

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341578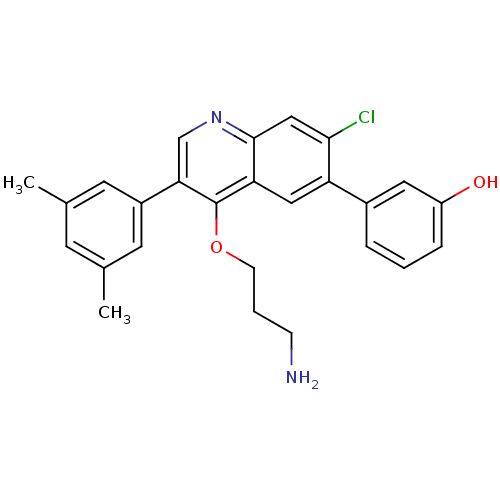 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341575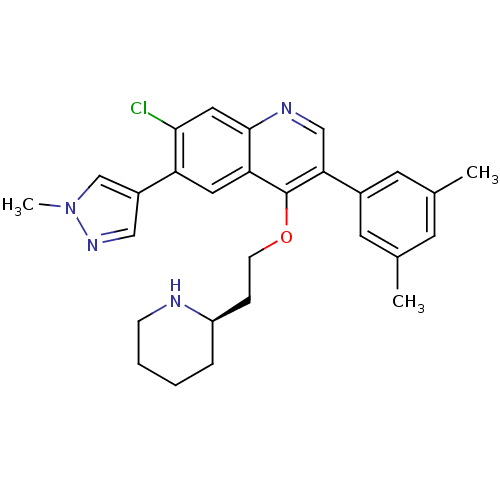 (7-Chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341572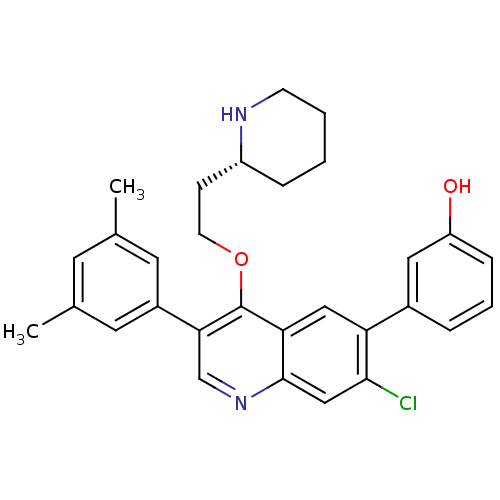 (3-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341574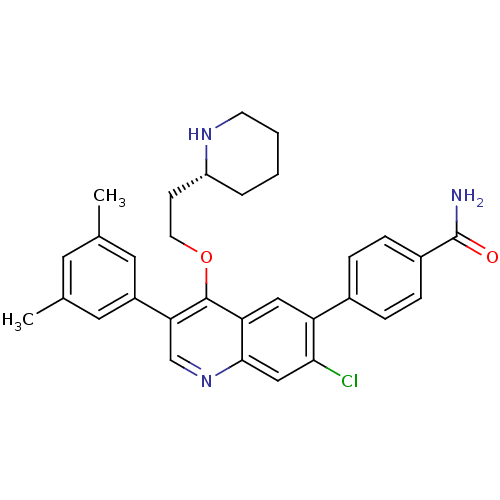 (3-{7-Chloro-3-(3,5-dimethylphenyl)-4-[2-(piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341562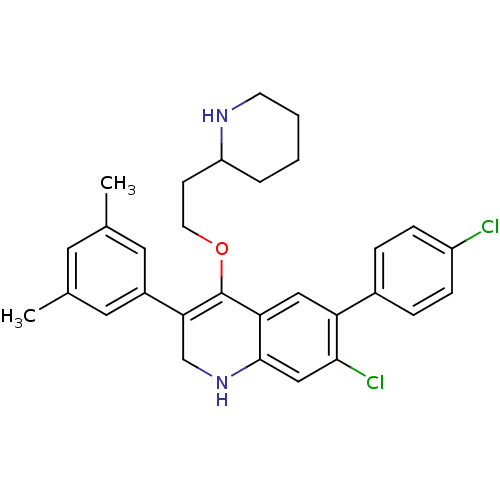 (7-chloro-6-(4-chlorophenyl)-3-(3,5-dimethylphenyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341577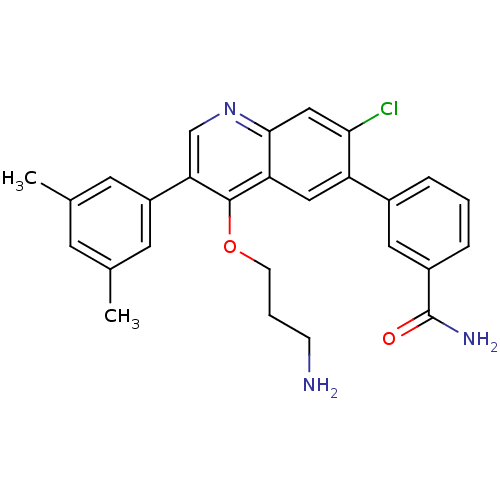 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341571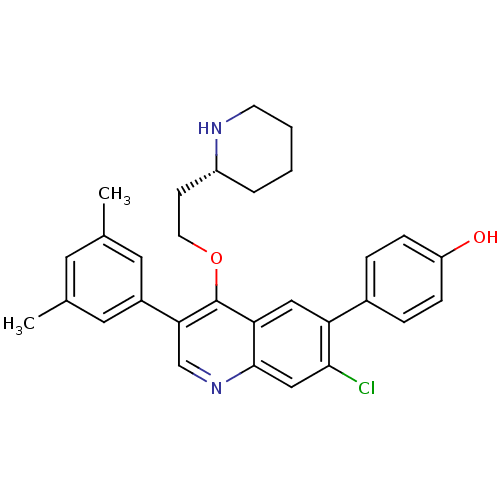 (4-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341573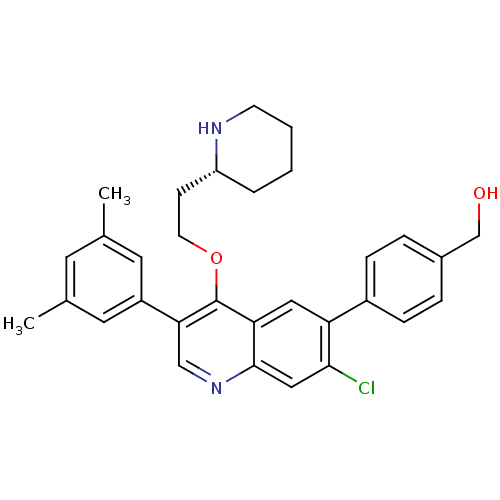 (CHEMBL1766098 | {4-[7-Chloro-3-(3,5-dimethylphenyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341570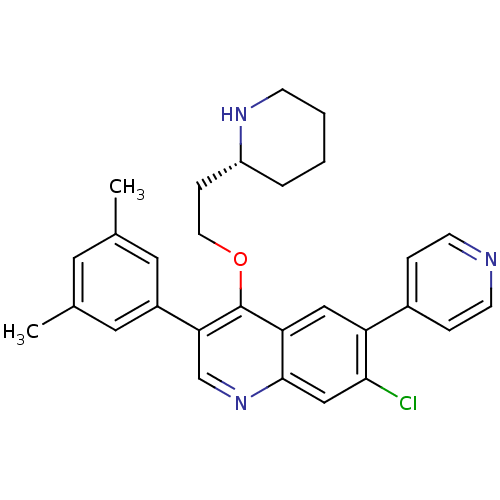 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341569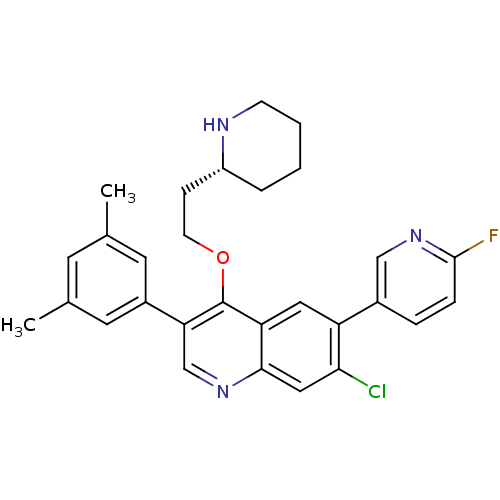 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-fluoropyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341568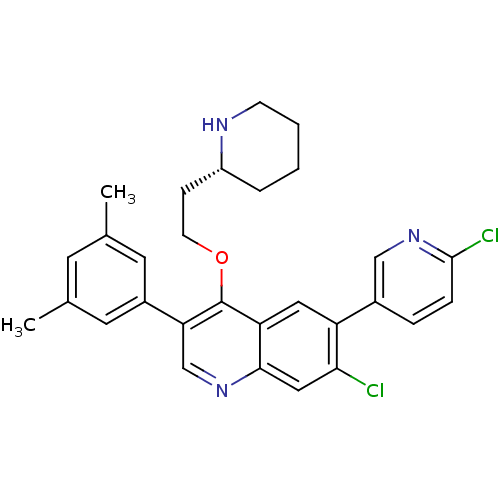 (7-Chloro-6-(6-chloropyridin-3-yl)-3-(3,5-dimethylp...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341564 (7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-piperid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341579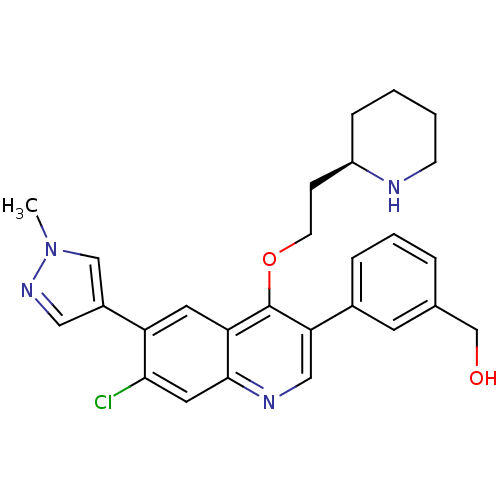 (CHEMBL1766104 | {3-[7-Chloro-6-(1-methyl-1H-pyrazo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341580 (CHEMBL1766105 | [(4-{2-[(2R)-Piperidin-2-yl]ethoxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341563 (7-chloro-3-(3,5-dimethylphenyl)-6-phenyl-4-(2-(pip...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341565 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341566 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341567 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-methoxypyridi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341576 ((S)-7-chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341560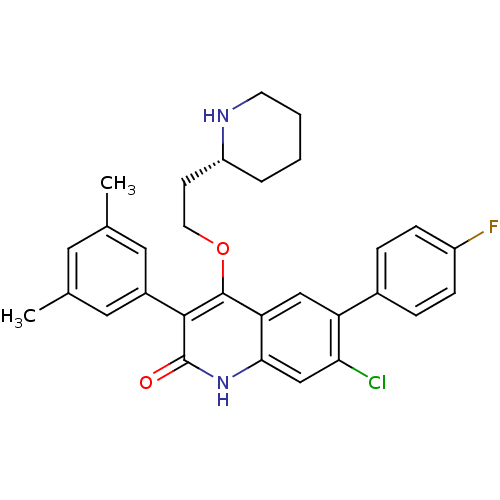 ((R)-7-chloro-3-(3,5-dimethylphenyl)-6-(4-fluorophe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341561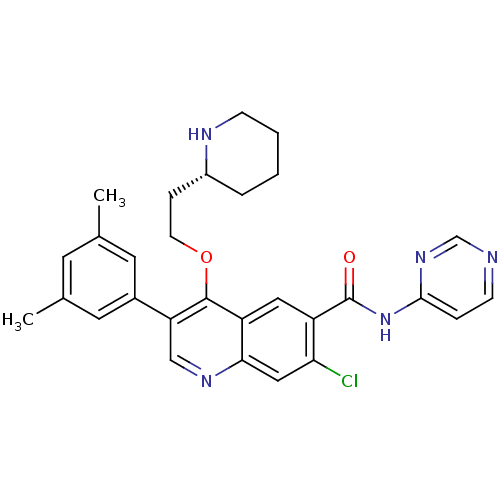 ((R)-7-chloro-3-(3,5-dimethylphenyl)-4-(2-(piperidi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50342649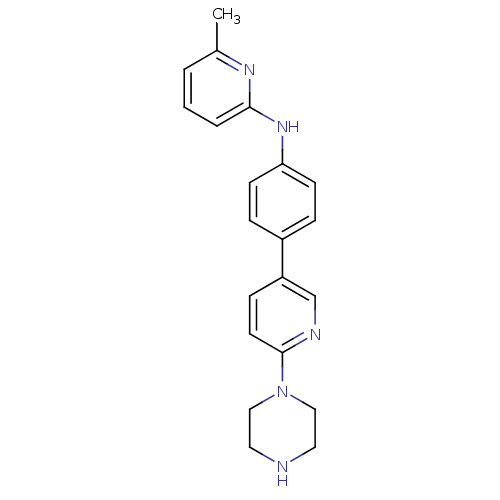 (6-methyl-N-(4-(6-(piperazin-1-yl)pyridin-3-yl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | Bioorg Med Chem Lett 21: 2646-9 (2011) Article DOI: 10.1016/j.bmcl.2010.12.115 BindingDB Entry DOI: 10.7270/Q2QV3MVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341559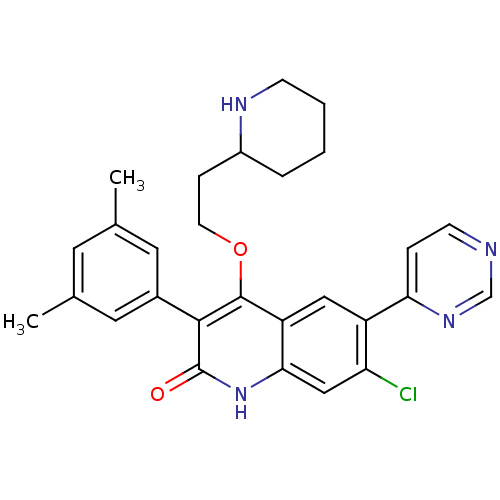 (7-chloro-3-(3,5-dimethylphenyl)-4-(2-(piperidin-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341565 (5-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341571 (4-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341577 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341574 (3-{7-Chloro-3-(3,5-dimethylphenyl)-4-[2-(piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50258579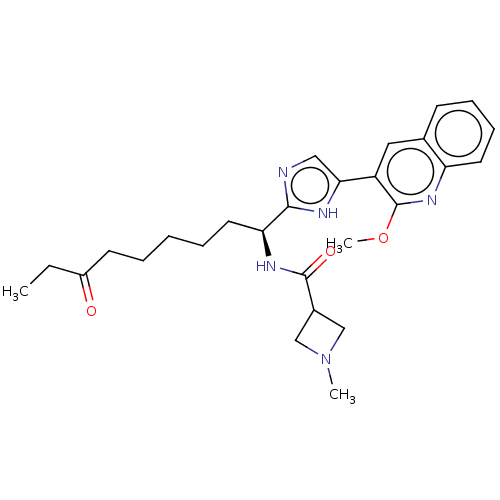 ((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50569630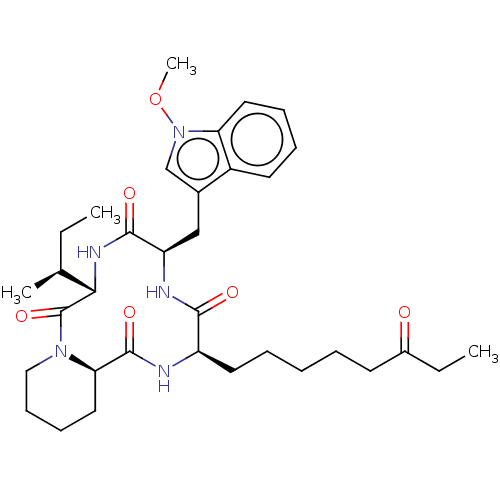 (CHEMBL4873847) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341572 (3-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50569630 (CHEMBL4873847) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50569643 (CHEMBL4867665) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50258579 ((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50195169 (2,4-dichloro-N-((4-(morpholine-4-carbonyl)-1-(prop...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co. Curated by ChEMBL | Assay Description Inhibition of human GlyT1 | Bioorg Med Chem Lett 16: 5968-72 (2006) Article DOI: 10.1016/j.bmcl.2006.08.131 BindingDB Entry DOI: 10.7270/Q2C82B31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248651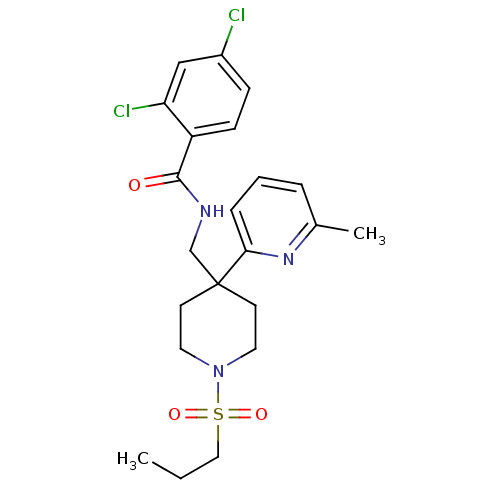 (2,4-dichloro-N-((4-(6-methylpyridin-2-yl)-1-(propy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human glycine transporter 1 | Bioorg Med Chem Lett 19: 1488-91 (2009) Article DOI: 10.1016/j.bmcl.2008.12.115 BindingDB Entry DOI: 10.7270/Q2V124P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248641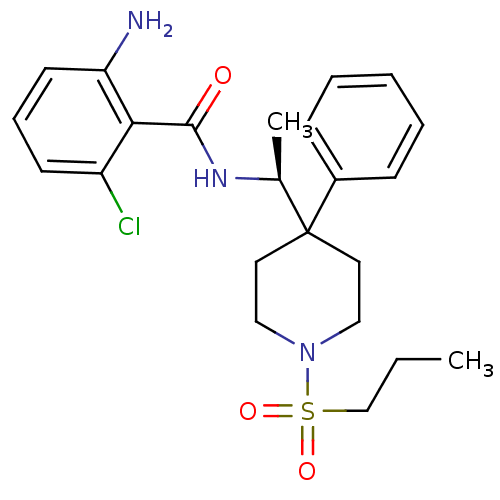 (2-amino-6-chloro-N-((4-phenyl-1-(propylsulfonyl)pi...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of glycine transporter 1 (unknown origin) by HTS assay | Bioorg Med Chem Lett 19: 1488-91 (2009) Article DOI: 10.1016/j.bmcl.2008.12.115 BindingDB Entry DOI: 10.7270/Q2V124P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248064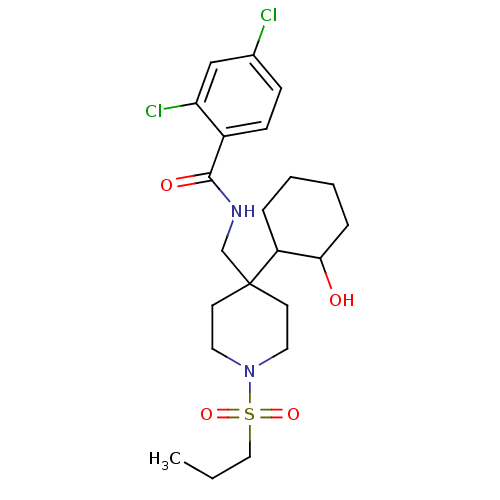 (2,4-dichloro-N-((4-(2-hydroxycyclohexyl)-1-(propyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of Glyt1 | Bioorg Med Chem Lett 19: 1492-5 (2009) Article DOI: 10.1016/j.bmcl.2009.01.015 BindingDB Entry DOI: 10.7270/Q2B27V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248062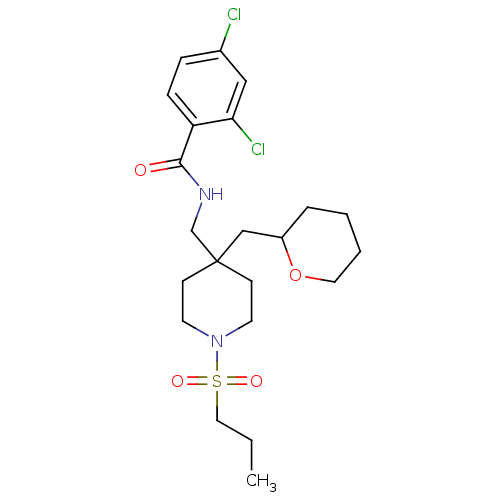 (2,4-dichloro-N-((1-(propylsulfonyl)-4-((tetrahydro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of Glyt1 | Bioorg Med Chem Lett 19: 1492-5 (2009) Article DOI: 10.1016/j.bmcl.2009.01.015 BindingDB Entry DOI: 10.7270/Q2B27V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248669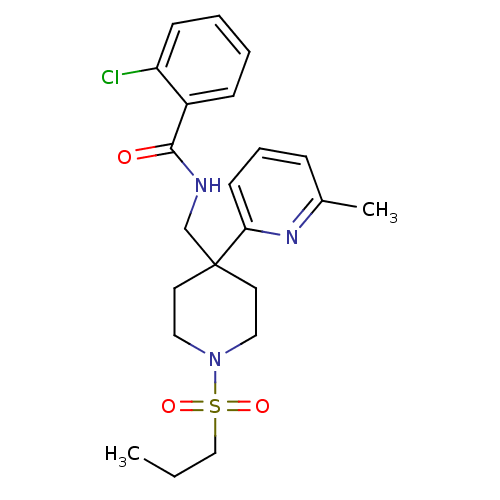 (2-chloro-N-((4-(6-methylpyridin-2-yl)-1-(propylsul...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human glycine transporter 1 | Bioorg Med Chem Lett 19: 1488-91 (2009) Article DOI: 10.1016/j.bmcl.2008.12.115 BindingDB Entry DOI: 10.7270/Q2V124P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248058 ((S)-2,4-dichloro-N-(1-(1-(propylsulfonyl)-4-(pyrid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human glycine transporter 1 | Bioorg Med Chem Lett 19: 1488-91 (2009) Article DOI: 10.1016/j.bmcl.2008.12.115 BindingDB Entry DOI: 10.7270/Q2V124P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50195169 (2,4-dichloro-N-((4-(morpholine-4-carbonyl)-1-(prop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co. Curated by ChEMBL | Assay Description Inhibition of rat GlyT1 | Bioorg Med Chem Lett 16: 5968-72 (2006) Article DOI: 10.1016/j.bmcl.2006.08.131 BindingDB Entry DOI: 10.7270/Q2C82B31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50341559 (7-chloro-3-(3,5-dimethylphenyl)-4-(2-(piperidin-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at GnRH receptor by GnRH | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248667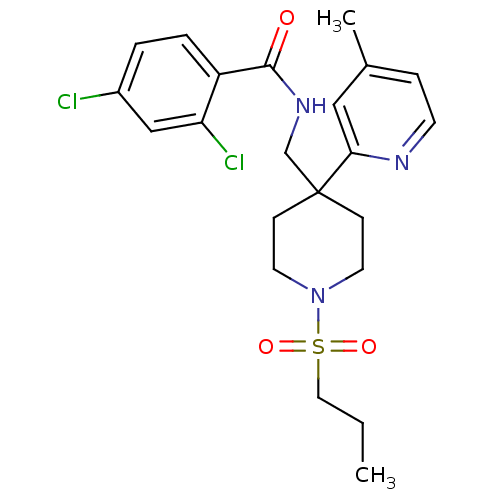 (2,4-dichloro-N-((4-(4-methylpyridin-2-yl)-1-(propy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human glycine transporter 1 | Bioorg Med Chem Lett 19: 1488-91 (2009) Article DOI: 10.1016/j.bmcl.2008.12.115 BindingDB Entry DOI: 10.7270/Q2V124P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248059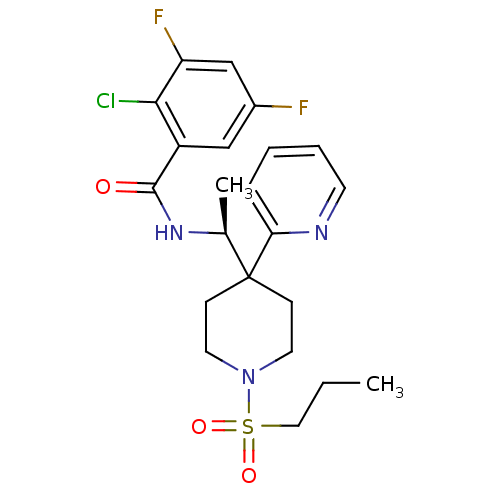 ((S)-2-chloro-3,5-difluoro-N-(1-(1-(propylsulfonyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human glycine transporter 1 | Bioorg Med Chem Lett 19: 1488-91 (2009) Article DOI: 10.1016/j.bmcl.2008.12.115 BindingDB Entry DOI: 10.7270/Q2V124P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50485997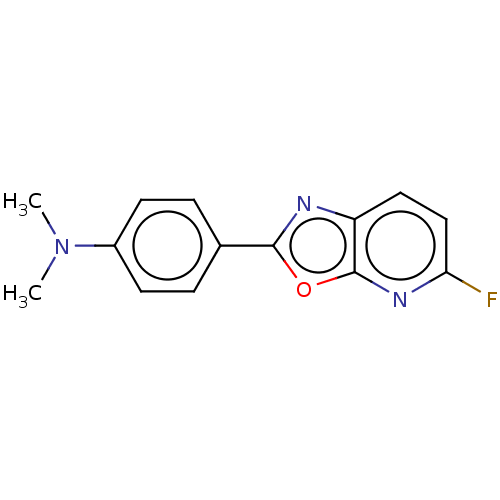 (CHEMBL2203379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human amyloid beta plaque | ACS Med Chem Lett 2: 498-502 (2011) Article DOI: 10.1021/ml200018n BindingDB Entry DOI: 10.7270/Q2TB19RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248100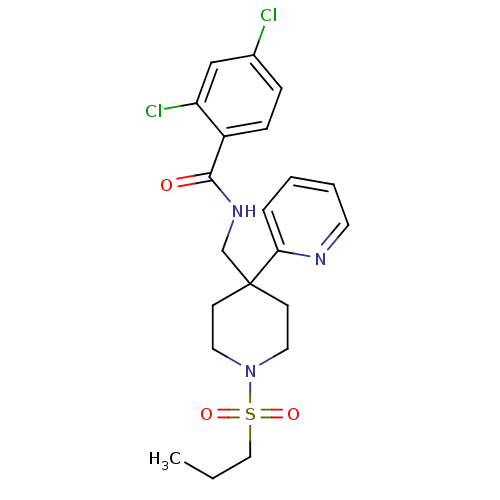 ((2,4-dichloro-N-((1-(propylsulfonyl)-4-(pyridin-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of Glyt1 | Bioorg Med Chem Lett 19: 1492-5 (2009) Article DOI: 10.1016/j.bmcl.2009.01.015 BindingDB Entry DOI: 10.7270/Q2B27V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (Homo sapiens (Human)) | BDBM50248100 ((2,4-dichloro-N-((1-(propylsulfonyl)-4-(pyridin-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of human glycine transporter 1 | Bioorg Med Chem Lett 19: 1488-91 (2009) Article DOI: 10.1016/j.bmcl.2008.12.115 BindingDB Entry DOI: 10.7270/Q2V124P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341569 (7-Chloro-3-(3,5-dimethylphenyl)-6-(6-fluoropyridin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50569643 (CHEMBL4867665) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341575 (7-Chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human SST2 expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP release after 40 mins by luminescence assay | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 659 total ) | Next | Last >> |