

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM719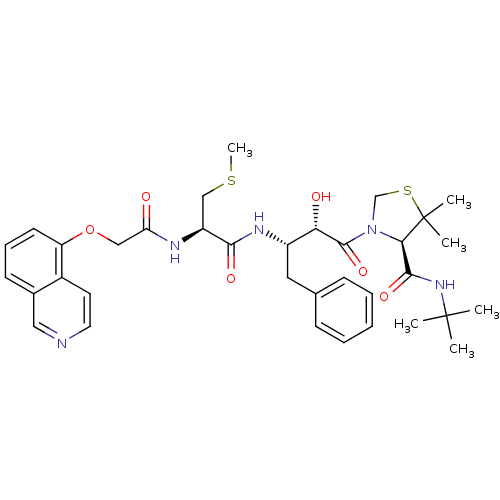 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00230 | -69.1 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute | Assay Description Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... | Antimicrob Agents Chemother 37: 810-7 (1993) Article DOI: 10.1128/aac.37.4.810 BindingDB Entry DOI: 10.7270/Q2KH0KHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM200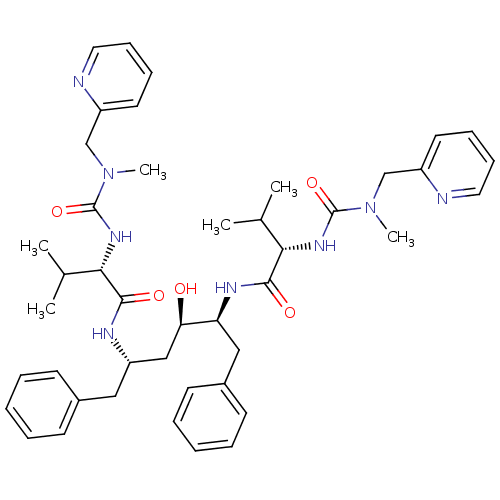 ((2S)-N-[(2S,3R,5S)-3-hydroxy-5-[(2S)-3-methyl-2-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.00400 | -66.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Am Chem Soc 116: 847-55 (1994) Article DOI: 10.1021/ja00082a004 BindingDB Entry DOI: 10.7270/Q2KK98Z1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM579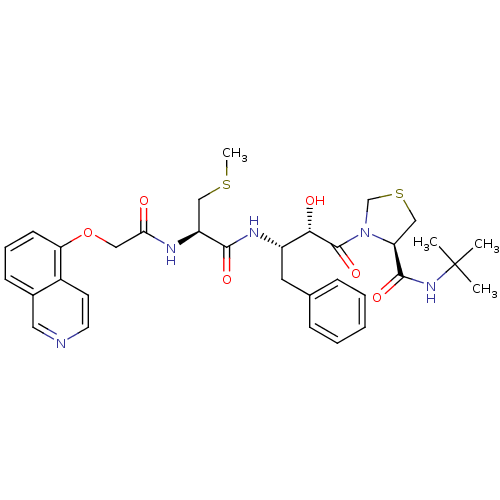 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00550 | -66.9 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute | Assay Description Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... | Antimicrob Agents Chemother 37: 810-7 (1993) Article DOI: 10.1128/aac.37.4.810 BindingDB Entry DOI: 10.7270/Q2KH0KHT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM793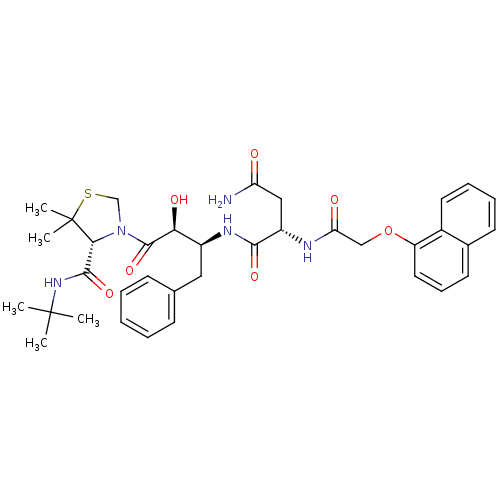 ((2S)-N-[(2S,3S)-4-[(4R)-4-(tert-butylcarbamoyl)-5,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00680 | -66.3 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute | Assay Description Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... | Antimicrob Agents Chemother 37: 810-7 (1993) Article DOI: 10.1128/aac.37.4.810 BindingDB Entry DOI: 10.7270/Q2KH0KHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073270 (CHEMBL333781 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM198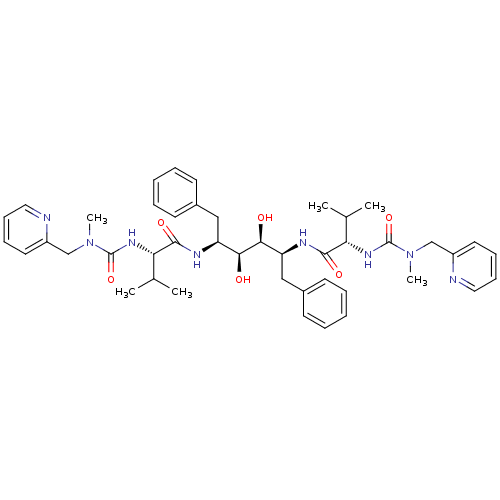 ((2S)-N-[(2S,3S,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article | 0.0110 | -63.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Am Chem Soc 116: 847-55 (1994) Article DOI: 10.1021/ja00082a004 BindingDB Entry DOI: 10.7270/Q2KK98Z1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM199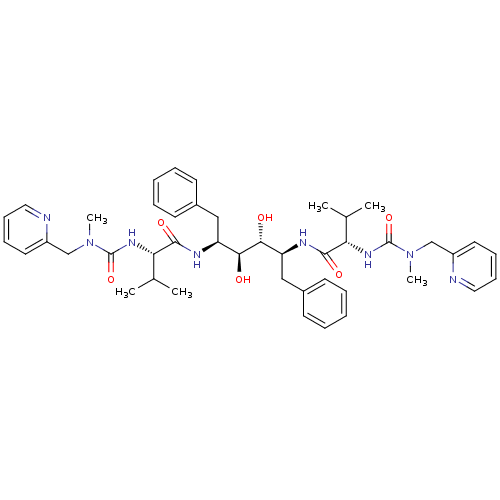 ((2S)-N-[(2S,3R,4S,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.0120 | -63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Am Chem Soc 116: 847-55 (1994) Article DOI: 10.1021/ja00082a004 BindingDB Entry DOI: 10.7270/Q2KK98Z1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073266 (CHEMBL119898 | {2-[(1S,3S,4S)-1-Benzyl-4-((2S,3S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073253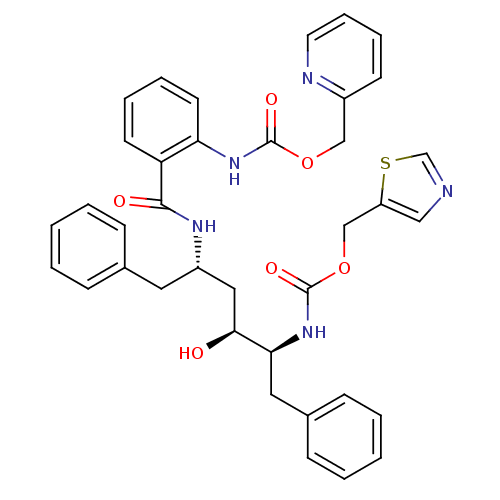 (CHEMBL278935 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073250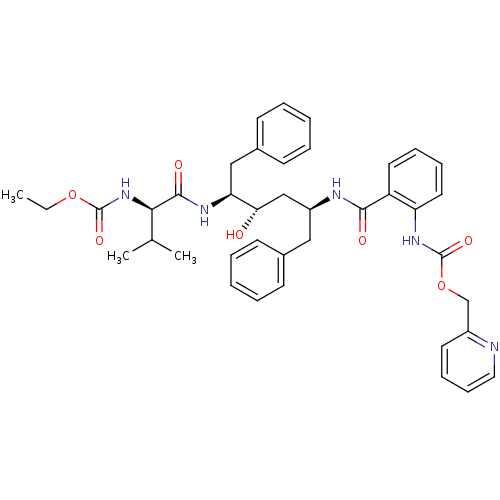 (CHEMBL333420 | {2-[(1S,3S,4S)-1-Benzyl-4-((R)-2-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50409174 (CHEMBL169119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073269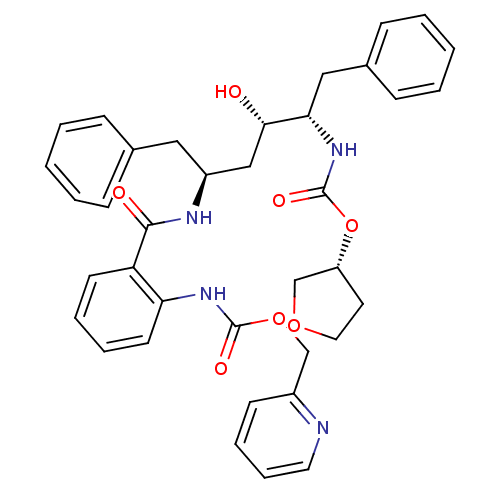 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-5-phenyl-4-[(R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254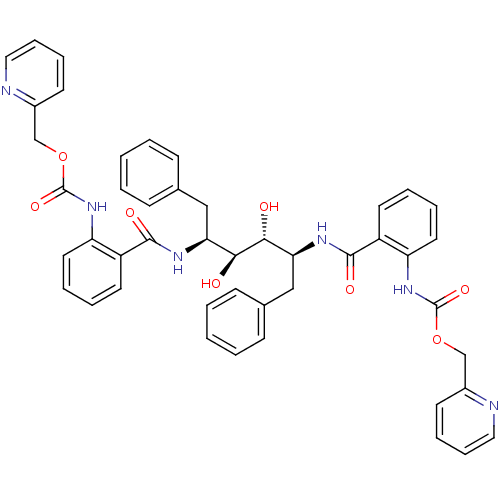 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073252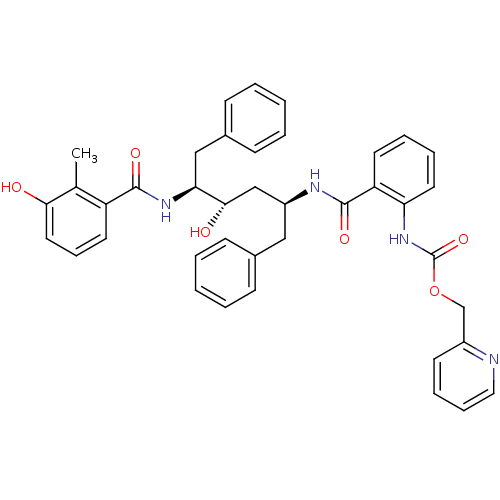 (CHEMBL324157 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527524 (CHEMBL4566742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285701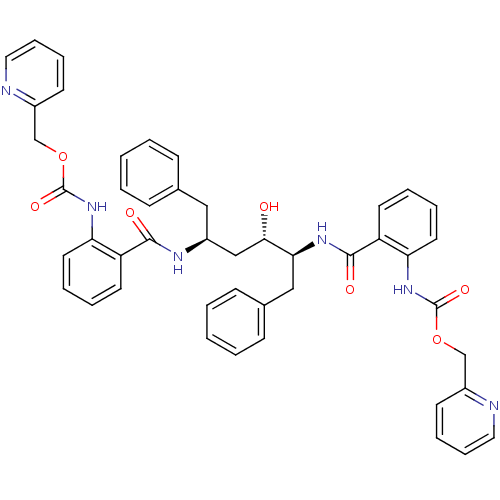 (Anthranilamide derivative | CHEMBL313767) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM465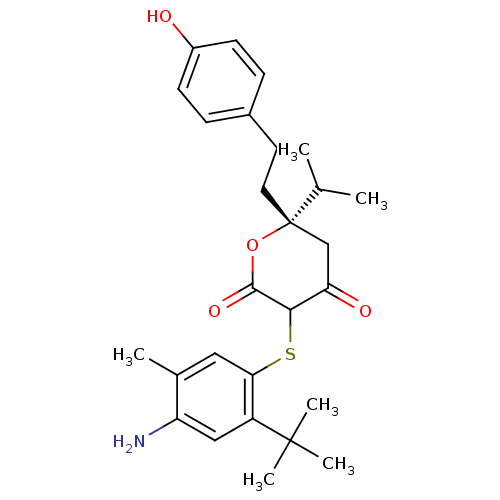 ((6S)-3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073265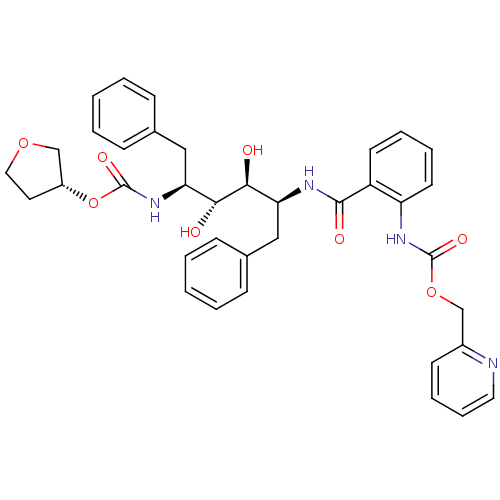 ((2-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-5-phenyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113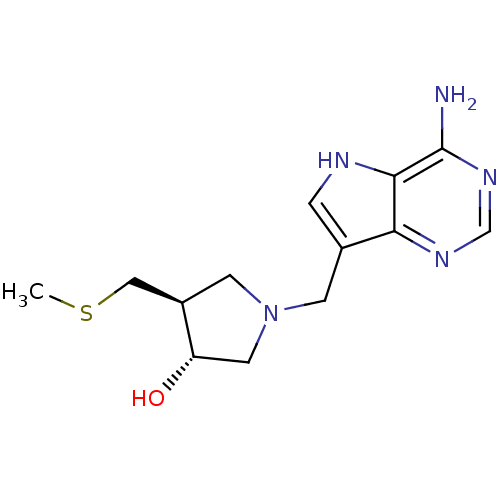 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP assessed as reduction in methylthioadenosine phosphorolysis/hydrolysis | Bioorg Med Chem 20: 5181-7 (2012) Article DOI: 10.1016/j.bmc.2012.07.006 BindingDB Entry DOI: 10.7270/Q2XG9S6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073268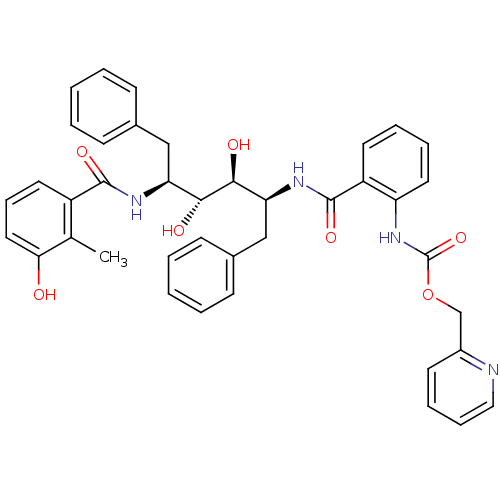 (CHEMBL331294 | {2-[(1S,2S,3R,4S)-1-Benzyl-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197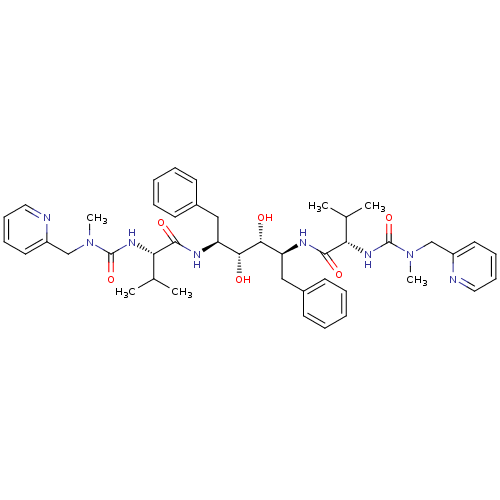 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.112 | -57.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
NCI-FCRDC | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Am Chem Soc 116: 847-55 (1994) Article DOI: 10.1021/ja00082a004 BindingDB Entry DOI: 10.7270/Q2KK98Z1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073263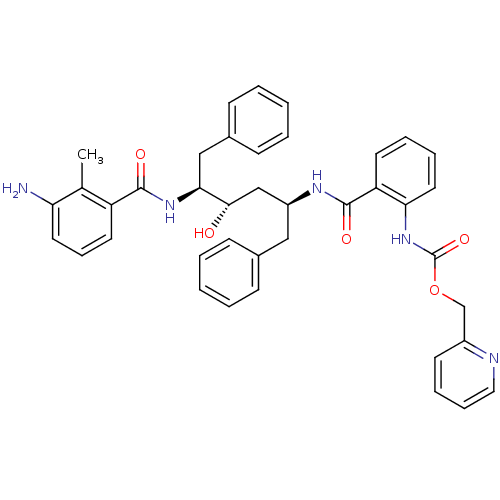 (CHEMBL117629 | {2-[(1S,3S,4S)-4-(3-Amino-2-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527552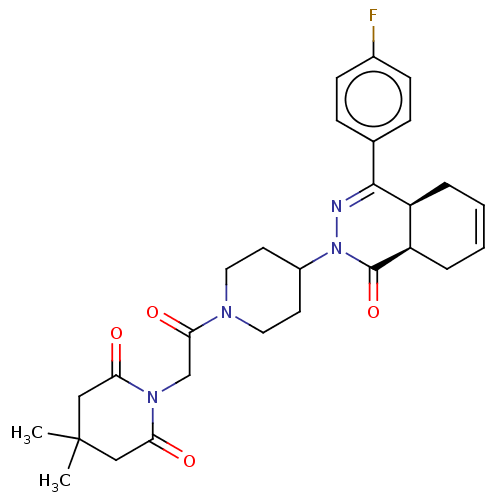 (CHEMBL4435111) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527532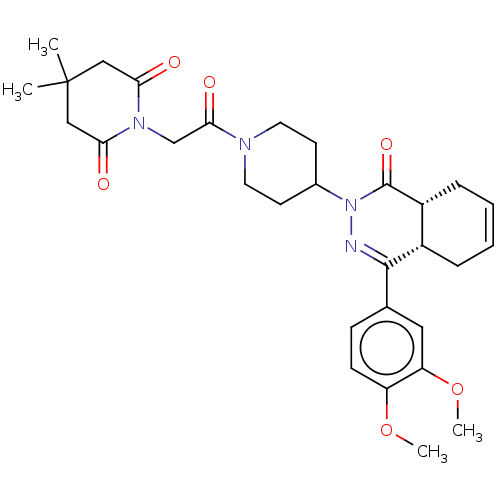 (CHEMBL4441748) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073244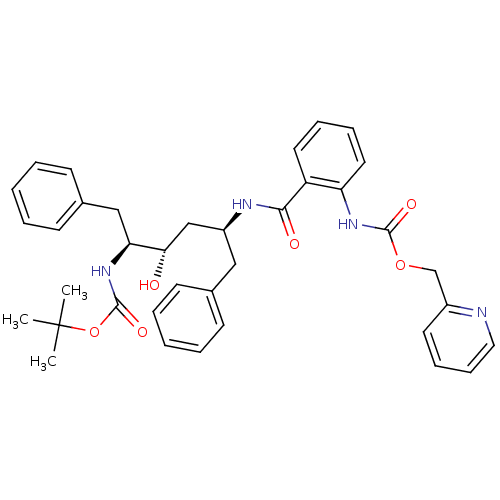 (CHEMBL332825 | [2-((1S,3S,4S)-1-Benzyl-4-tert-buto...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073262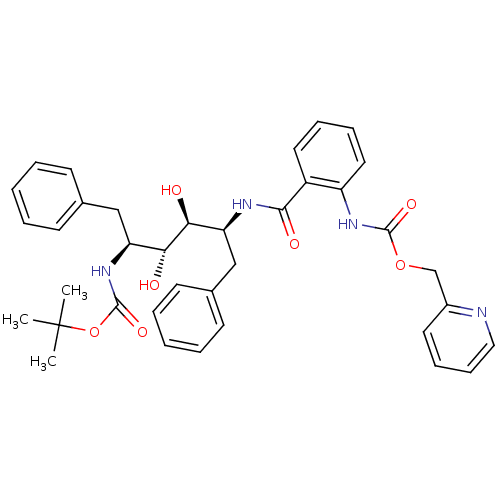 (CHEMBL333290 | [2-((1S,2S,3R,4S)-1-Benzyl-4-tert-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527531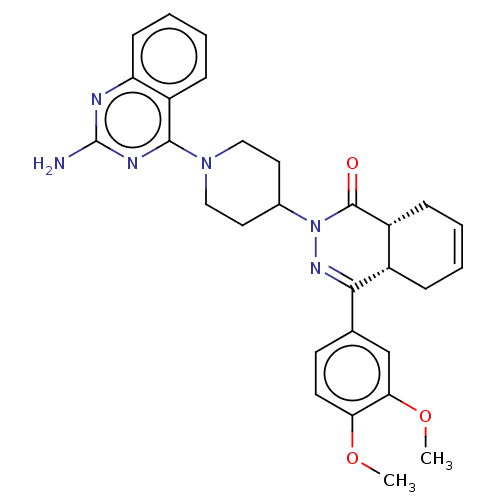 (CHEMBL4524402) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527537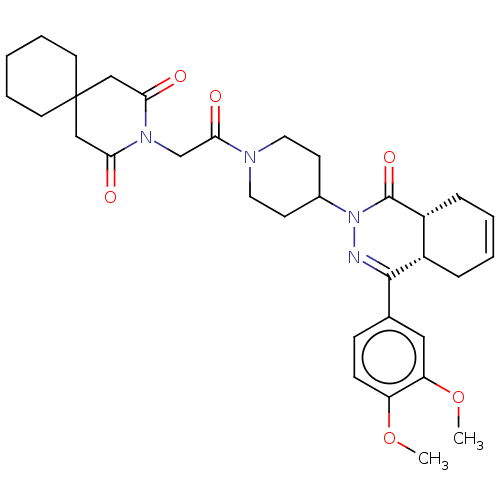 (CHEMBL4453005) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064201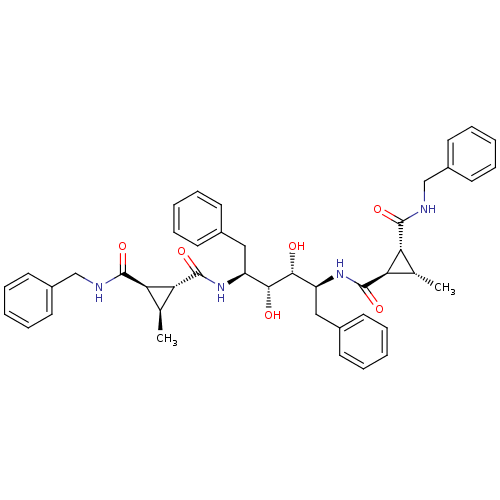 (1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoyl-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527528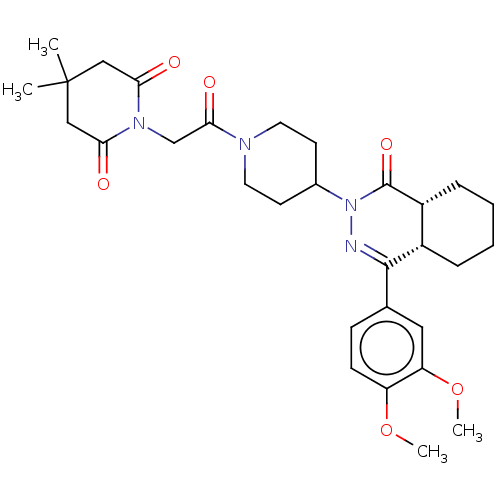 (CHEMBL4441871) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527550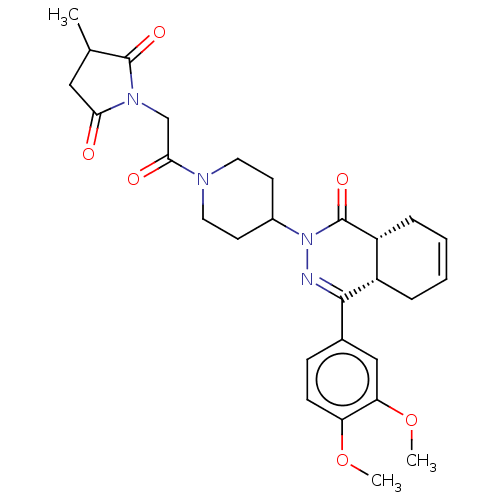 (CHEMBL4461456) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064202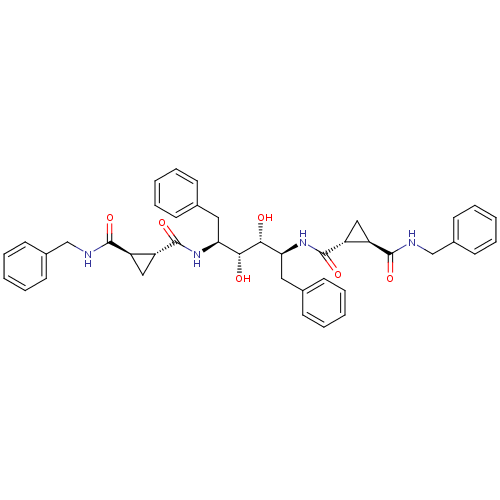 (1N-benzyl-2N-[1-benzyl-4-(2-benzylcarbamoylcyclopr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM469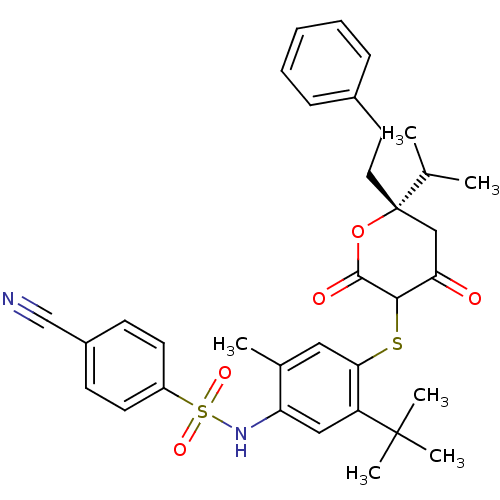 (CHEMBL2110206 | Dihydropyran-2-one deriv. 74 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM469 (CHEMBL2110206 | Dihydropyran-2-one deriv. 74 | N-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM402 (CHEMBL354027 | Dihydropyran-2-one deriv. 7 | N-[5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | -57.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM402 (CHEMBL354027 | Dihydropyran-2-one deriv. 7 | N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527535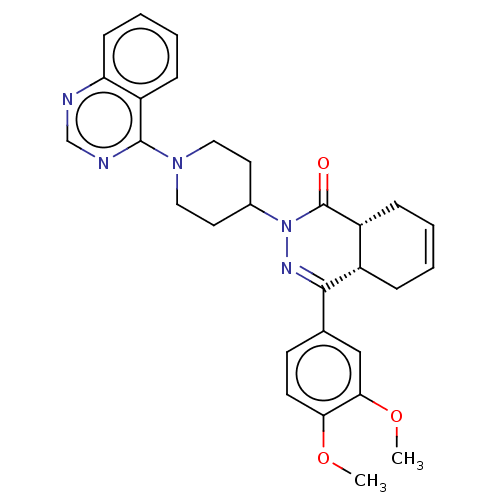 (CHEMBL4589927) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527533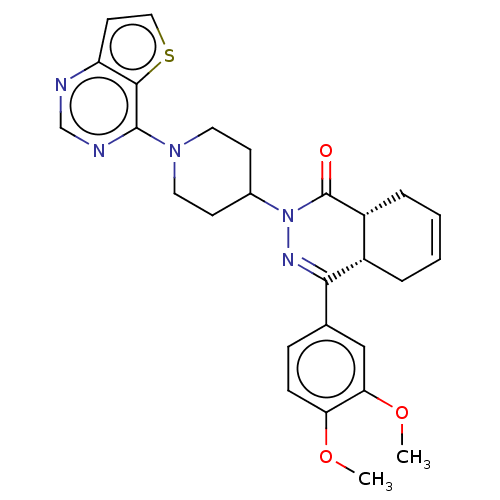 (CHEMBL4592553) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285698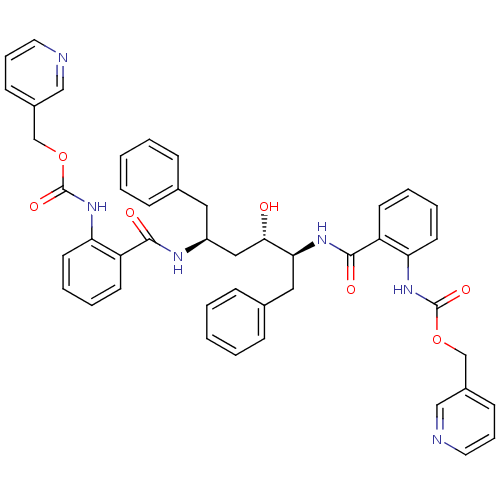 (Anthranilamide derivative | CHEMBL315500) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073248 (CHEMBL119745 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527555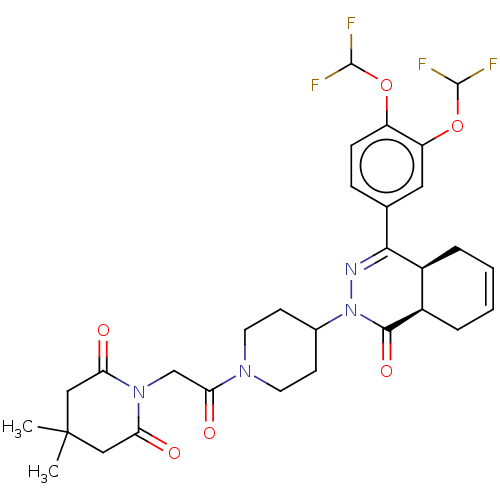 (CHEMBL4530777) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM470 (CHEMBL2110205 | Dihydropyran-2-one deriv. 75 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064199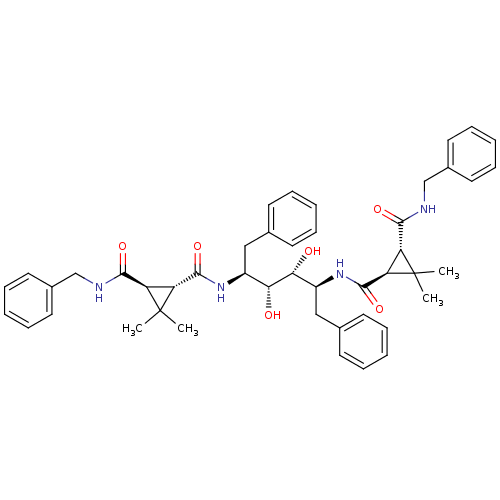 (1N-benzyl-2N-[1-benzyl-4-(3-benzylcarbamoyl-2,2-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick Biomedical Supercomputing Center Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured against wild-type HIV-1 protease | J Med Chem 41: 1581-97 (1998) Article DOI: 10.1021/jm980033d BindingDB Entry DOI: 10.7270/Q27S7MWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527567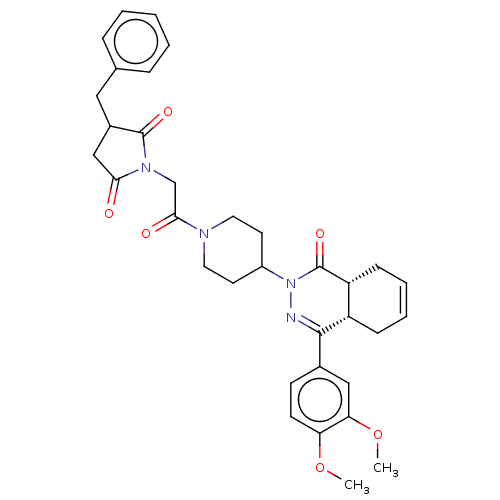 (CHEMBL4473426) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527560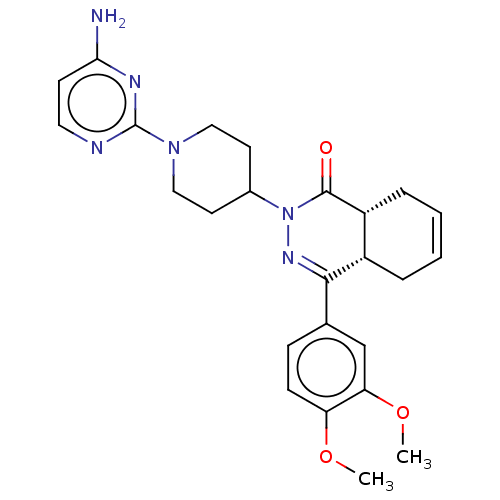 (CHEMBL4444578) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1995 total ) | Next | Last >> |