

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127171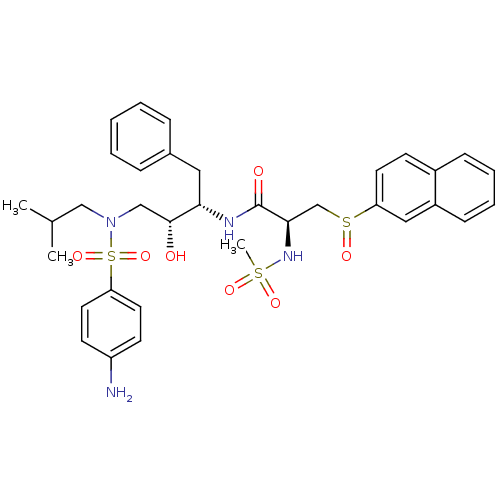 (CHEMBL299578 | N-{3-[(4-Amino-benzenesulfonyl)-iso...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262566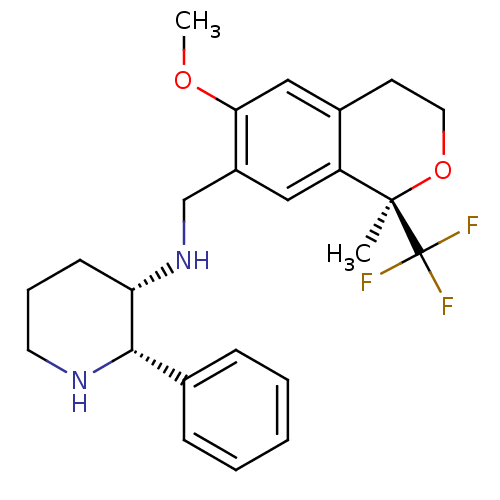 ((2S,3S)-3-[(1R)-6-Methoxy-1-methyl-1-trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127172 (4-Amino-N-[3-benzyl-2-hydroxy-6-methanesulfonylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50067935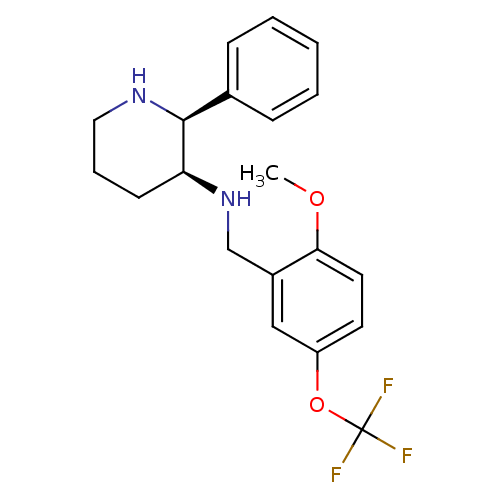 ((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262395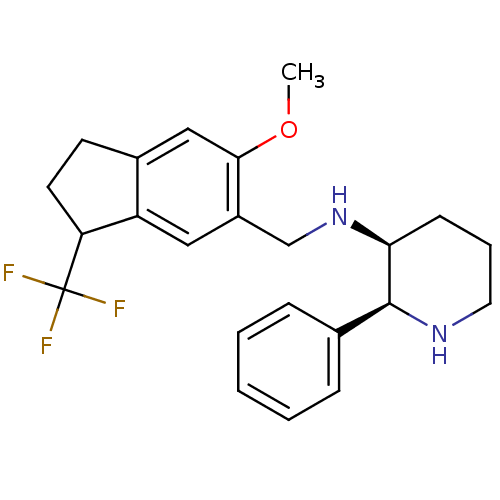 (CHEMBL478620 | R/S-(2S,3S)-N-((6-methoxy-3-(triflu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262280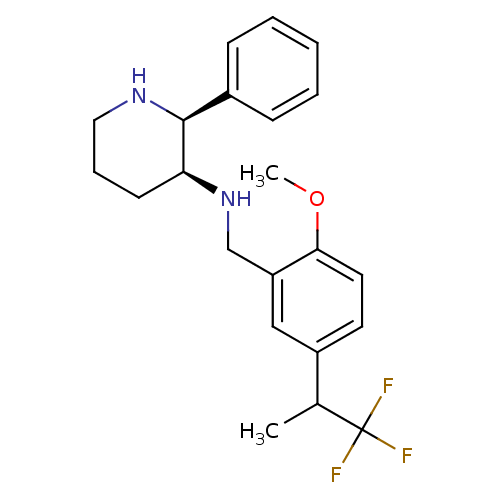 (CHEMBL513351 | R/S-(2S,3S)-N-(2-methoxy-5-(1,1,1-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262510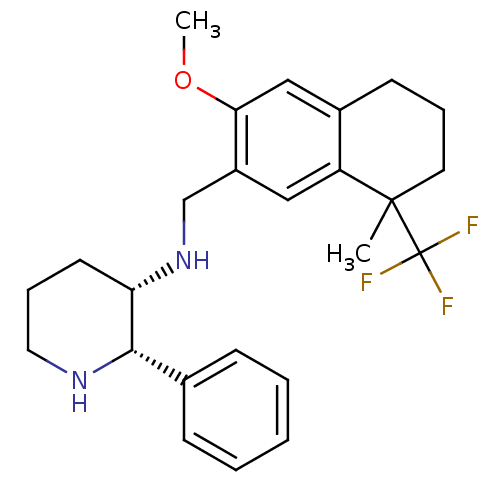 (CHEMBL477365 | R/S-(2S,3S)-3-[((6-Methoxy-1-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262567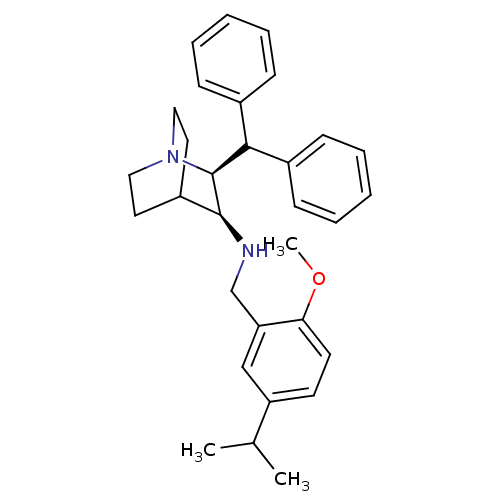 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262279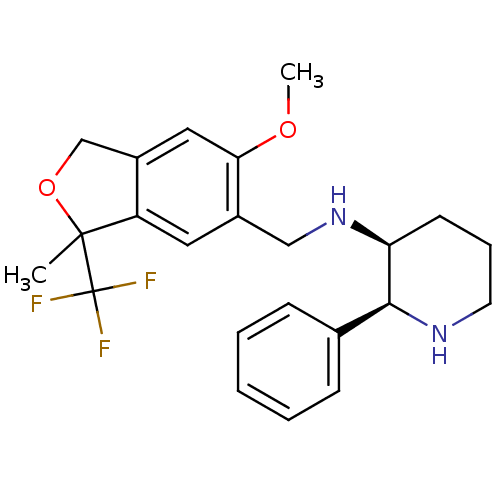 ((2S,3S)-N-((6-methoxy-3-methyl-3-(trifluoromethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50224198 ((2S,3S)-N-(2-methoxy-5-(1,1,1-trifluoro-2-methylpr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262337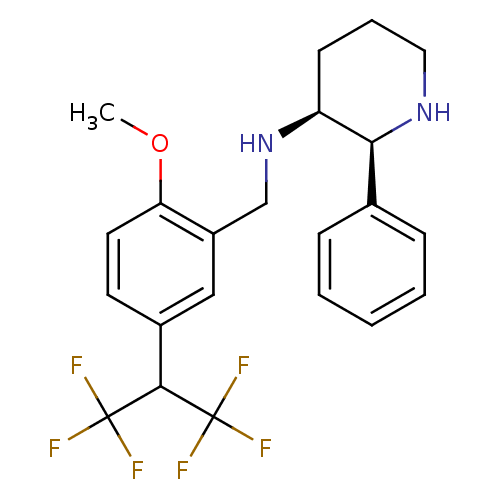 ((2S,3S)-N-(5-(1,1,1,3,3,3-hexafluoropropan-2-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Rattus norvegicus) | BDBM50420231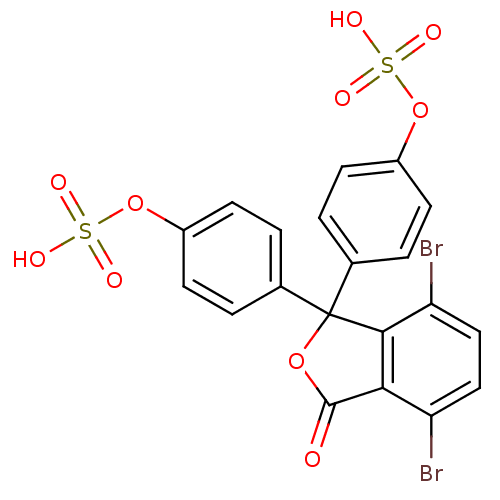 (CHEMBL2074600) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in Oat1-expressing LLC-PK1 cells | J Pharmacol Exp Ther 300: 746-53 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9XW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Rattus norvegicus) | BDBM50420231 (CHEMBL2074600) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Pravastatin uptake in Oat3-expressing LLC-PK1 cells | J Pharmacol Exp Ther 300: 746-53 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9XW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50262281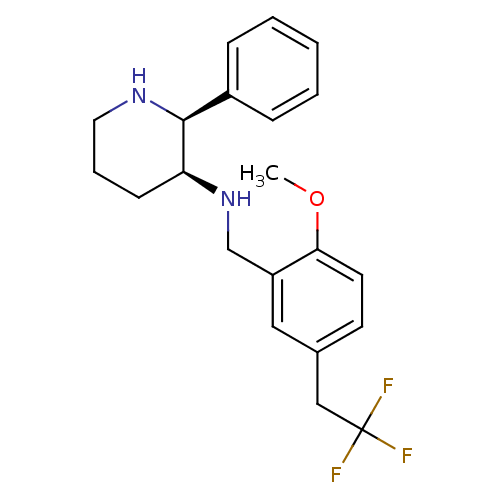 ((2S,3S)-3-[2-Methoxy-5-(2,2,2-trifluoroethyl)benzy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human CYP2D6 using bufuralol as substrate | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Rattus norvegicus) | BDBM50022787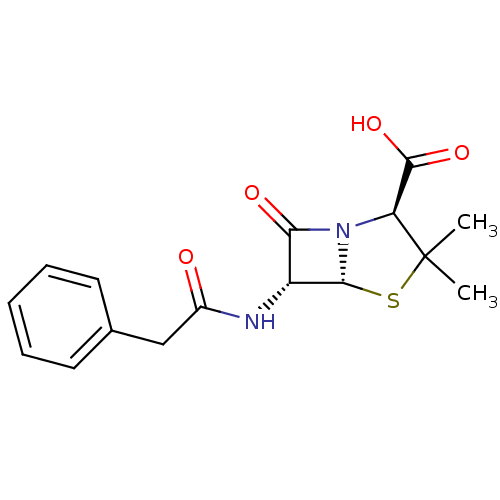 ((+)-3,3-Dimethyl-7-oxo-6-phenylacetylamino-4-thia-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 5.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Pravastatin uptake in Oat3-expressing LLC-PK1 cells | J Pharmacol Exp Ther 300: 746-53 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9XW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Rattus norvegicus) | BDBM50022787 ((+)-3,3-Dimethyl-7-oxo-6-phenylacetylamino-4-thia-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in Oat1-expressing LLC-PK1 cells | J Pharmacol Exp Ther 300: 746-53 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9XW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 6 (Rattus norvegicus) | BDBM20688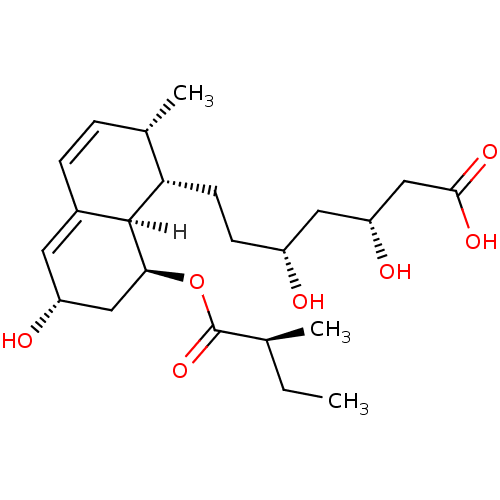 ((3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.15E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in Oat1-expressing LLC-PK1 cells | J Pharmacol Exp Ther 300: 746-53 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9XW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Rattus norvegicus) | BDBM50270006 (2-(4-aminobenzamido)acetate | AMINOHIPPURATE) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 1.35E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Pravastatin uptake in Oat3-expressing LLC-PK1 cells | J Pharmacol Exp Ther 300: 746-53 (2002) BindingDB Entry DOI: 10.7270/Q2FQ9XW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262279 ((2S,3S)-N-((6-methoxy-3-methyl-3-(trifluoromethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50224198 ((2S,3S)-N-(2-methoxy-5-(1,1,1-trifluoro-2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50261732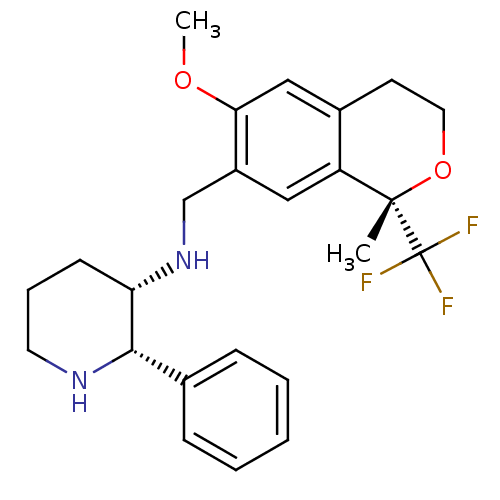 ((2S,3S)-N-(((S)-6-methoxy-1-methyl-1-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262510 (CHEMBL477365 | R/S-(2S,3S)-3-[((6-Methoxy-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262510 (CHEMBL477365 | R/S-(2S,3S)-3-[((6-Methoxy-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262280 (CHEMBL513351 | R/S-(2S,3S)-N-(2-methoxy-5-(1,1,1-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50067935 ((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262453 (CHEMBL479049 | R/S-(2S,3S)-N-((3-methoxy-8-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262336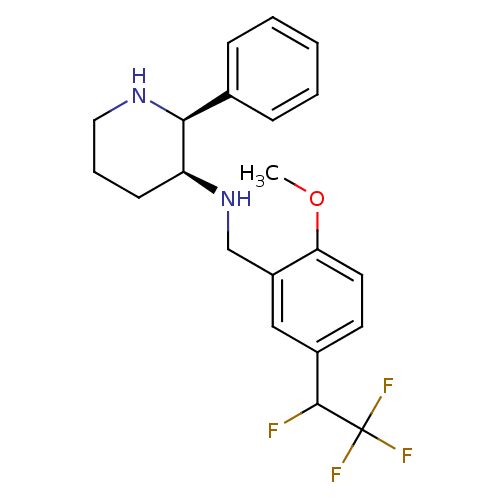 (CHEMBL468163 | R/S-(2S,3S)-N-(2-methoxy-5-(1,2,2,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262394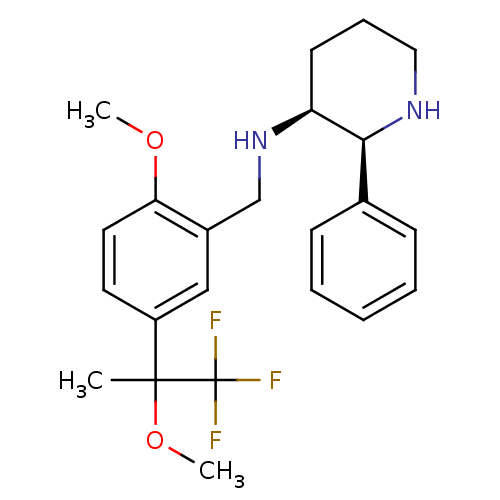 (CHEMBL515935 | R/S-(2S,3S)-N-(2-methoxy-5-(1,1,1-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262395 (CHEMBL478620 | R/S-(2S,3S)-N-((6-methoxy-3-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262395 (CHEMBL478620 | R/S-(2S,3S)-N-((6-methoxy-3-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50262281 ((2S,3S)-3-[2-Methoxy-5-(2,2,2-trifluoroethyl)benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from NK1 receptor in human IM9 cells | Bioorg Med Chem 16: 7193-205 (2008) Article DOI: 10.1016/j.bmc.2008.06.047 BindingDB Entry DOI: 10.7270/Q2RV0NHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50138191 ((1S,5S,6R,7R)-7-Chloro-5-methyl-2-aza-bicyclo[4.1....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of inducible nitric oxide synthase (iNOS) in mice | Bioorg Med Chem Lett 14: 313-6 (2003) BindingDB Entry DOI: 10.7270/Q2XP74C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202356 (CHEMBL218806 | cyclo(-D-Tyr-D-MeArg-L-Arg-L-Nal-Gl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50159214 (CHEMBL438934) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 expressed in CHO cells | J Med Chem 48: 380-91 (2005) Article DOI: 10.1021/jm049429h BindingDB Entry DOI: 10.7270/Q27S7N98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202350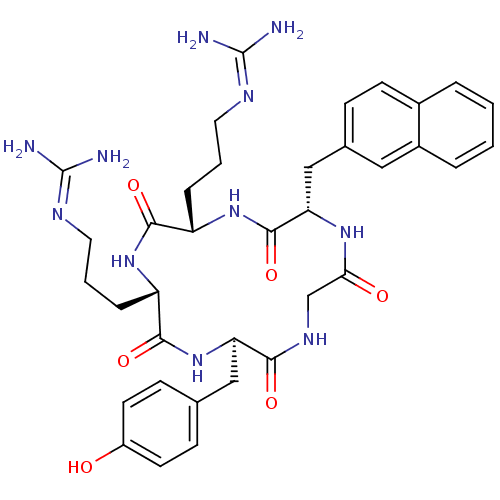 (CHEMBL219474 | cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-Gly-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166087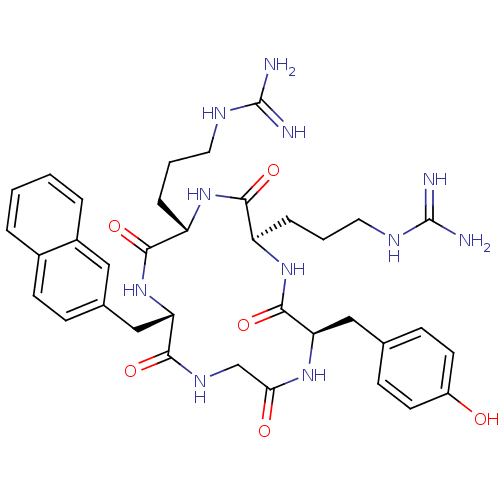 (CHEMBL436097 | N-{3-[(2R,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50159213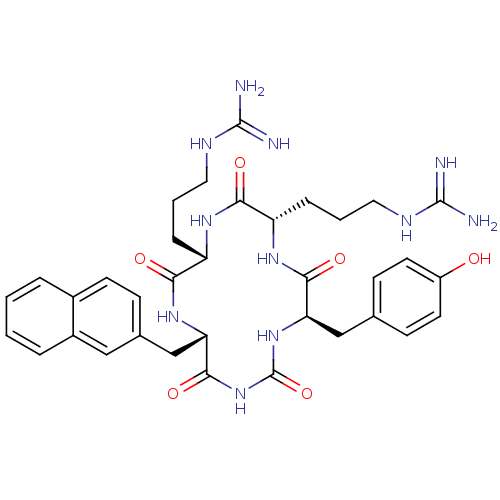 (CHEMBL370001 | N-{3-[10-(3-Guanidino-propyl)-4-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 expressed in CHO cells | J Med Chem 48: 380-91 (2005) Article DOI: 10.1021/jm049429h BindingDB Entry DOI: 10.7270/Q27S7N98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50249878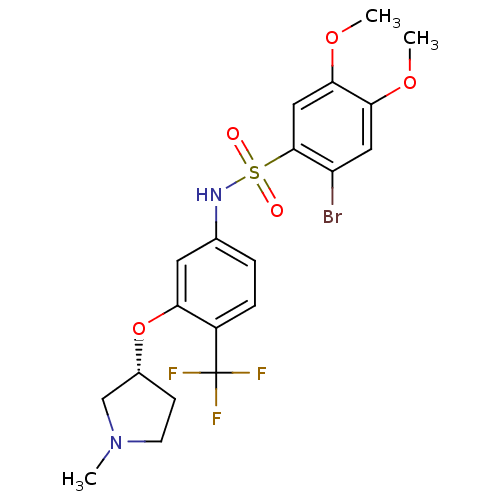 ((R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-U2 from human recombinant urotensin2 receptor expressed in human Chem-2 cells after 4 hrs by scintillation proximity assay | Bioorg Med Chem Lett 23: 2177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.01.105 BindingDB Entry DOI: 10.7270/Q2XS5WSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202346 (CHEMBL219339 | cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-Gly-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166106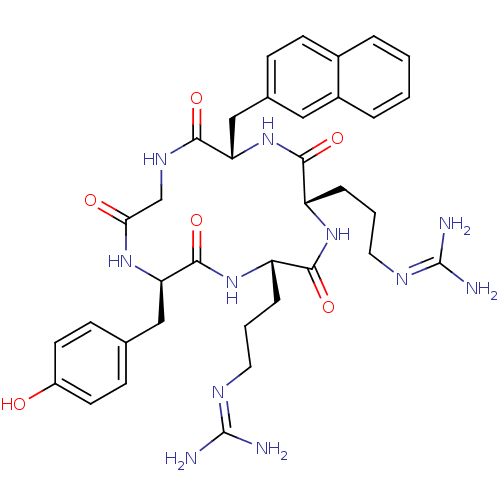 (CHEMBL436283 | N-{3-[(2S,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50161394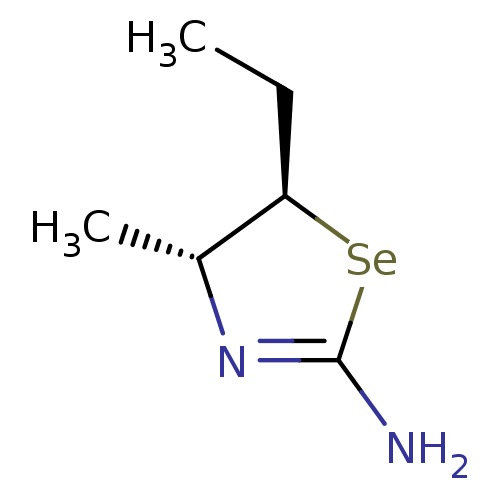 ((4R,5R)-5-Ethyl-4-methyl-selenazolidin-(2Z)-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against inducible nitric acid synthase | Bioorg Med Chem Lett 15: 1361-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.013 BindingDB Entry DOI: 10.7270/Q2G160B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50161393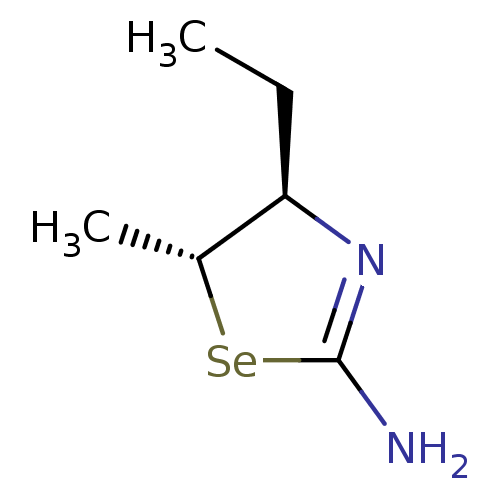 ((4R,5R)-4-Ethyl-5-methyl-selenazolidin-(2Z)-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration against inducible nitric acid synthase | Bioorg Med Chem Lett 15: 1361-6 (2005) Article DOI: 10.1016/j.bmcl.2005.01.013 BindingDB Entry DOI: 10.7270/Q2G160B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166096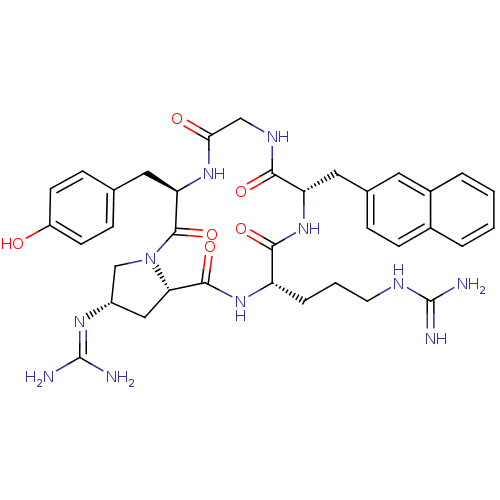 (CHEMBL372874 | N-[(2S,5R,11S,14S,16aS)-14-(3-Guani...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166079 (CHEMBL192183 | N-[(2R,5R,11S,14S,16aS)-14-(3-Guani...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202345 (CHEMBL373636 | cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-D-Al...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202359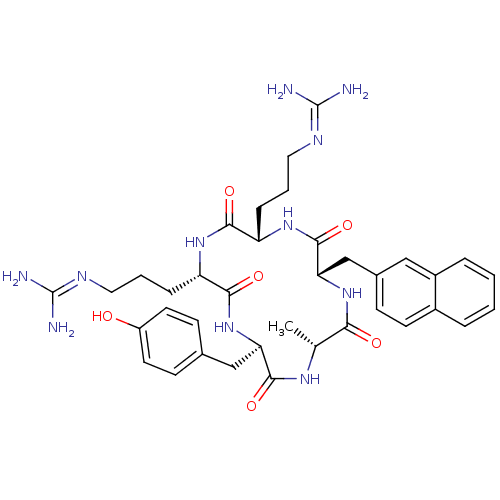 (CHEMBL375990 | cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-D-Al...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50249878 ((R)-2-bromo-4,5-dimethoxy-N-(3-(1-methylpyrrolidin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at recombinant human urotensin2 receptor expressed in CHO cells assessed as inhibition of urotensin2-stimulated Ca2+ mobilization... | Bioorg Med Chem Lett 23: 2177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.01.105 BindingDB Entry DOI: 10.7270/Q2XS5WSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 285 total ) | Next | Last >> |