[nH]c(nc12)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H22N4O/c1-26-12-14-27(15-13-26)20-11-5-10-19-21(20)25-23(24-19)22(28)18-9-4-7-16-6-2-3-8-17(16)18/h2-11H,12-15H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 receptor expressed in COS7 cells assessed as inhibition of seratonin-induced cAMP accumulation by HTRF assay |
J Med Chem 53: 1357-69 (2010)
Article DOI: 10.1021/jm901672k
BindingDB Entry DOI: 10.7270/Q2Z320KQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(MOUSE) | BDBM50304001

(CHEMBL584690 | [4-(4-Methylpiperazin-1-yl)-1H-benz...)Show SMILES CN1CCN(CC1)c1cccc2[nH]c(nc12)C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H22N4O/c1-26-12-14-27(15-13-26)20-11-5-10-19-21(20)25-23(24-19)22(28)18-9-4-7-16-6-2-3-8-17(16)18/h2-11H,12-15H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse wild type 5HT6 receptor expressed in COS7 cells assessed as inhibition of seratonin-induced cAMP accumulation by HTRF as... |
J Med Chem 53: 1357-69 (2010)
Article DOI: 10.1021/jm901672k
BindingDB Entry DOI: 10.7270/Q2Z320KQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM50007863
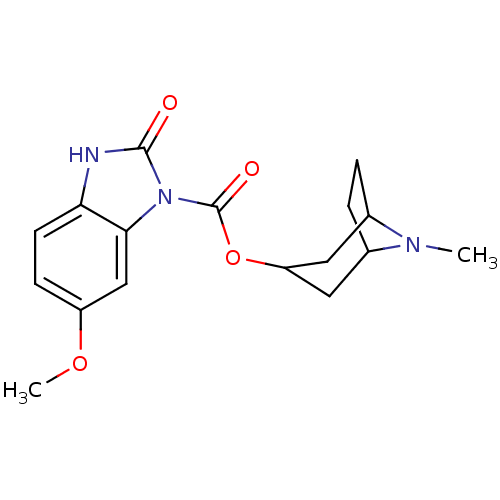
(6-Methoxy-2-oxo-2,3-dihydro-benzoimidazole-1-carbo...)Show SMILES COc1ccc2[nH]c(=O)n(C(=O)OC3CC4CCC(C3)N4C)c2c1 |TLB:12:13:20:16.17| Show InChI InChI=1S/C17H21N3O4/c1-19-10-3-4-11(19)8-13(7-10)24-17(22)20-15-9-12(23-2)5-6-14(15)18-16(20)21/h5-6,9-11,13H,3-4,7-8H2,1-2H3,(H,18,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515598

(CHEMBL4526049)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(Cc2ccc(O)c(O)c2)CC1 Show InChI InChI=1S/C22H27ClN2O4/c1-29-22-12-18(24)17(23)11-16(22)19(26)4-2-14-6-8-25(9-7-14)13-15-3-5-20(27)21(28)10-15/h3,5,10-12,14,27-28H,2,4,6-9,13,24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM50056401
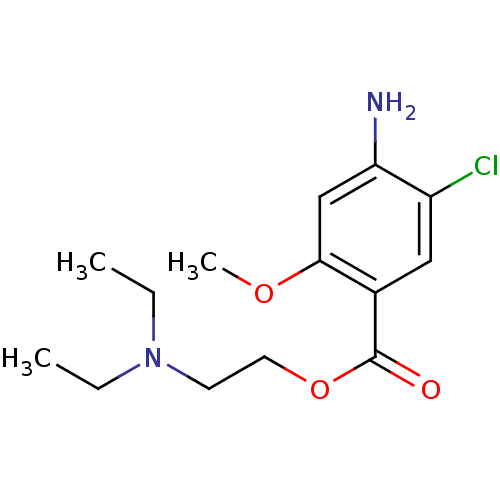
(2-diethylaminoethyl [2-methoxy-4-amino-5-chloro]be...)Show InChI InChI=1S/C14H21ClN2O3/c1-4-17(5-2)6-7-20-14(18)10-8-11(15)12(16)9-13(10)19-3/h8-9H,4-7,16H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079362

(CHEMBL3417009 | US9663465, 9)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(CC2CCCCC2)CC1 Show InChI InChI=1S/C22H33ClN2O2/c1-27-22-14-20(24)19(23)13-18(22)21(26)8-7-16-9-11-25(12-10-16)15-17-5-3-2-4-6-17/h13-14,16-17H,2-12,15,24H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
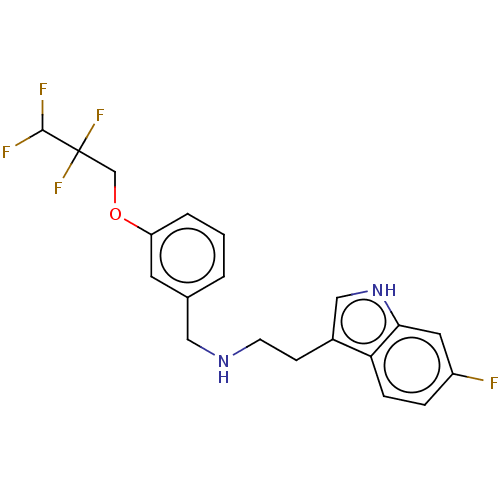
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membrane measured after 60 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113059
BindingDB Entry DOI: 10.7270/Q2KP85V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM50005833
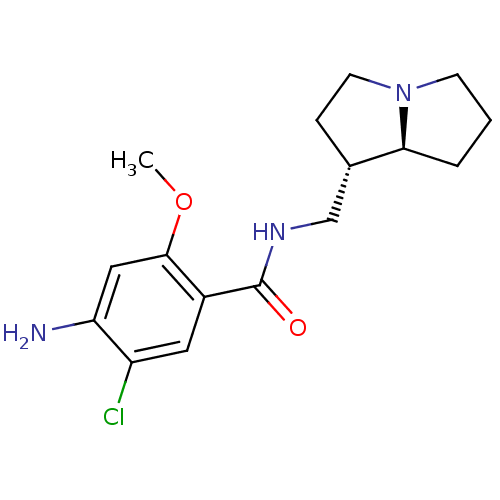
((exo)4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-9-10-4-6-20-5-2-3-14(10)20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM50056401

(2-diethylaminoethyl [2-methoxy-4-amino-5-chloro]be...)Show InChI InChI=1S/C14H21ClN2O3/c1-4-17(5-2)6-7-20-14(18)10-8-11(15)12(16)9-13(10)19-3/h8-9H,4-7,16H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(MOUSE) | BDBM50005833

((exo)4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-9-10-4-6-20-5-2-3-14(10)20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
FEBS Lett 398: 19-25 (1996)
Article DOI: 10.1016/s0014-5793(96)01132-5
BindingDB Entry DOI: 10.7270/Q228065Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079368
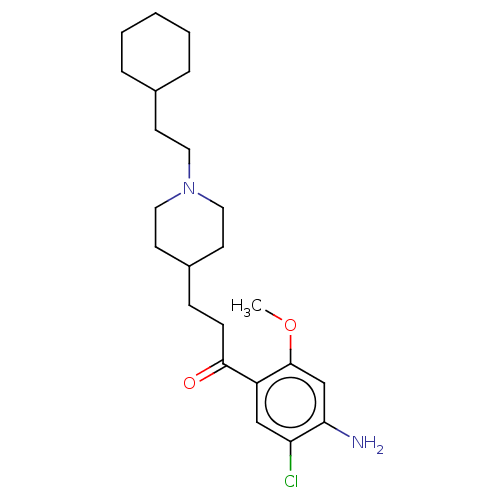
(CHEMBL3416999 | US9663465, 16)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(CCC2CCCCC2)CC1 Show InChI InChI=1S/C23H35ClN2O2/c1-28-23-16-21(25)20(24)15-19(23)22(27)8-7-18-10-13-26(14-11-18)12-9-17-5-3-2-4-6-17/h15-18H,2-14,25H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079364
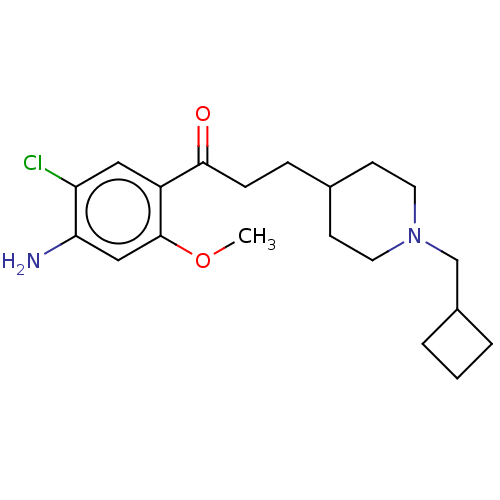
(CHEMBL3416995 | US9663465, 7)Show InChI InChI=1S/C20H29ClN2O2/c1-25-20-12-18(22)17(21)11-16(20)19(24)6-5-14-7-9-23(10-8-14)13-15-3-2-4-15/h11-12,14-15H,2-10,13,22H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM84950

(CAS_183782 | NSC_183782 | RS 67333)Show InChI InChI=1S/C19H29ClN2O2/c1-3-4-9-22-10-7-14(8-11-22)5-6-18(23)15-12-16(20)17(21)13-19(15)24-2/h12-14H,3-11,21H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50007863

(6-Methoxy-2-oxo-2,3-dihydro-benzoimidazole-1-carbo...)Show SMILES COc1ccc2[nH]c(=O)n(C(=O)OC3CC4CCC(C3)N4C)c2c1 |TLB:12:13:20:16.17| Show InChI InChI=1S/C17H21N3O4/c1-19-10-3-4-11(19)8-13(7-10)24-17(22)20-15-9-12(23-2)5-6-14(15)18-16(20)21/h5-6,9-11,13H,3-4,7-8H2,1-2H3,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UPR 9023
Curated by PDSP Ki Database
| |
Neuroreport 8: 3189-96 (1997)
Article DOI: 10.1097/00001756-199710200-00002
BindingDB Entry DOI: 10.7270/Q2TD9VWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50562651

(CHEMBL4788250) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membrane measured after 60 mins |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113059
BindingDB Entry DOI: 10.7270/Q2KP85V3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515600
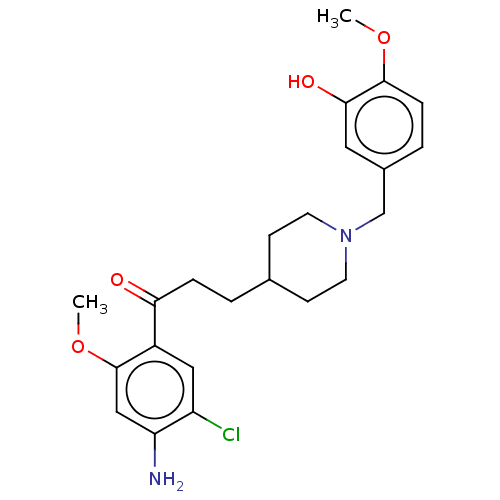
(CHEMBL4580044)Show SMILES COc1ccc(CN2CCC(CCC(=O)c3cc(Cl)c(N)cc3OC)CC2)cc1O Show InChI InChI=1S/C23H29ClN2O4/c1-29-22-6-4-16(11-21(22)28)14-26-9-7-15(8-10-26)3-5-20(27)17-12-18(24)19(25)13-23(17)30-2/h4,6,11-13,15,28H,3,5,7-10,14,25H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50304003

(1-Naphthyl(4-piperazin-1-yl-1H-benzimidazol-2-yl)m...)Show SMILES O=C(c1nc2c(cccc2[nH]1)N1CCNCC1)c1cccc2ccccc12 Show InChI InChI=1S/C22H20N4O/c27-21(17-8-3-6-15-5-1-2-7-16(15)17)22-24-18-9-4-10-19(20(18)25-22)26-13-11-23-12-14-26/h1-10,23H,11-14H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells by liquid scintillation counting |
J Med Chem 53: 1357-69 (2010)
Article DOI: 10.1021/jm901672k
BindingDB Entry DOI: 10.7270/Q2Z320KQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079370
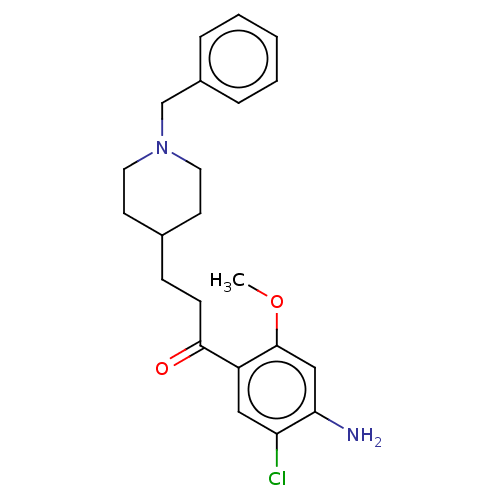
(CHEMBL3417001)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C22H27ClN2O2/c1-27-22-14-20(24)19(23)13-18(22)21(26)8-7-16-9-11-25(12-10-16)15-17-5-3-2-4-6-17/h2-6,13-14,16H,7-12,15,24H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50079363
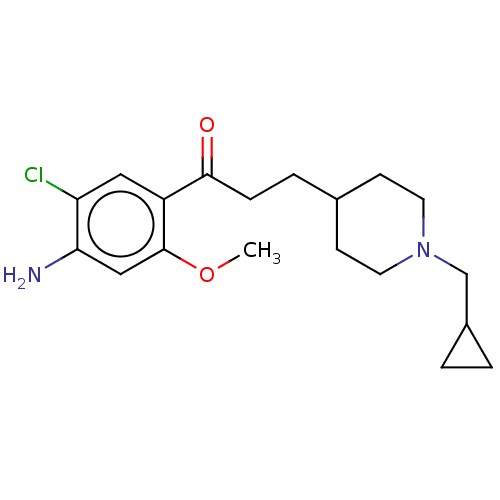
(CHEMBL3414597 | US9663465, 6)Show InChI InChI=1S/C19H27ClN2O2/c1-24-19-11-17(21)16(20)10-15(19)18(23)5-4-13-6-8-22(9-7-13)12-14-2-3-14/h10-11,13-14H,2-9,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay |
J Med Chem 58: 3172-87 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00115
BindingDB Entry DOI: 10.7270/Q2HQ41MH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data