

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035429 (4'-[2-Butyl-6-(3-cyclohexyl-ureido)-benzoimidazol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035458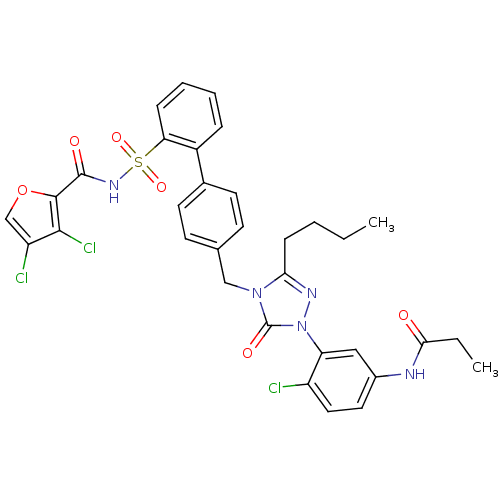 (CHEMBL138690 | N-[3-(3-Butyl-4-{2'-[(3,4-dichloro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035434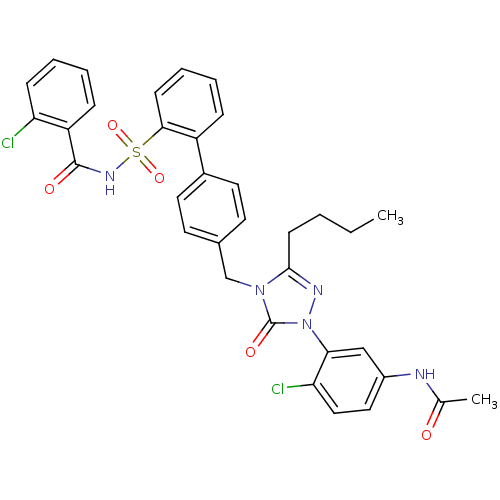 (CHEMBL265797 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT1 receptor from rabbit aorta | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035434 (CHEMBL265797 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50030726 (3-[4-(2'-Benzoylsulfamoyl-3-fluoro-biphenyl-4-ylme...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II type 2 receptor in rat adrenal membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50030685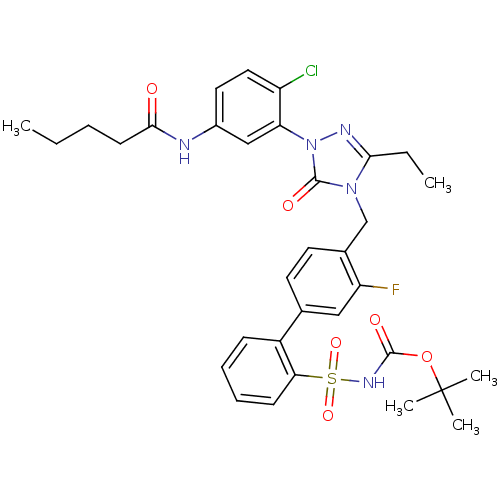 (CHEMBL338888 | Pentanoic acid {4-chloro-3-[3-ethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II type 2 receptor in rat adrenal membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030692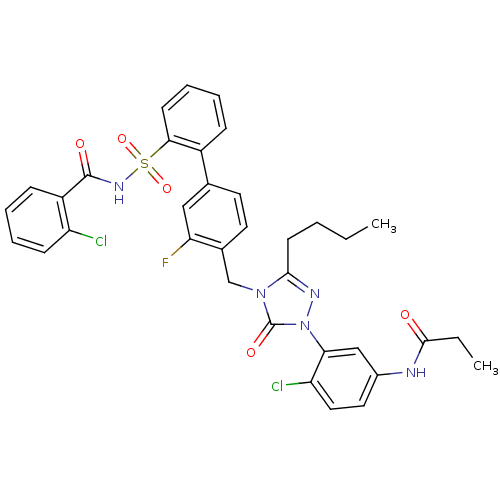 (CHEMBL339672 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030692 (CHEMBL339672 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035444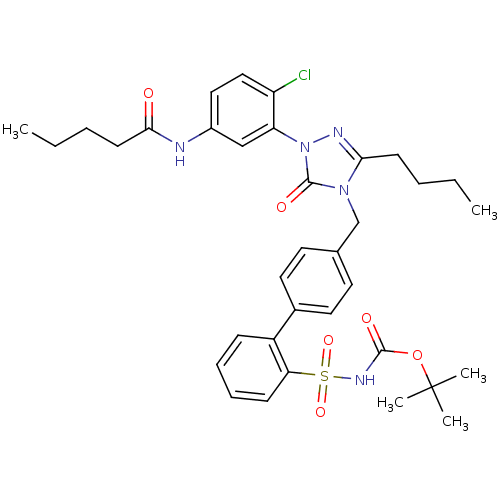 (4-[[2'-[N-(tert-Butoxycarbonyl)sulfamoyl]biphenyl-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035454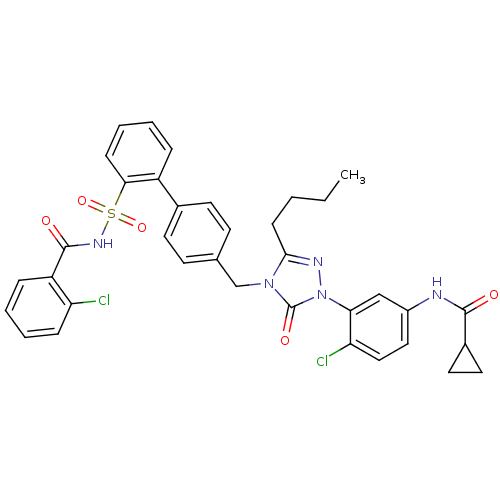 (CHEMBL343309 | Cyclopropanecarboxylic acid (3-{3-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50030721 (4-Bromo-N-butyl-3-{4-[2'-(2,5-dichloro-benzoylsulf...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II type 2 receptor in rat adrenal membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283765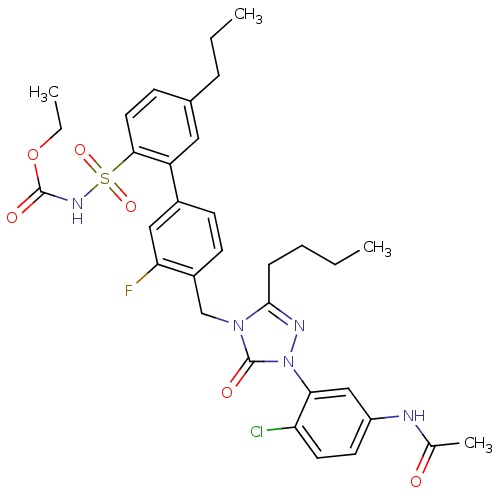 (CHEMBL321313 | N-{3-[3-Butyl-4-(3-fluoro-2'-(ethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT2 receptor from rat midbrain | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282276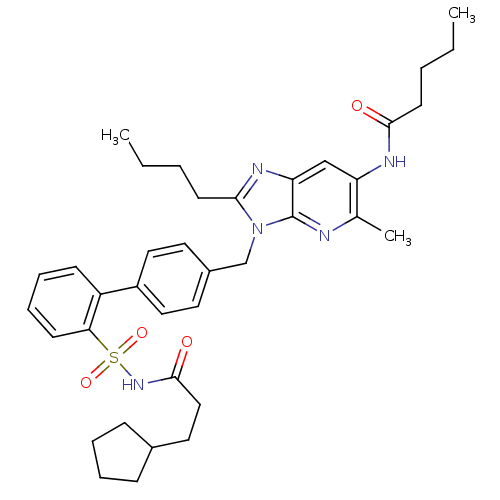 (CHEMBL304947 | Pentanoic acid {2-butyl-3-[2'-(3-cy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282274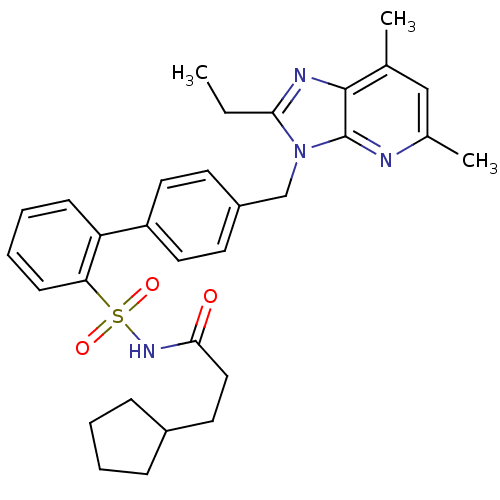 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030684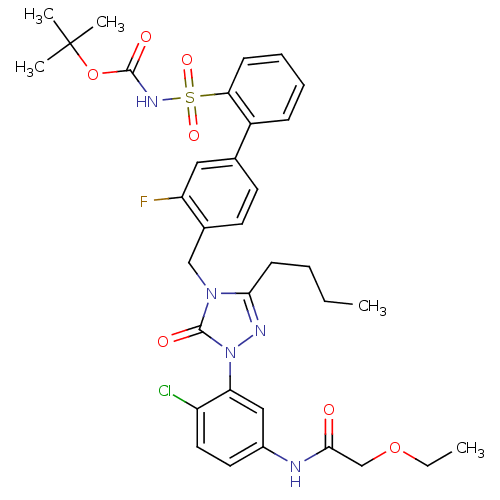 (CHEMBL339722 | N-{3-[3-Butyl-4-(3-fluoro-2-(N-t-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030684 (CHEMBL339722 | N-{3-[3-Butyl-4-(3-fluoro-2-(N-t-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50030693 (CHEMBL338687 | Pentanoic acid {4-bromo-3-[3-ethyl-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 2 in rat midbrain membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50041969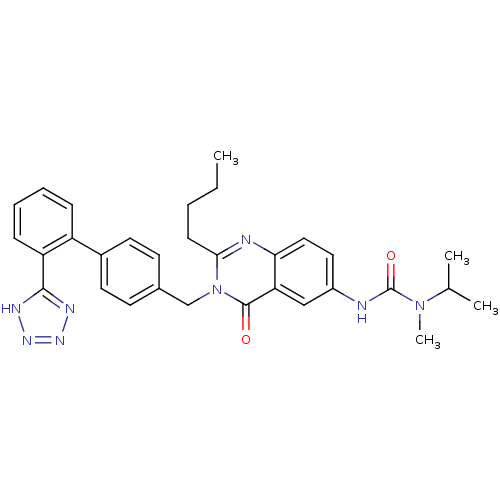 (3-{2-Butyl-4-oxo-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1-Ile8-AII (without BSA) from type 1 Angiotensin II receptor of rabbit aorta | Bioorg Med Chem Lett 3: 1299-1304 (1993) Article DOI: 10.1016/S0960-894X(00)80335-2 BindingDB Entry DOI: 10.7270/Q29P31KG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283305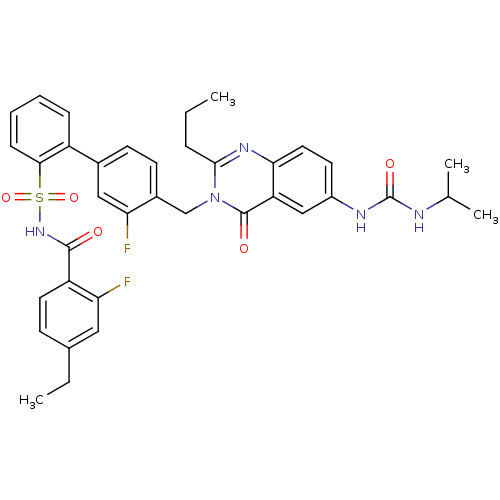 (3'-Fluoro-4'-[6-(3-isopropyl-ureido)-4-oxo-2-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration against Angiotensin II receptor, type 1 of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035445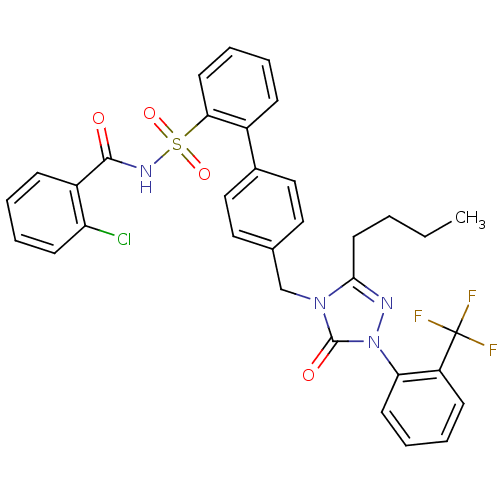 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT1 receptor from rabbit aorta | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283763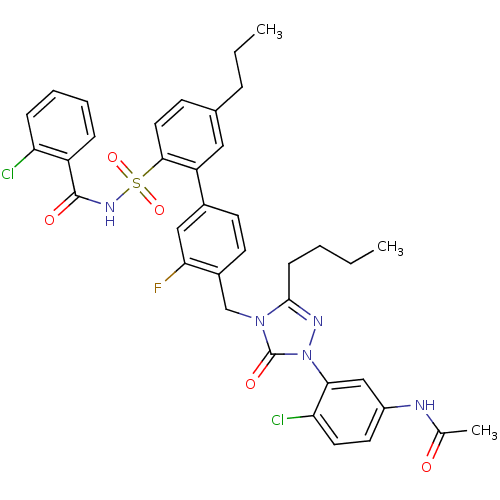 (CHEMBL321531 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration against AT1 receptor from rat adrenal tissues | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282277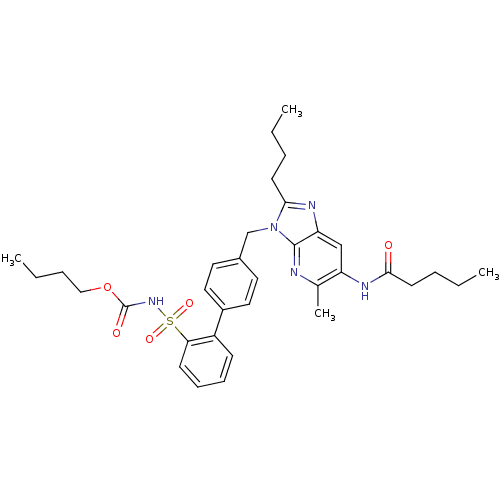 (CHEMBL306407 | Imidazo[4,5-b]pyridine derivative) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035456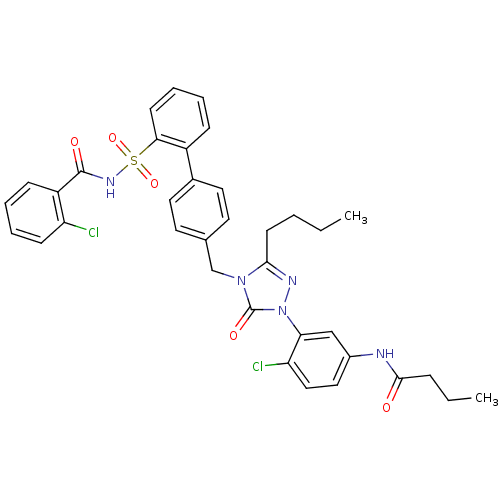 (CHEMBL341934 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035445 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035439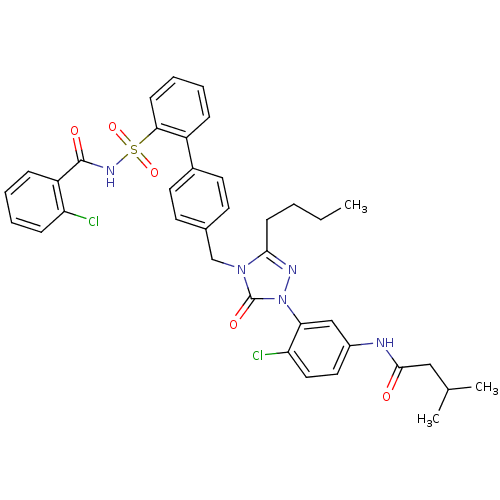 (CHEMBL343142 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282281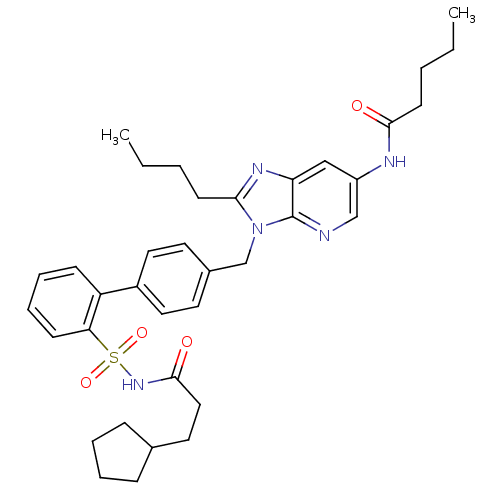 (CHEMBL68618 | Pentanoic acid {2-butyl-3-[2'-(3-cyc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity determined by its ability to displace the specific binding ligand [125I]-Sar1, Ile8-AII from Angiotensin II receptor, type ... | Bioorg Med Chem Lett 4: 17-22 (1994) Article DOI: 10.1016/S0960-894X(01)81115-X BindingDB Entry DOI: 10.7270/Q2G73DPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50030711 (CHEMBL338027 | L-163958 | Pentanoic acid {4-bromo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 2 in rat midbrain membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035430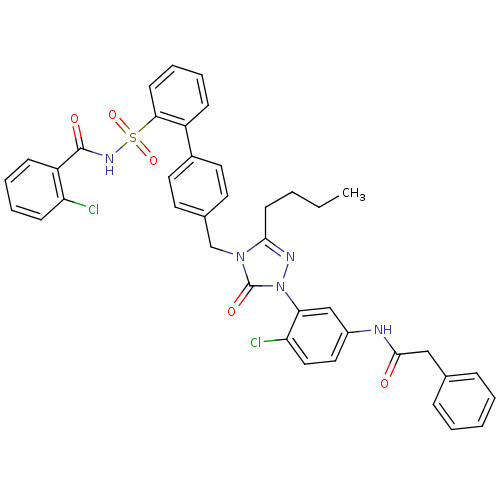 (CHEMBL140708 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030729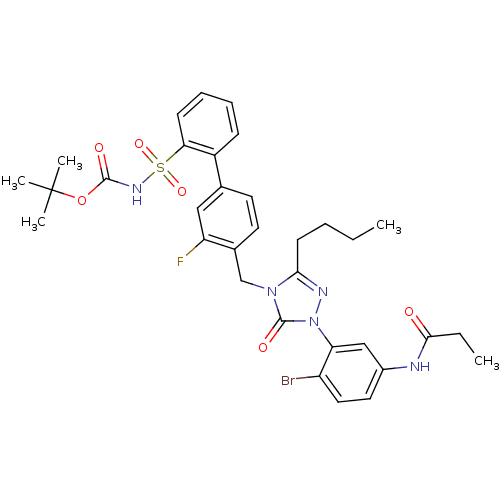 (CHEMBL124771 | N-{4-Bromo-3-[3-butyl-4-(3-fluoro-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282470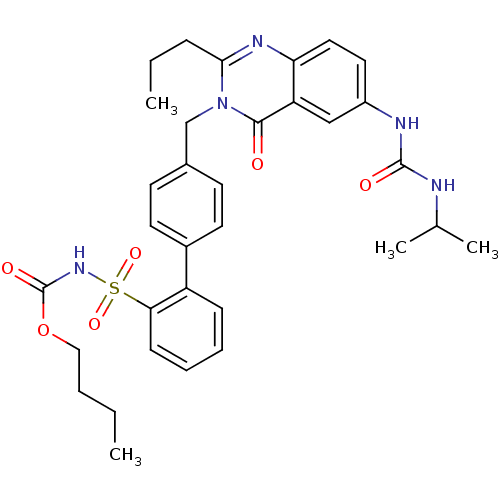 (CHEMBL353037 | Quinazolinone Analog) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro for binding affinity against Angiotensin II receptor, type 1 in rabbit aortic membrane | Bioorg Med Chem Lett 4: 81-86 (1994) Article DOI: 10.1016/S0960-894X(01)81126-4 BindingDB Entry DOI: 10.7270/Q2D79BBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50283319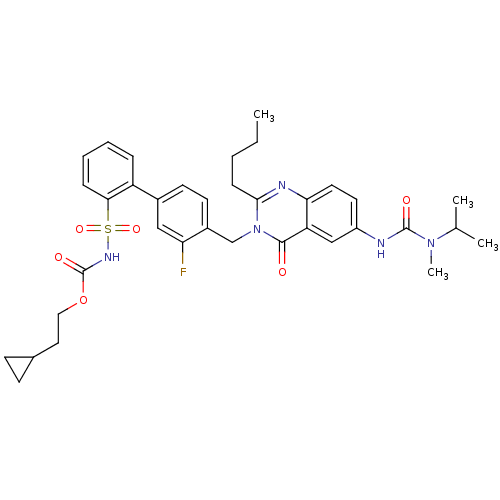 (2-Butyl-3-(3-fluoro-2'-(2-cyclopropylethyloxycarbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II receptor, type 1 of rabbit aorta membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283317 (2-Propyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030687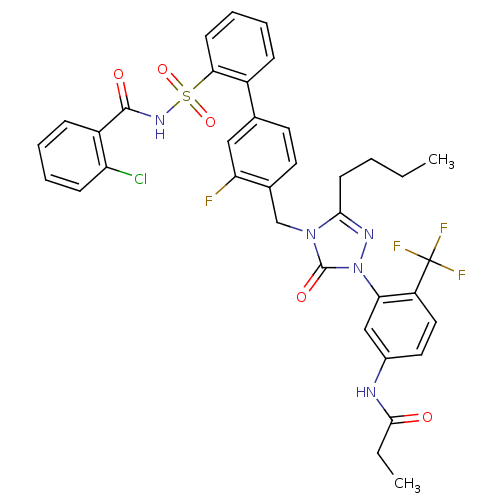 (CHEMBL339256 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030728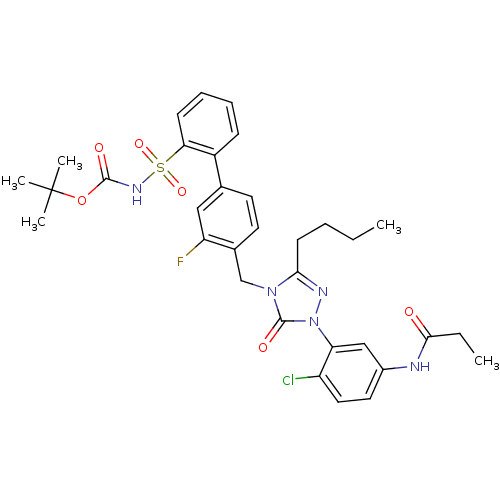 (CHEMBL339605 | N-{3-[3-Butyl-4-(3-fluoro-2'-(N-t-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030718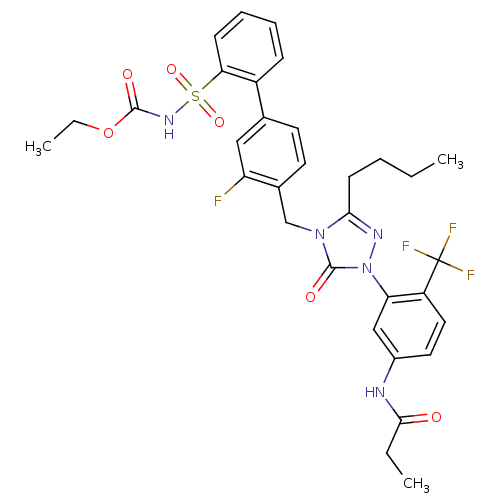 (CHEMBL332576 | N-{3-[3-Butyl-4-(3-fluoro-2-(N-t-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283310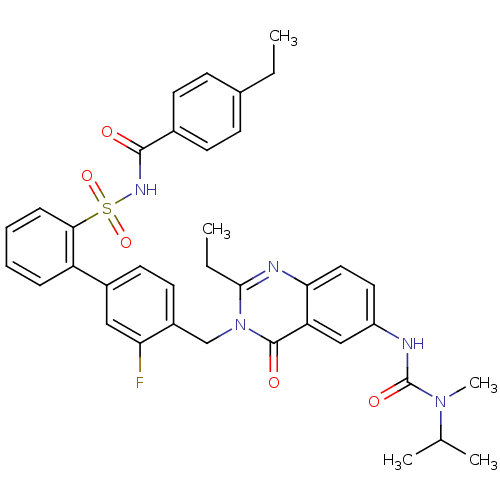 (4'-[2-Ethyl-6-(3-isopropyl-3-methyl-ureido)-4-oxo-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283300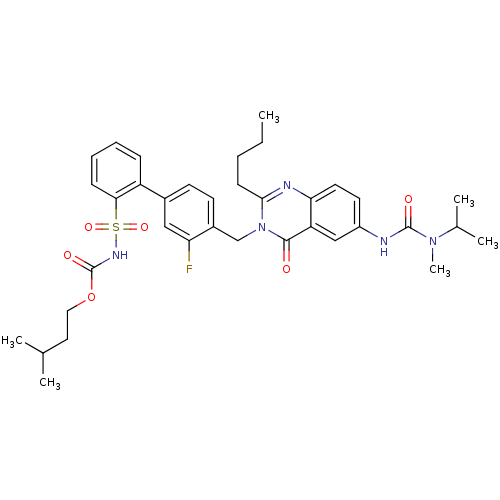 (2-Butyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283312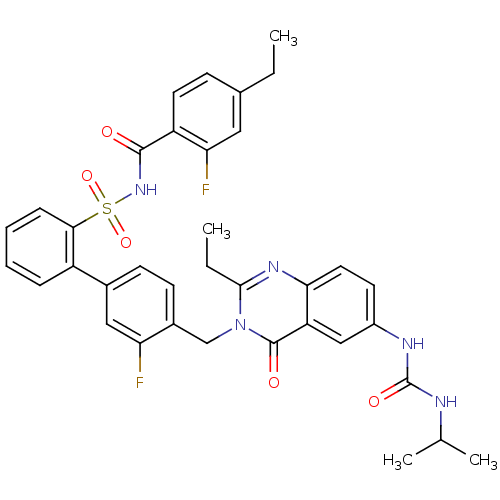 (4'-[2-Ethyl-6-(3-isopropyl-ureido)-4-oxo-4H-quinaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against AT2 receptor of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50030720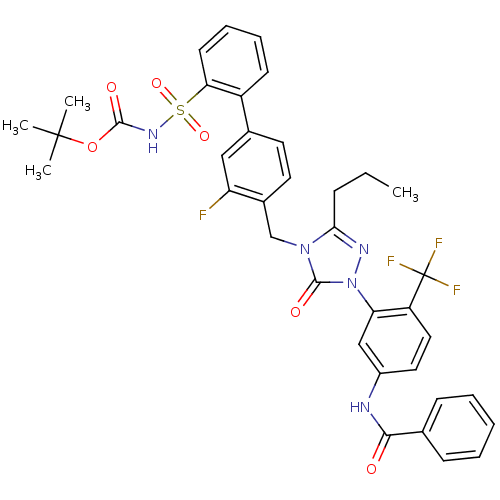 (CHEMBL122158 | N-{3-[4-(3-Fluoro-2'-(N-t-butyloxyc...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II type 2 receptor in rat adrenal membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030698 (CHEMBL435811 | N-{3-[3-Butyl-4-(3-fluoro-2'-(N-t-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030710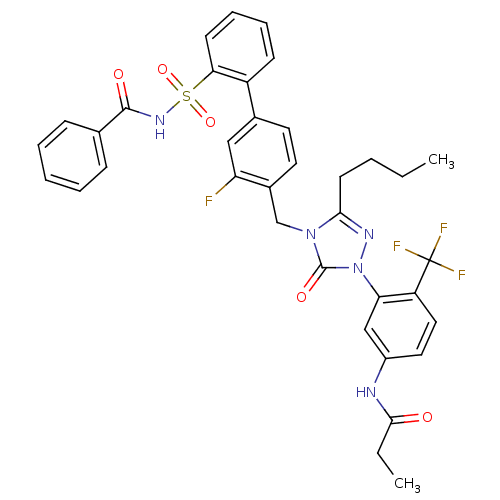 (CHEMBL332909 | N-{3-[4-(2'-Benzoylsulfamoyl-3-fluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030731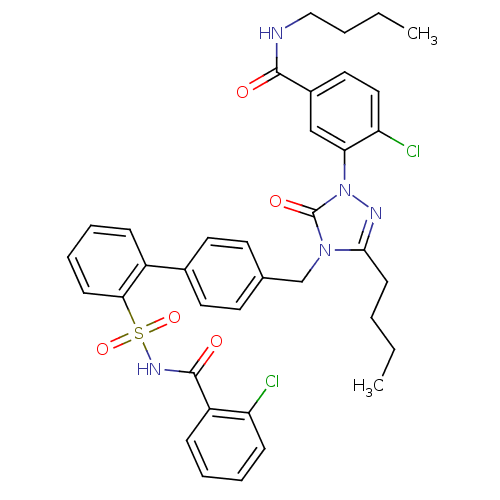 (CHEMBL338101 | N-Butyl-3-{3-butyl-4-[2'-(2-chloro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030731 (CHEMBL338101 | N-Butyl-3-{3-butyl-4-[2'-(2-chloro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035462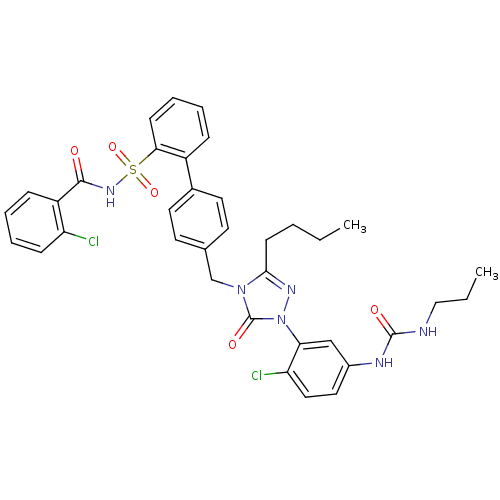 (4'-{3-Butyl-1-[2-chloro-5-(3-propyl-ureido)-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... | J Med Chem 37: 4464-78 (1995) BindingDB Entry DOI: 10.7270/Q2WD3ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030698 (CHEMBL435811 | N-{3-[3-Butyl-4-(3-fluoro-2'-(N-t-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50030710 (CHEMBL332909 | N-{3-[4-(2'-Benzoylsulfamoyl-3-fluo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50030700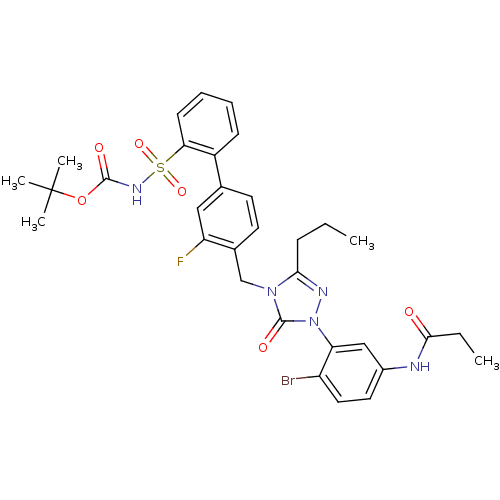 (CHEMBL436396 | N-{4-Bromo-3-[4-(3-fluoro-2'-(N-t-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 2 in rat midbrain membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283763 (CHEMBL321531 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT2 receptor from rat midbrain | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50283762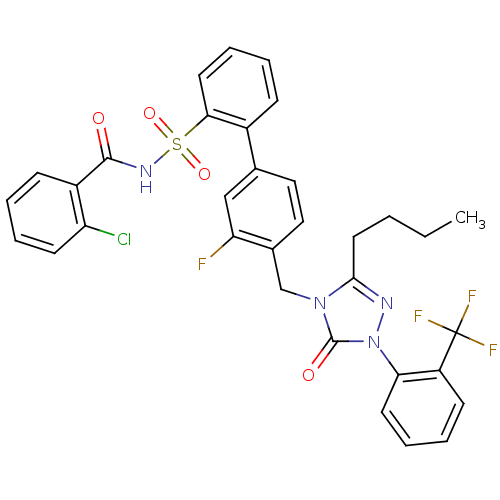 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT1 receptor from rabbit aorta | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50283766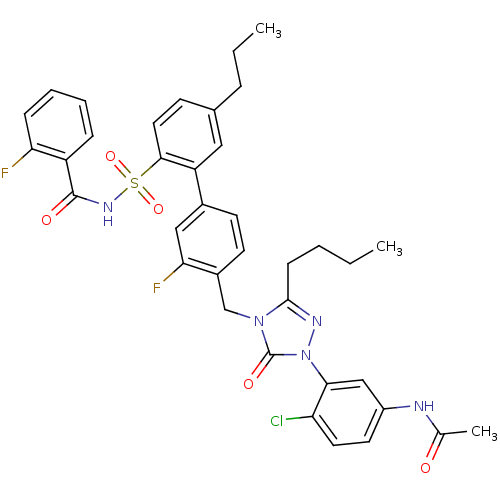 (CHEMBL313868 | N-(3-{3-Butyl-4-[3-fluoro-2'-(2-flu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT2 receptor from rat midbrain | Bioorg Med Chem Lett 4: 2787-2792 (1994) Article DOI: 10.1016/S0960-894X(01)80595-3 BindingDB Entry DOI: 10.7270/Q2WS8T68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1176 total ) | Next | Last >> |