

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50002087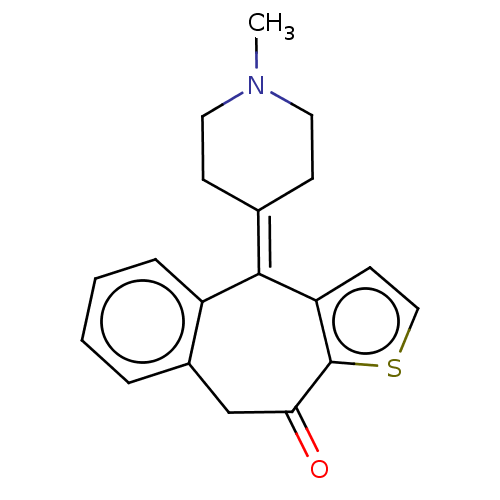 (4-(1-Methyl-piperidin-4-ylidene)-4,9-dihydro-1-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417376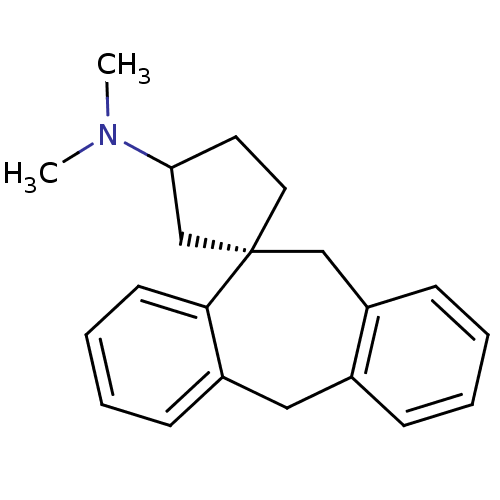 (CHEMBL1278116) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50330747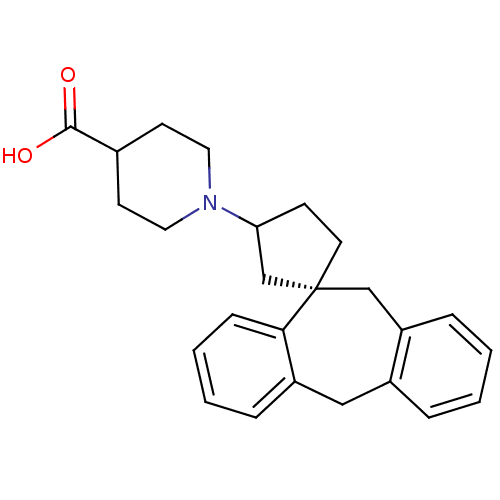 ((-)-1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50330752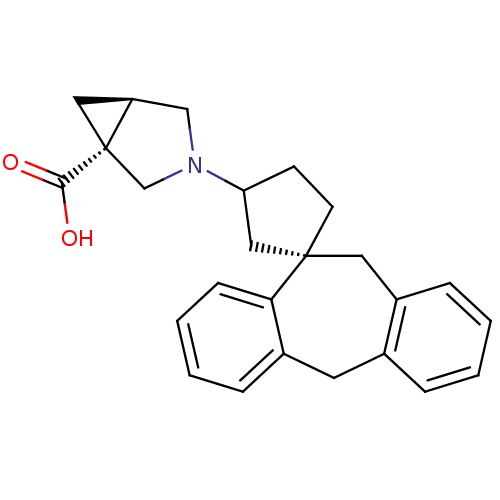 (3-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50330751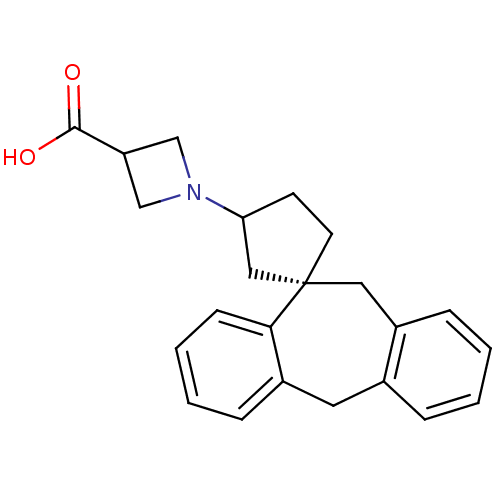 (1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50330751 (1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50330748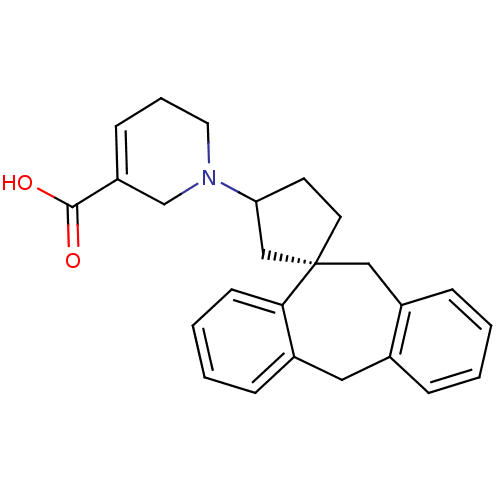 (1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417355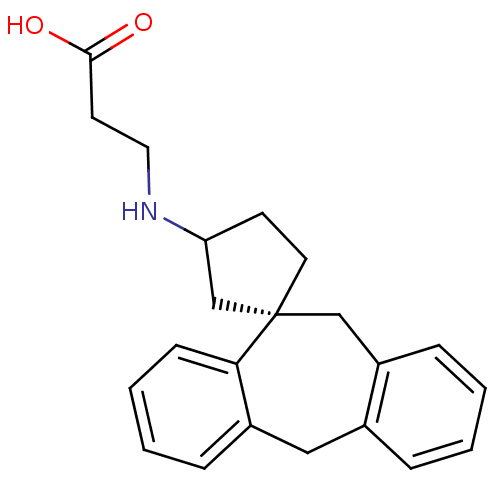 (CHEMBL1278114) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50330753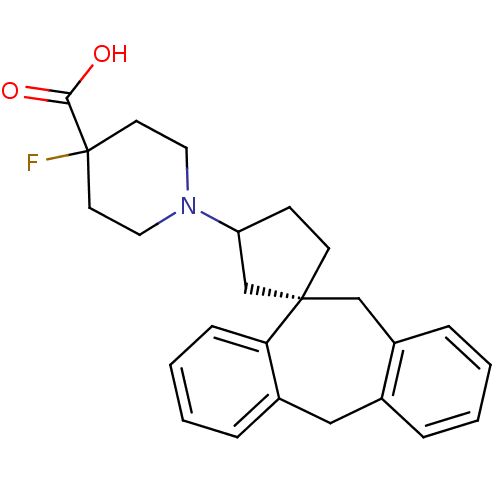 (1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417365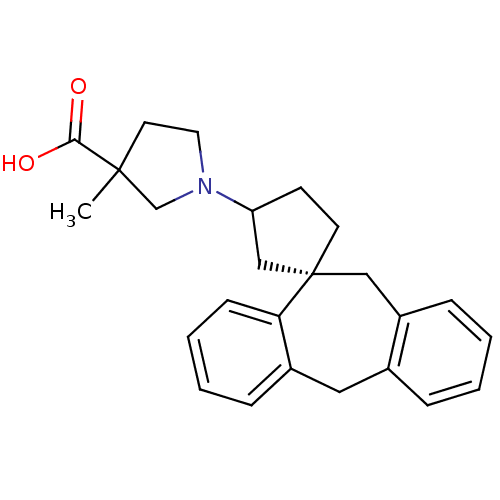 (CHEMBL1278202) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50330749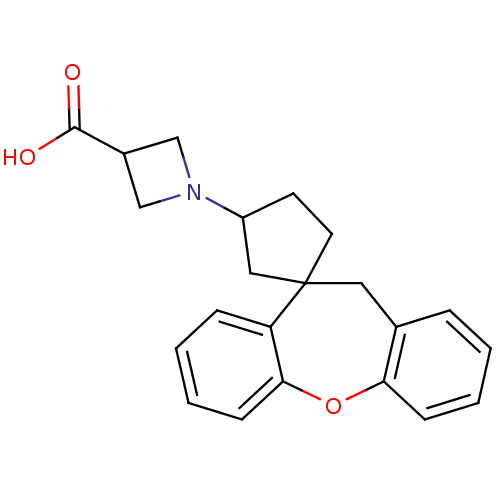 (1-(11'H-Spiro[cyclopentane-1,10'-dibenzo[b,f]oxepi...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417373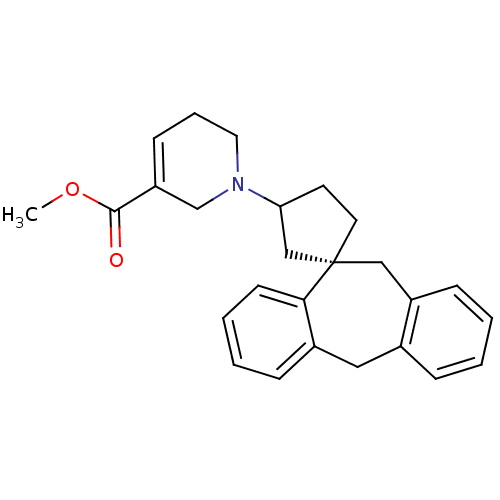 (CHEMBL1278020) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417366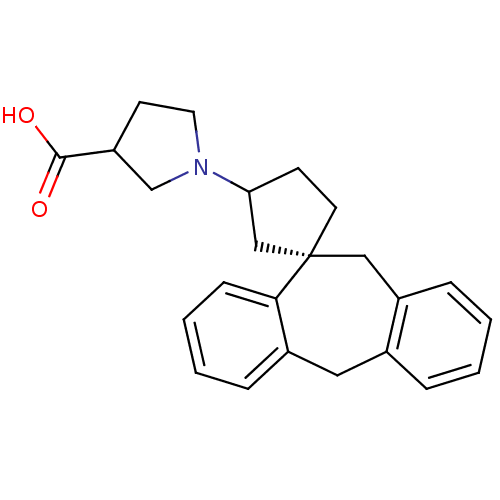 (CHEMBL1278201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417369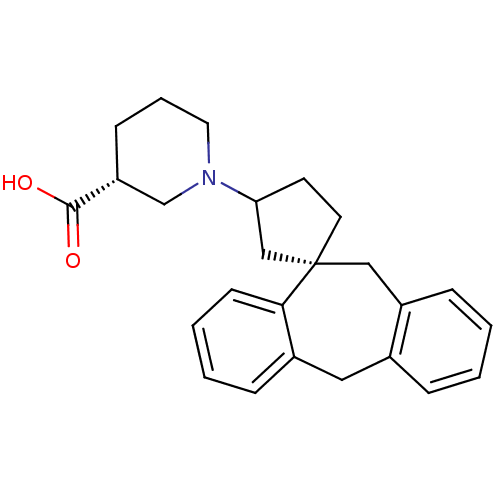 (CHEMBL1277584) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (RAT) | BDBM50330750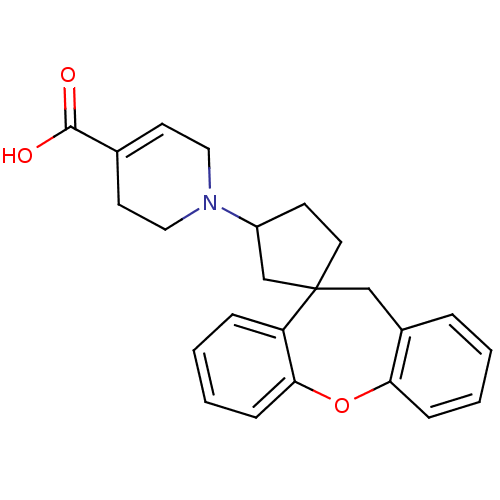 (CHEMBL1277126) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]pyrilamine from histamine H1 receptor in Sprague-Dawley rat cortical membrane by liquid scintillation counting | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50330748 (1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50330748 (1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417370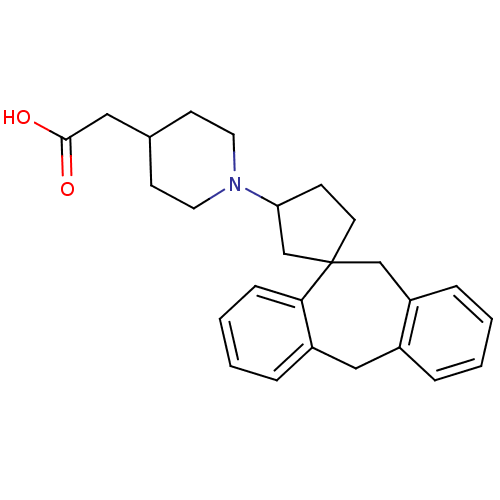 (CHEMBL1277585) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417375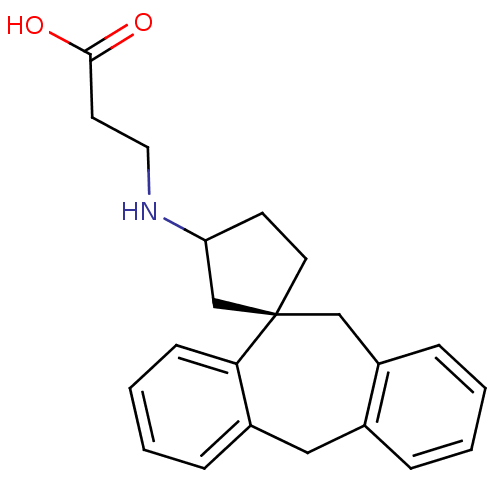 (CHEMBL1278115) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417364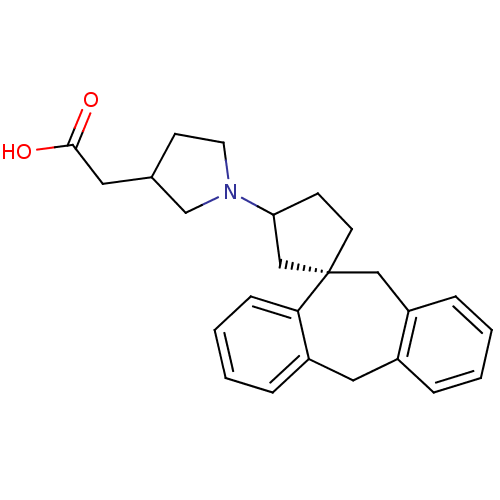 (CHEMBL1276859) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50330752 (3-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417372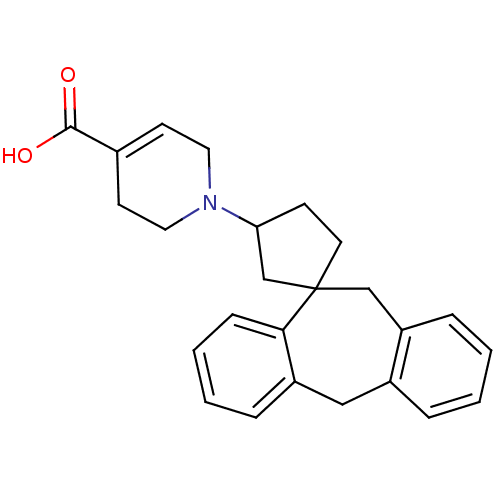 (CHEMBL1277854) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50330753 (1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417363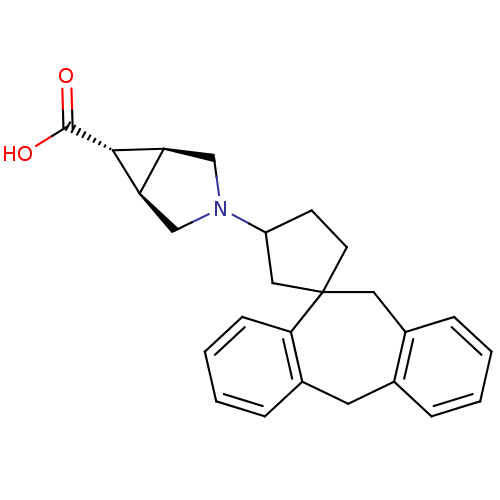 (CHEMBL1276860) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417381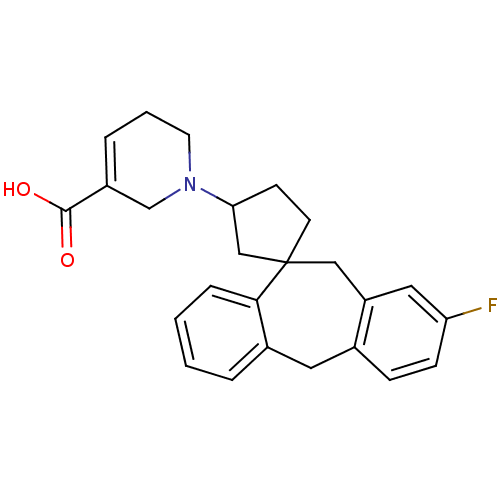 (CHEMBL1277855) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50330747 ((-)-1-((1S)-5',11'-Dihydrospiro[cyclopentane-1,10'...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417371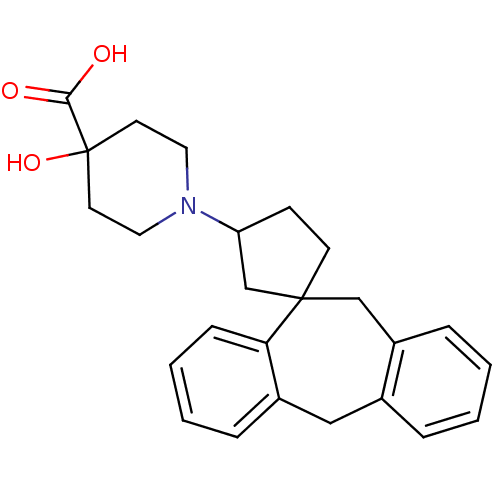 (CHEMBL1277679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50330750 (CHEMBL1277126) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417380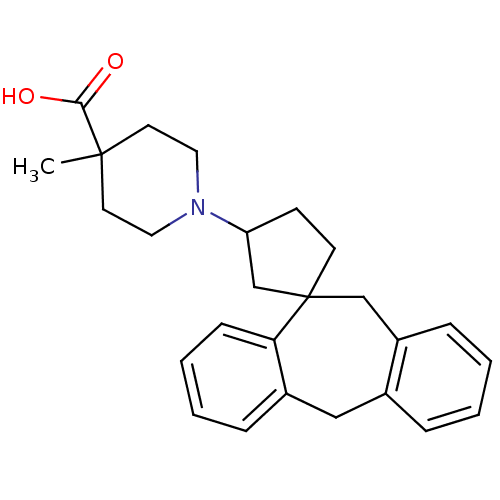 (CHEMBL1277769) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50330749 (1-(11'H-Spiro[cyclopentane-1,10'-dibenzo[b,f]oxepi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417382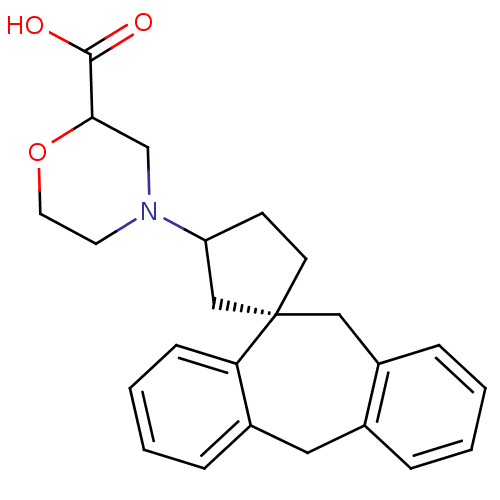 (CHEMBL1278112) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417378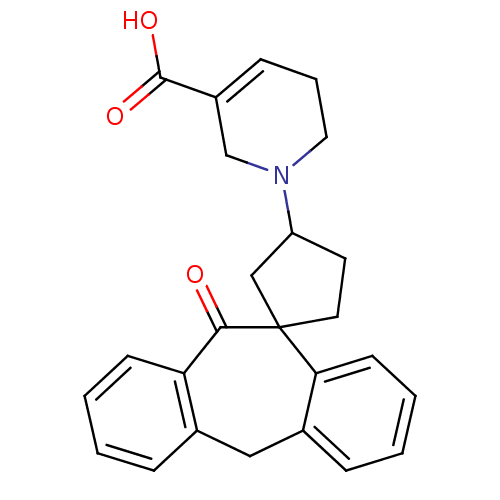 (CHEMBL1277400) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417359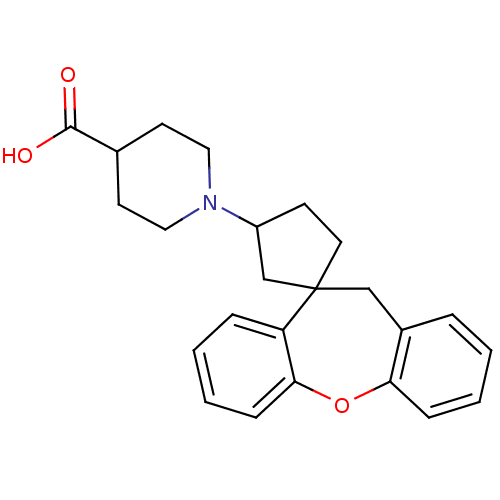 (CHEMBL1277216) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417360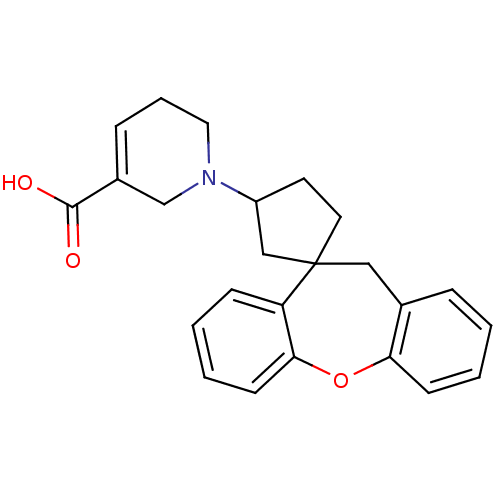 (CHEMBL1277125) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417367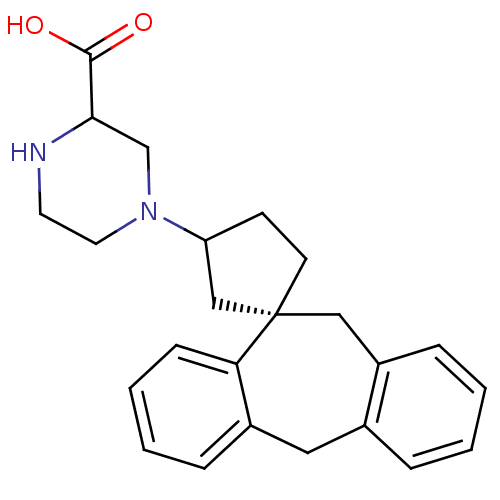 (CHEMBL1278113) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417358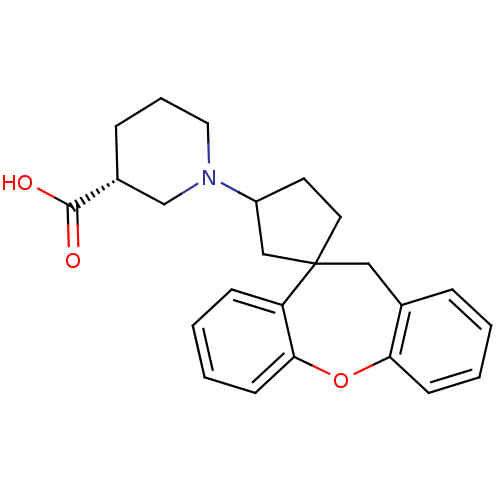 (CHEMBL1277311) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417374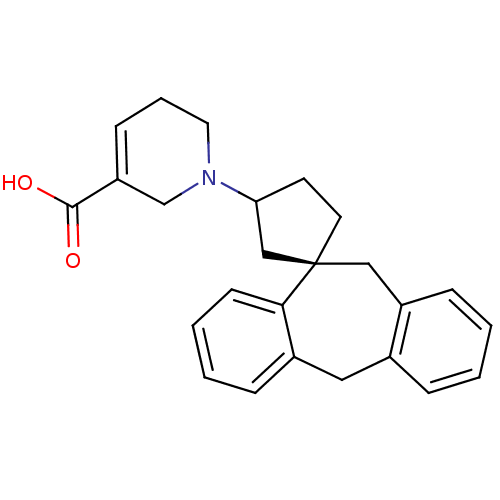 (CHEMBL1278021) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417379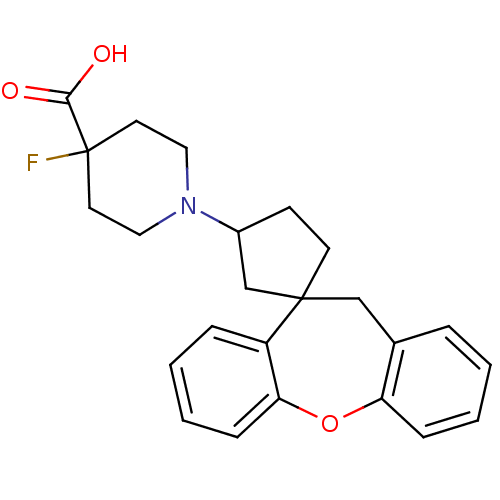 (CHEMBL1277217) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417377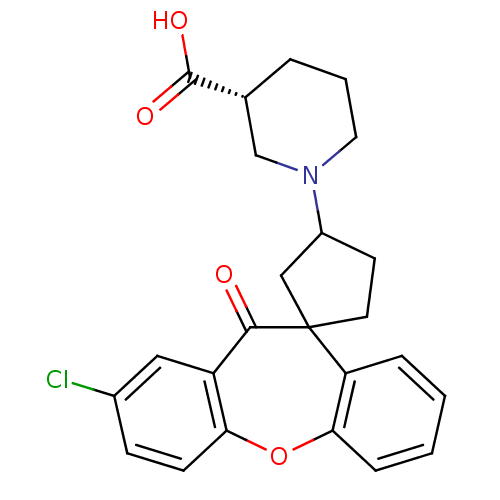 (CHEMBL1277492) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417361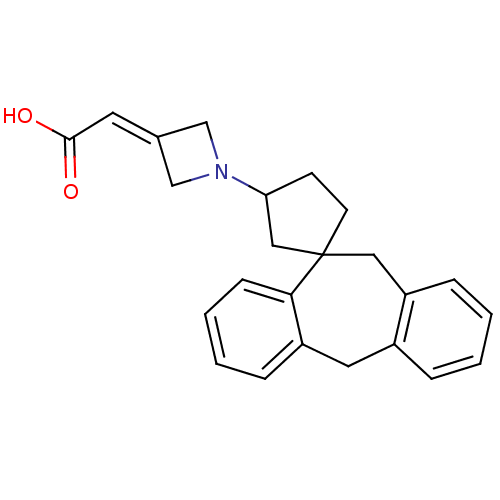 (CHEMBL1277035) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417356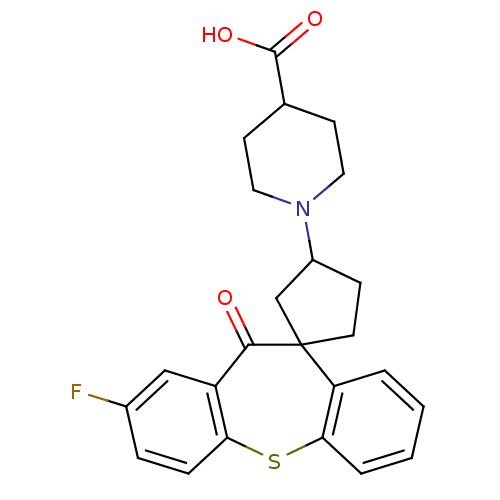 (CHEMBL1277491) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417362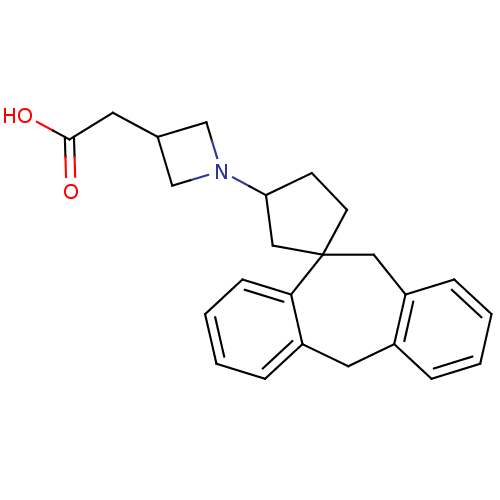 (CHEMBL1277034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417368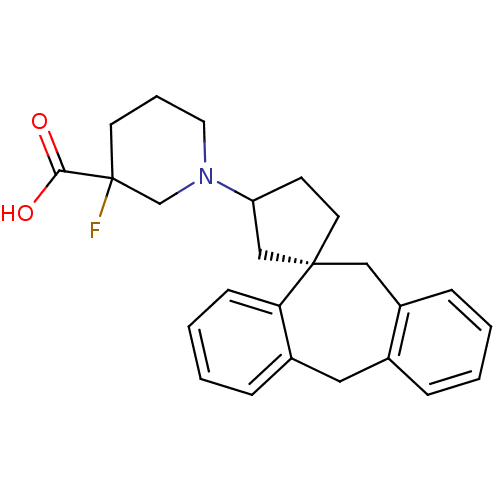 (CHEMBL1278019) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50095027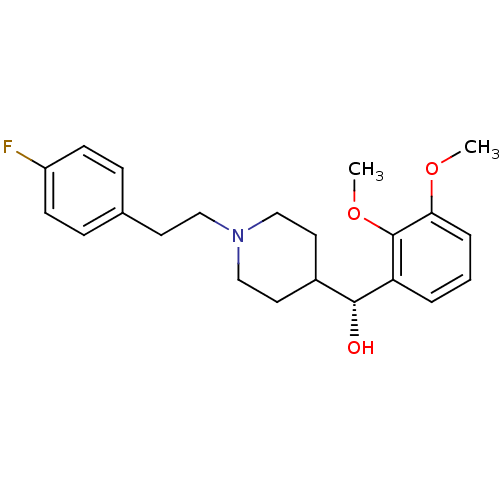 ((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50417357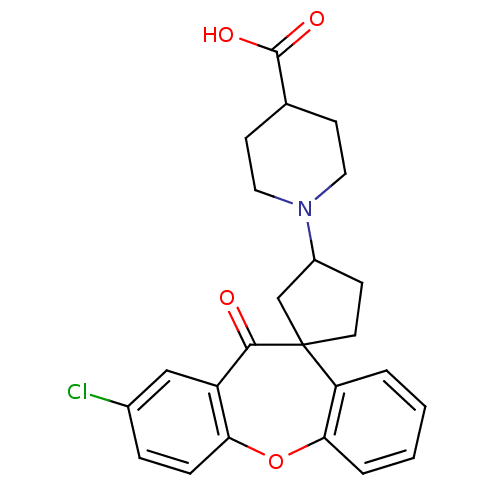 (CHEMBL1277401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human histamine H1 receptor expressed in CHO cells by FLIPR assay | J Med Chem 53: 7778-95 (2010) Article DOI: 10.1021/jm100856p BindingDB Entry DOI: 10.7270/Q2FQ9WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259706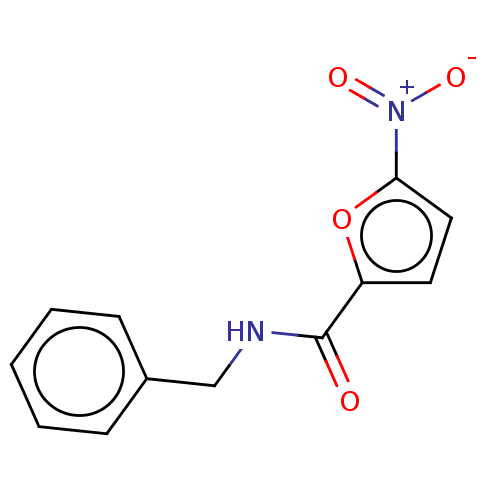 (CHEMBL2313314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259707 (CHEMBL4091464) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259704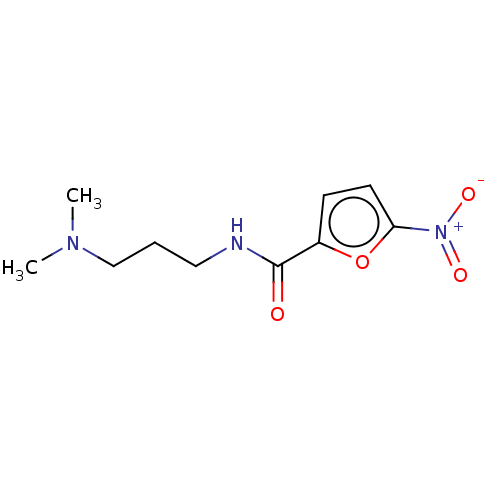 (CHEMBL4066587) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259705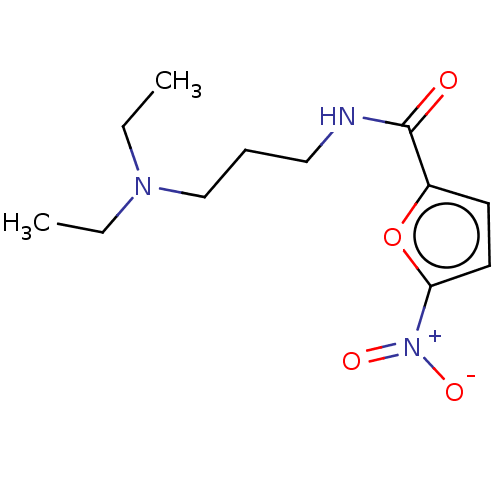 (CHEMBL4088638) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50259703 (CHEMBL4084206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Litoral Curated by ChEMBL | Assay Description Uncompetitive inhibition of Trypanosoma cruzi trypanothione reductase using T(SH)2 as substrate at pH 7.5 in presence of NADPH by photometric method | Eur J Med Chem 125: 1088-1097 (2017) Article DOI: 10.1016/j.ejmech.2016.10.055 BindingDB Entry DOI: 10.7270/Q2TM7DKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 223 total ) | Next | Last >> |