 Found 759 hits with Last Name = 'sparks' and Initial = 'sm'
Found 759 hits with Last Name = 'sparks' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502507
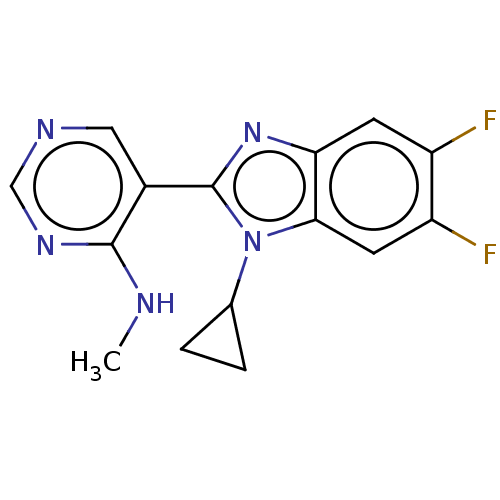
(CHEMBL4475659)Show InChI InChI=1S/C15H13F2N5/c1-18-14-9(6-19-7-20-14)15-21-12-4-10(16)11(17)5-13(12)22(15)8-2-3-8/h4-8H,2-3H2,1H3,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502502

(CHEMBL4528905)Show SMILES Fc1cc2nc(-c3cncnc3NCCC(F)(F)F)n(C3CC3)c2cc1F Show InChI InChI=1S/C17H14F5N5/c18-11-5-13-14(6-12(11)19)27(9-1-2-9)16(26-13)10-7-23-8-25-15(10)24-4-3-17(20,21)22/h5-9H,1-4H2,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1803
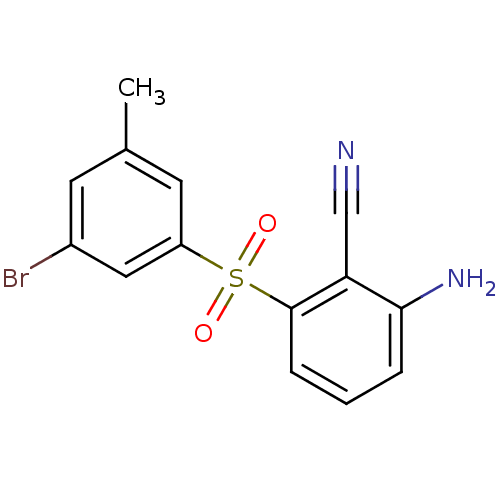
(2-Amino-6-arylthiobenzonitrile deriv. 3w | 2-amino...)Show InChI InChI=1S/C14H11BrN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27747

((2S,3R)-3-(tert-butoxy)-2-{[2-({[4-(cyclopropylmet...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(CC2CC2)cc1C)-c1cccc(F)c1)C(O)=O |r| Show InChI InChI=1S/C34H40FN3O5/c1-19-14-23(16-22-10-11-22)15-20(2)29(19)38-33(42)36-28-18-25(24-8-7-9-26(35)17-24)12-13-27(28)31(39)37-30(32(40)41)21(3)43-34(4,5)6/h7-9,12-15,17-18,21-22,30H,10-11,16H2,1-6H3,(H,37,39)(H,40,41)(H2,36,38,42)/t21-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27730
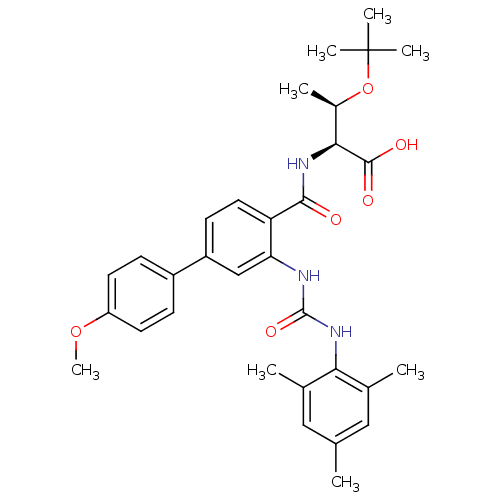
((2S,3R)-3-(tert-butoxy)-2-{[4-(4-methoxyphenyl)-2-...)Show SMILES COc1ccc(cc1)-c1ccc(C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)c(NC(=O)Nc2c(C)cc(C)cc2C)c1 |r| Show InChI InChI=1S/C32H39N3O6/c1-18-15-19(2)27(20(3)16-18)35-31(39)33-26-17-23(22-9-12-24(40-8)13-10-22)11-14-25(26)29(36)34-28(30(37)38)21(4)41-32(5,6)7/h9-17,21,28H,1-8H3,(H,34,36)(H,37,38)(H2,33,35,39)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27745
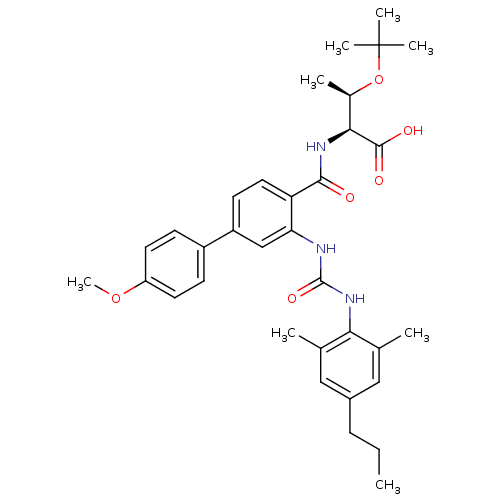
((2S,3R)-3-(tert-butoxy)-2-[(2-{[(2,6-dimethyl-4-pr...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2ccc(OC)cc2)c(C)c1 |r| Show InChI InChI=1S/C34H43N3O6/c1-9-10-23-17-20(2)29(21(3)18-23)37-33(41)35-28-19-25(24-11-14-26(42-8)15-12-24)13-16-27(28)31(38)36-30(32(39)40)22(4)43-34(5,6)7/h11-19,22,30H,9-10H2,1-8H3,(H,36,38)(H,39,40)(H2,35,37,41)/t22-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27743

((2S,3R)-3-(tert-butoxy)-2-{[2-({[4-(cyclopropylmet...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(F)cc1NC(=O)Nc1c(C)cc(CC2CC2)cc1C)C(O)=O |r| Show InChI InChI=1S/C28H36FN3O5/c1-15-11-19(13-18-7-8-18)12-16(2)23(15)32-27(36)30-22-14-20(29)9-10-21(22)25(33)31-24(26(34)35)17(3)37-28(4,5)6/h9-12,14,17-18,24H,7-8,13H2,1-6H3,(H,31,33)(H,34,35)(H2,30,32,36)/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27746

((2S,3R)-3-(tert-butoxy)-2-[(2-{[(2,6-dimethyl-4-pr...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2cccc(F)c2)c(C)c1 |r| Show InChI InChI=1S/C33H40FN3O5/c1-8-10-22-15-19(2)28(20(3)16-22)37-32(41)35-27-18-24(23-11-9-12-25(34)17-23)13-14-26(27)30(38)36-29(31(39)40)21(4)42-33(5,6)7/h9,11-18,21,29H,8,10H2,1-7H3,(H,36,38)(H,39,40)(H2,35,37,41)/t21-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
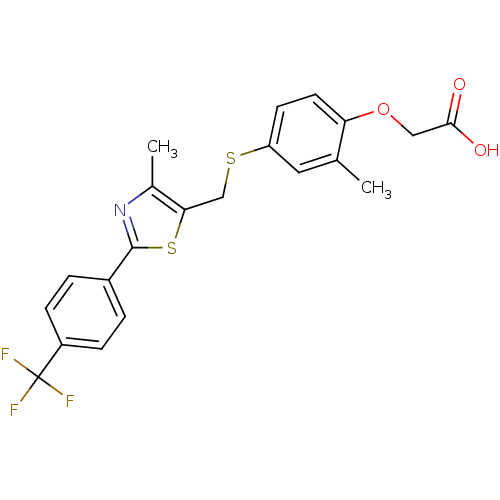
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2345-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.077
BindingDB Entry DOI: 10.7270/Q2M61MHT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1804
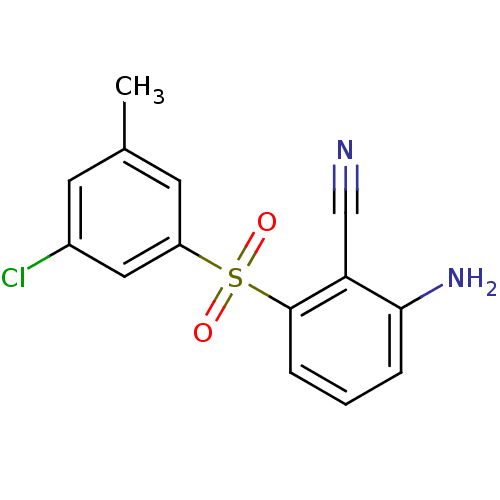
(2-Amino-6-arylthiobenzonitrile deriv. 3x | 2-amino...)Show InChI InChI=1S/C14H11ClN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50255975

((S)-2-cyclohexyl-2-(3-(3-(2,6-dichloro-4-(trifluor...)Show SMILES OC(=O)[C@@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cc(OC(F)(F)F)cc1Cl)C1CCCCC1 |r| Show InChI InChI=1S/C27H24Cl2F3N3O5/c28-19-12-17(40-27(30,31)32)13-20(29)23(19)35-26(39)33-21-11-16-9-5-4-8-15(16)10-18(21)24(36)34-22(25(37)38)14-6-2-1-3-7-14/h4-5,8-14,22H,1-3,6-7H2,(H,34,36)(H,37,38)(H2,33,35,39)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256329
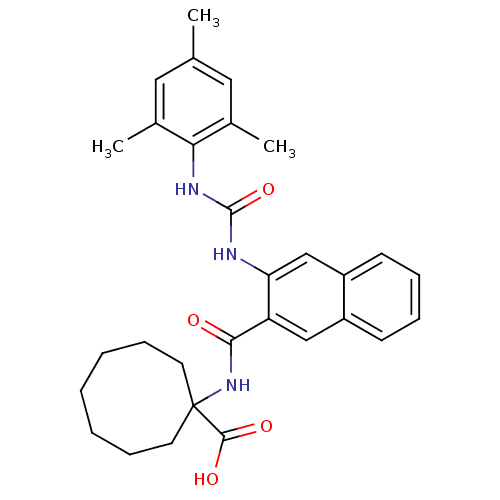
(1-(3-(3-mesitylureido)-2-naphthamido)cyclooctaneca...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC2(CCCCCCC2)C(O)=O)c(C)c1 Show InChI InChI=1S/C30H35N3O4/c1-19-15-20(2)26(21(3)16-19)32-29(37)31-25-18-23-12-8-7-11-22(23)17-24(25)27(34)33-30(28(35)36)13-9-5-4-6-10-14-30/h7-8,11-12,15-18H,4-6,9-10,13-14H2,1-3H3,(H,33,34)(H,35,36)(H2,31,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502506
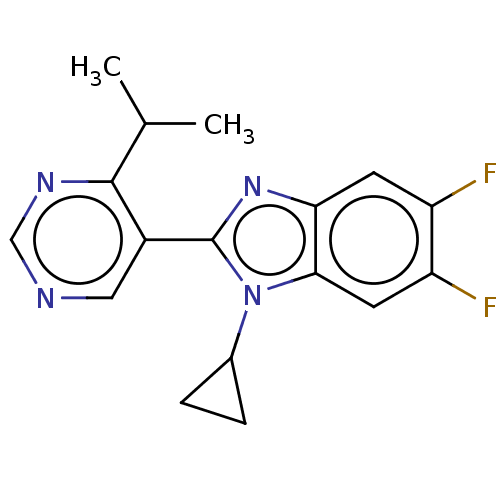
(CHEMBL4460841)Show InChI InChI=1S/C17H16F2N4/c1-9(2)16-11(7-20-8-21-16)17-22-14-5-12(18)13(19)6-15(14)23(17)10-3-4-10/h5-10H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256669
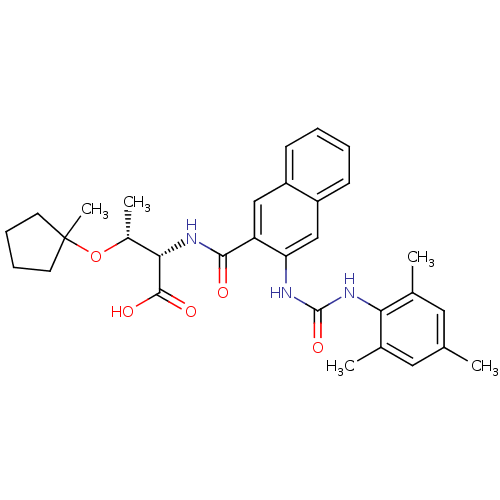
((2S,3R)-2-(3-(3-mesitylureido)-2-naphthamido)-3-(1...)Show SMILES C[C@@H](OC1(C)CCCC1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C31H37N3O5/c1-18-14-19(2)26(20(3)15-18)34-30(38)32-25-17-23-11-7-6-10-22(23)16-24(25)28(35)33-27(29(36)37)21(4)39-31(5)12-8-9-13-31/h6-7,10-11,14-17,21,27H,8-9,12-13H2,1-5H3,(H,33,35)(H,36,37)(H2,32,34,38)/t21-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50255977
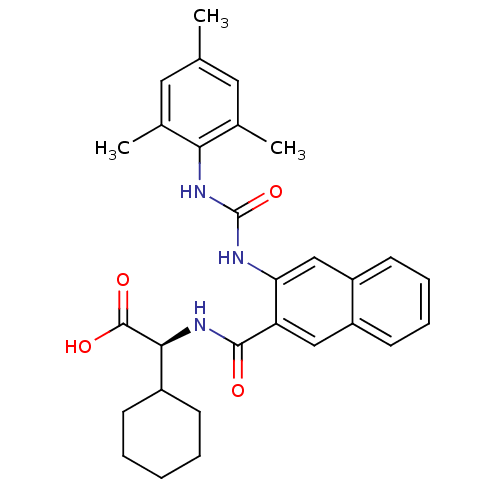
((S)-2-cyclohexyl-2-(3-(3-mesitylureido)-2-naphtham...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@H](C2CCCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C29H33N3O4/c1-17-13-18(2)25(19(3)14-17)32-29(36)30-24-16-22-12-8-7-11-21(22)15-23(24)27(33)31-26(28(34)35)20-9-5-4-6-10-20/h7-8,11-16,20,26H,4-6,9-10H2,1-3H3,(H,31,33)(H,34,35)(H2,30,32,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50418124
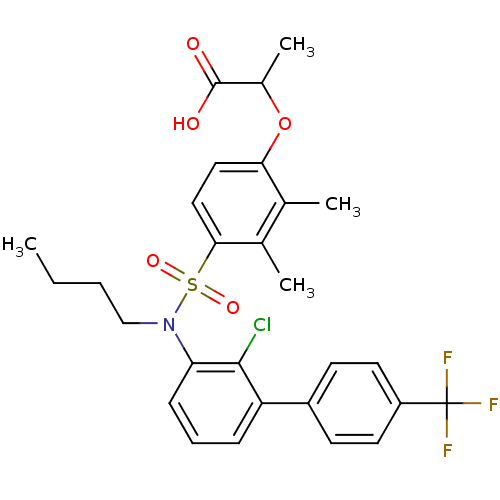
(CHEMBL1760428)Show SMILES CCCCN(c1cccc(-c2ccc(cc2)C(F)(F)F)c1Cl)S(=O)(=O)c1ccc(OC(C)C(O)=O)c(C)c1C Show InChI InChI=1S/C28H29ClF3NO5S/c1-5-6-16-33(39(36,37)25-15-14-24(17(2)18(25)3)38-19(4)27(34)35)23-9-7-8-22(26(23)29)20-10-12-21(13-11-20)28(30,31)32/h7-15,19H,5-6,16H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2345-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.077
BindingDB Entry DOI: 10.7270/Q2M61MHT |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27734
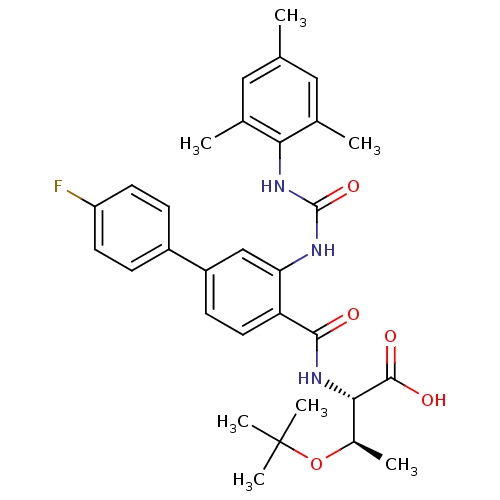
((2S,3R)-3-(tert-butoxy)-2-{[4-(4-fluorophenyl)-2-{...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(C)cc1C)-c1ccc(F)cc1)C(O)=O |r| Show InChI InChI=1S/C31H36FN3O5/c1-17-14-18(2)26(19(3)15-17)35-30(39)33-25-16-22(21-8-11-23(32)12-9-21)10-13-24(25)28(36)34-27(29(37)38)20(4)40-31(5,6)7/h8-16,20,27H,1-7H3,(H,34,36)(H,37,38)(H2,33,35,39)/t20-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27726
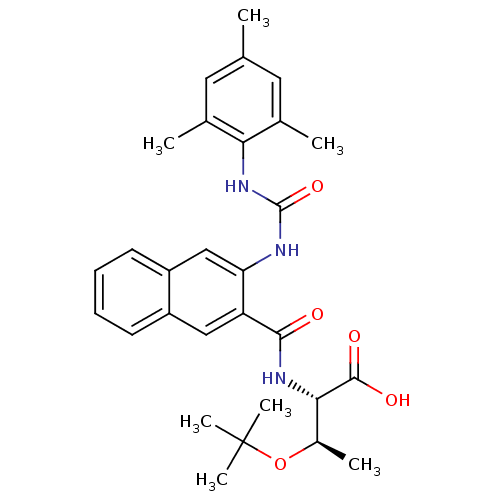
((2S,3R)-3-(tert-butoxy)-2-[(3-{[(2,4,6-trimethylph...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C29H35N3O5/c1-16-12-17(2)24(18(3)13-16)32-28(36)30-23-15-21-11-9-8-10-20(21)14-22(23)26(33)31-25(27(34)35)19(4)37-29(5,6)7/h8-15,19,25H,1-7H3,(H,31,33)(H,34,35)(H2,30,32,36)/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27744
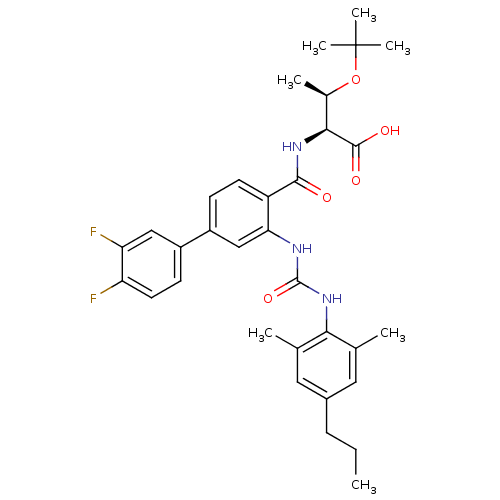
((2S,3R)-3-(tert-butoxy)-2-{[4-(3,4-difluorophenyl)...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2ccc(F)c(F)c2)c(C)c1 |r| Show InChI InChI=1S/C33H39F2N3O5/c1-8-9-21-14-18(2)28(19(3)15-21)38-32(42)36-27-17-23(22-11-13-25(34)26(35)16-22)10-12-24(27)30(39)37-29(31(40)41)20(4)43-33(5,6)7/h10-17,20,29H,8-9H2,1-7H3,(H,37,39)(H,40,41)(H2,36,38,42)/t20-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27726

((2S,3R)-3-(tert-butoxy)-2-[(3-{[(2,4,6-trimethylph...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C29H35N3O5/c1-16-12-17(2)24(18(3)13-16)32-28(36)30-23-15-21-11-9-8-10-20(21)14-22(23)26(33)31-25(27(34)35)19(4)37-29(5,6)7/h8-15,19,25H,1-7H3,(H,31,33)(H,34,35)(H2,30,32,36)/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1802
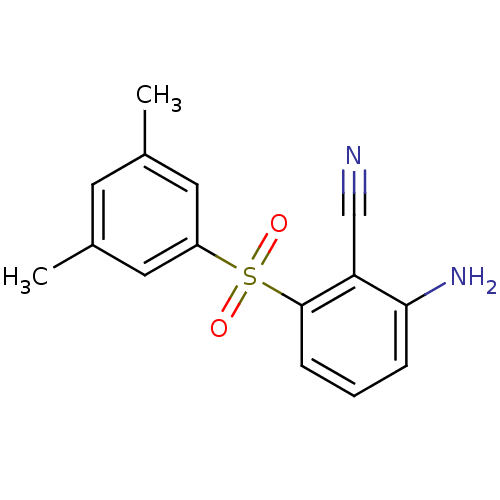
(2-Amino-6-arylthiobenzonitrile deriv. 3v, 739W94 |...)Show InChI InChI=1S/C15H14N2O2S/c1-10-6-11(2)8-12(7-10)20(18,19)15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50418148

(CHEMBL1760411)Show SMILES CCCCN(c1cccc(c1Cl)-c1ccc(Cl)cc1)S(=O)(=O)c1ccc(OC(C)C(O)=O)c(C)c1C Show InChI InChI=1S/C27H29Cl2NO5S/c1-5-6-16-30(23-9-7-8-22(26(23)29)20-10-12-21(28)13-11-20)36(33,34)25-15-14-24(17(2)18(25)3)35-19(4)27(31)32/h7-15,19H,5-6,16H2,1-4H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2345-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.077
BindingDB Entry DOI: 10.7270/Q2M61MHT |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Rattus norvegicus) | BDBM50044874
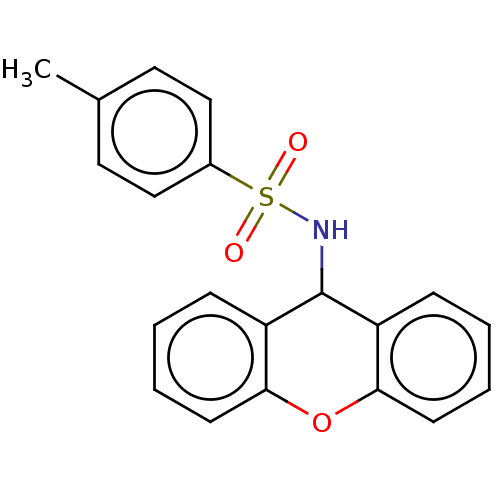
(CHEMBL3311302)Show InChI InChI=1S/C20H17NO3S/c1-14-10-12-15(13-11-14)25(22,23)21-20-16-6-2-4-8-18(16)24-19-9-5-3-7-17(19)20/h2-13,20-21H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat FFA4 receptor expressed in U2OS cells |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Free fatty acid receptor 4
(Mus musculus) | BDBM50044874

(CHEMBL3311302)Show InChI InChI=1S/C20H17NO3S/c1-14-10-12-15(13-11-14)25(22,23)21-20-16-6-2-4-8-18(16)24-19-9-5-3-7-17(19)20/h2-13,20-21H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of mouse FFA4 receptor expressed in U2OS cells |
Bioorg Med Chem Lett 24: 3100-3 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.012
BindingDB Entry DOI: 10.7270/Q2CC1292 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27731
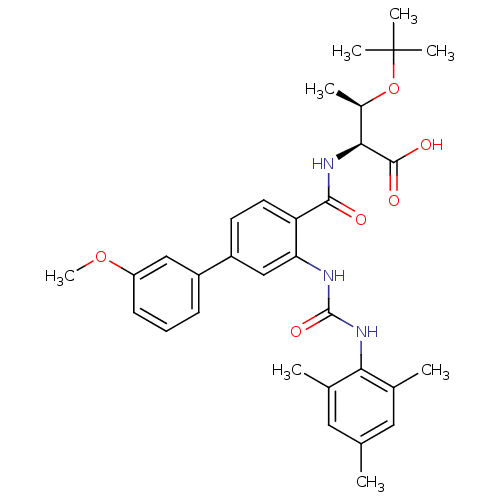
((2S,3R)-3-(tert-butoxy)-2-{[4-(3-methoxyphenyl)-2-...)Show SMILES COc1cccc(c1)-c1ccc(C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)c(NC(=O)Nc2c(C)cc(C)cc2C)c1 |r| Show InChI InChI=1S/C32H39N3O6/c1-18-14-19(2)27(20(3)15-18)35-31(39)33-26-17-23(22-10-9-11-24(16-22)40-8)12-13-25(26)29(36)34-28(30(37)38)21(4)41-32(5,6)7/h9-17,21,28H,1-8H3,(H,34,36)(H,37,38)(H2,33,35,39)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502516

(CHEMBL4470436)Show InChI InChI=1S/C16H14F2N4/c1-2-13-10(7-19-8-20-13)16-21-14-5-11(17)12(18)6-15(14)22(16)9-3-4-9/h5-9H,2-4H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502509
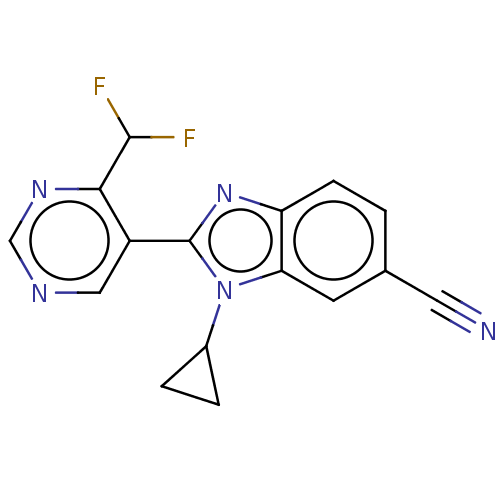
(CHEMBL4548610)Show InChI InChI=1S/C16H11F2N5/c17-15(18)14-11(7-20-8-21-14)16-22-12-4-1-9(6-19)5-13(12)23(16)10-2-3-10/h1,4-5,7-8,10,15H,2-3H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256573
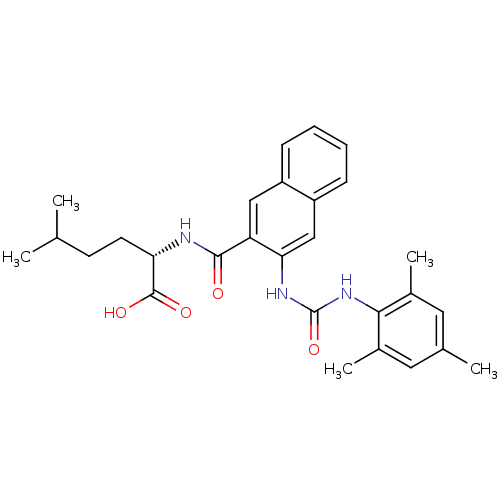
((S)-2-(3-(3-mesitylureido)-2-naphthamido)-5-methyl...)Show SMILES CC(C)CC[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C28H33N3O4/c1-16(2)10-11-23(27(33)34)29-26(32)22-14-20-8-6-7-9-21(20)15-24(22)30-28(35)31-25-18(4)12-17(3)13-19(25)5/h6-9,12-16,23H,10-11H2,1-5H3,(H,29,32)(H,33,34)(H2,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27729
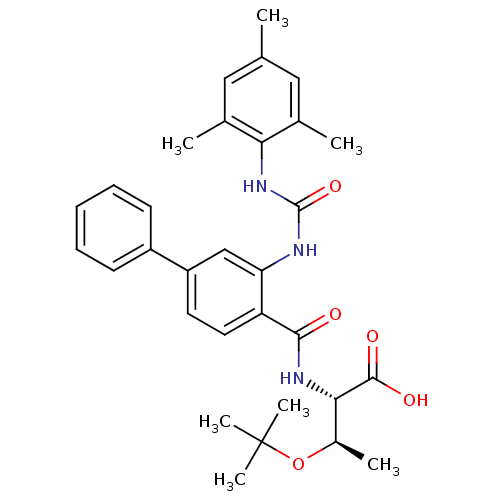
((2S,3R)-3-(tert-butoxy)-2-[(4-phenyl-2-{[(2,4,6-tr...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(C)cc1C)-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C31H37N3O5/c1-18-15-19(2)26(20(3)16-18)34-30(38)32-25-17-23(22-11-9-8-10-12-22)13-14-24(25)28(35)33-27(29(36)37)21(4)39-31(5,6)7/h8-17,21,27H,1-7H3,(H,33,35)(H,36,37)(H2,32,34,38)/t21-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27742
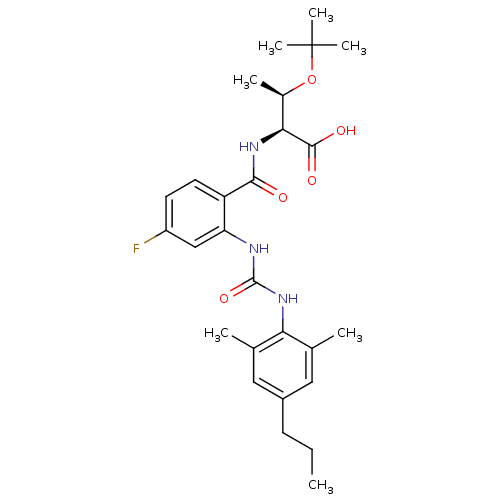
((2S,3R)-3-(tert-butoxy)-2-[(2-{[(2,6-dimethyl-4-pr...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(F)ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C27H36FN3O5/c1-8-9-18-12-15(2)22(16(3)13-18)31-26(35)29-21-14-19(28)10-11-20(21)24(32)30-23(25(33)34)17(4)36-27(5,6)7/h10-14,17,23H,8-9H2,1-7H3,(H,30,32)(H,33,34)(H2,29,31,35)/t17-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256169
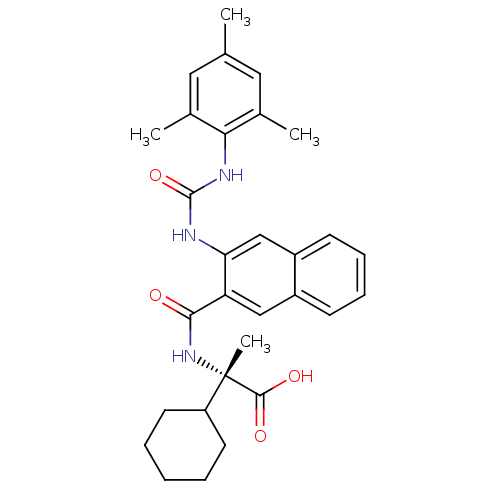
((S)-2-cyclohexyl-2-(3-(3-mesitylureido)-2-naphtham...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@](C)(C2CCCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C30H35N3O4/c1-18-14-19(2)26(20(3)15-18)32-29(37)31-25-17-22-11-9-8-10-21(22)16-24(25)27(34)33-30(4,28(35)36)23-12-6-5-7-13-23/h8-11,14-17,23H,5-7,12-13H2,1-4H3,(H,33,34)(H,35,36)(H2,31,32,37)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1805
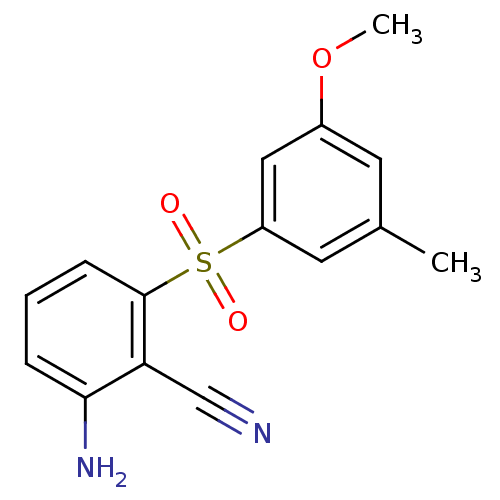
(2-Amino-6-arylthiobenzonitrile deriv. 3y | 2-amino...)Show InChI InChI=1S/C15H14N2O3S/c1-10-6-11(20-2)8-12(7-10)21(18,19)15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502517
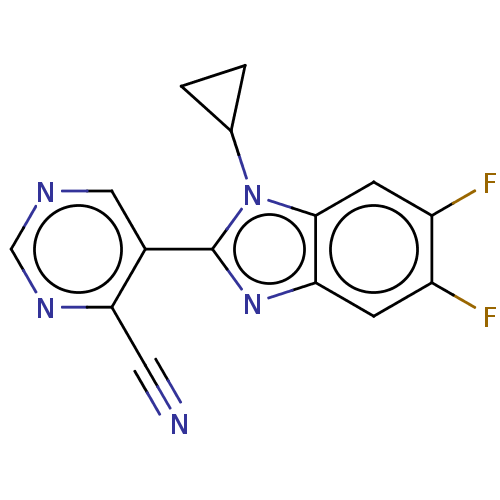
(CHEMBL4458025)Show InChI InChI=1S/C15H9F2N5/c16-10-3-12-14(4-11(10)17)22(8-1-2-8)15(21-12)9-6-19-7-20-13(9)5-18/h3-4,6-8H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27735
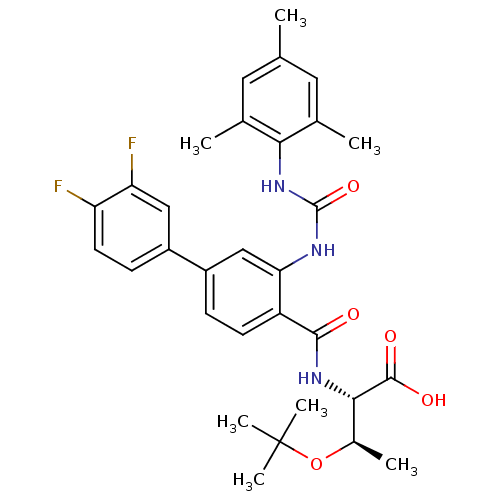
((2S,3R)-3-(tert-butoxy)-2-{[4-(3,4-difluorophenyl)...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(C)cc1C)-c1ccc(F)c(F)c1)C(O)=O |r| Show InChI InChI=1S/C31H35F2N3O5/c1-16-12-17(2)26(18(3)13-16)36-30(40)34-25-15-21(20-9-11-23(32)24(33)14-20)8-10-22(25)28(37)35-27(29(38)39)19(4)41-31(5,6)7/h8-15,19,27H,1-7H3,(H,35,37)(H,38,39)(H2,34,36,40)/t19-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27740

((2S)-2-cyclohexyl-2-{[2-({[4-(cyclopropylmethyl)-2...)Show SMILES Cc1cc(CC2CC2)cc(C)c1NC(=O)Nc1cc(F)ccc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C28H34FN3O4/c1-16-12-19(14-18-8-9-18)13-17(2)24(16)32-28(36)30-23-15-21(29)10-11-22(23)26(33)31-25(27(34)35)20-6-4-3-5-7-20/h10-13,15,18,20,25H,3-9,14H2,1-2H3,(H,31,33)(H,34,35)(H2,30,32,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50418129
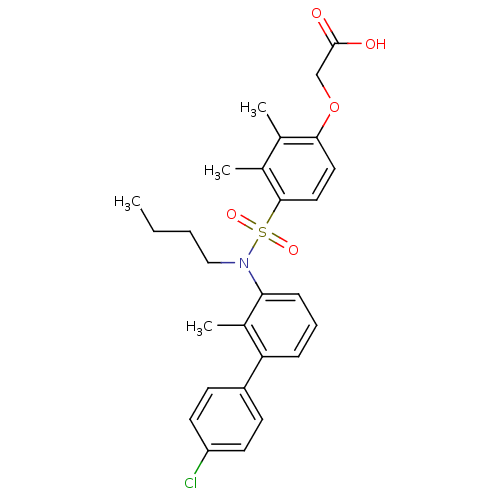
(CHEMBL1760268)Show SMILES CCCCN(c1cccc(c1C)-c1ccc(Cl)cc1)S(=O)(=O)c1ccc(OCC(O)=O)c(C)c1C Show InChI InChI=1S/C27H30ClNO5S/c1-5-6-16-29(24-9-7-8-23(20(24)4)21-10-12-22(28)13-11-21)35(32,33)26-15-14-25(18(2)19(26)3)34-17-27(30)31/h7-15H,5-6,16-17H2,1-4H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2345-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.077
BindingDB Entry DOI: 10.7270/Q2M61MHT |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256071
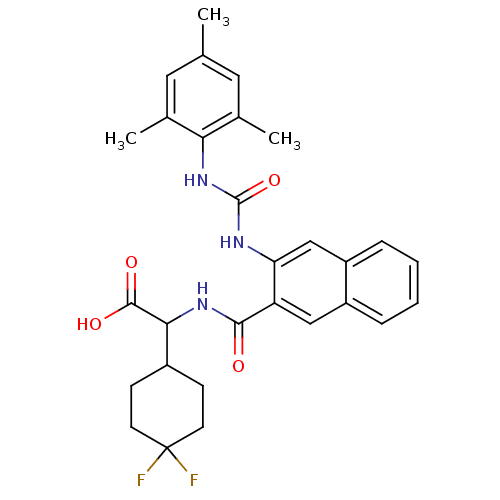
(2-(4,4-difluorocyclohexyl)-2-(3-(3-mesitylureido)-...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC(C2CCC(F)(F)CC2)C(O)=O)c(C)c1 Show InChI InChI=1S/C29H31F2N3O4/c1-16-12-17(2)24(18(3)13-16)34-28(38)32-23-15-21-7-5-4-6-20(21)14-22(23)26(35)33-25(27(36)37)19-8-10-29(30,31)11-9-19/h4-7,12-15,19,25H,8-11H2,1-3H3,(H,33,35)(H,36,37)(H2,32,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256330
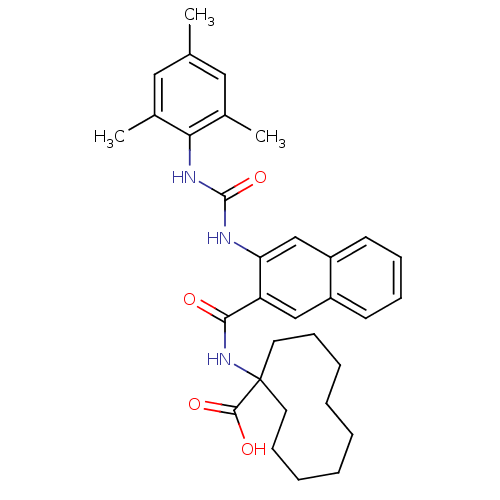
(1-(3-(3-mesitylureido)-2-naphthamido)cyclodecaneca...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC2(CCCCCCCCC2)C(O)=O)c(C)c1 Show InChI InChI=1S/C32H39N3O4/c1-21-17-22(2)28(23(3)18-21)34-31(39)33-27-20-25-14-10-9-13-24(25)19-26(27)29(36)35-32(30(37)38)15-11-7-5-4-6-8-12-16-32/h9-10,13-14,17-20H,4-8,11-12,15-16H2,1-3H3,(H,35,36)(H,37,38)(H2,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256012

((S)-2-cyclopentyl-2-(3-(3-mesitylureido)-2-naphtha...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@H](C2CCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C28H31N3O4/c1-16-12-17(2)24(18(3)13-16)31-28(35)29-23-15-21-11-7-6-10-20(21)14-22(23)26(32)30-25(27(33)34)19-8-4-5-9-19/h6-7,10-15,19,25H,4-5,8-9H2,1-3H3,(H,30,32)(H,33,34)(H2,29,31,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27733

((2S,3R)-3-(tert-butoxy)-2-{[4-(3-fluorophenyl)-2-{...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(C)cc1C)-c1cccc(F)c1)C(O)=O |r| Show InChI InChI=1S/C31H36FN3O5/c1-17-13-18(2)26(19(3)14-17)35-30(39)33-25-16-22(21-9-8-10-23(32)15-21)11-12-24(25)28(36)34-27(29(37)38)20(4)40-31(5,6)7/h8-16,20,27H,1-7H3,(H,34,36)(H,37,38)(H2,33,35,39)/t20-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256710
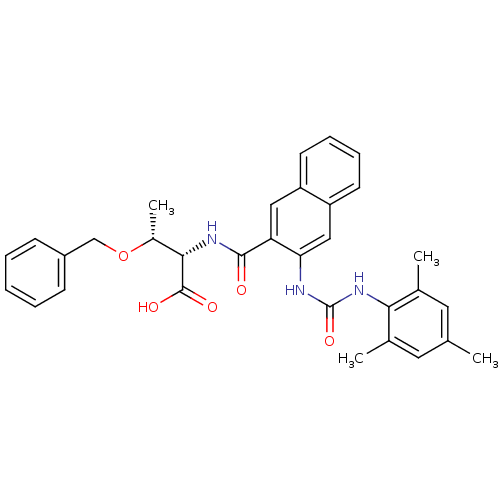
((2S,3R)-3-(benzyloxy)-2-(3-(3-mesitylureido)-2-nap...)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C32H33N3O5/c1-19-14-20(2)28(21(3)15-19)35-32(39)33-27-17-25-13-9-8-12-24(25)16-26(27)30(36)34-29(31(37)38)22(4)40-18-23-10-6-5-7-11-23/h5-17,22,29H,18H2,1-4H3,(H,34,36)(H,37,38)(H2,33,35,39)/t22-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50418135
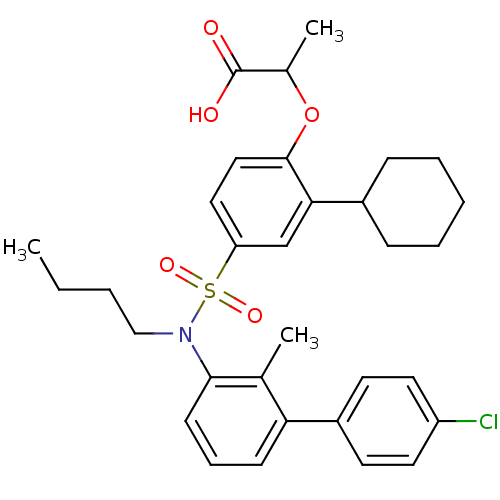
(CHEMBL1760274)Show SMILES CCCCN(c1cccc(c1C)-c1ccc(Cl)cc1)S(=O)(=O)c1ccc(OC(C)C(O)=O)c(c1)C1CCCCC1 Show InChI InChI=1S/C32H38ClNO5S/c1-4-5-20-34(30-13-9-12-28(22(30)2)25-14-16-26(33)17-15-25)40(37,38)27-18-19-31(39-23(3)32(35)36)29(21-27)24-10-7-6-8-11-24/h9,12-19,21,23-24H,4-8,10-11,20H2,1-3H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2345-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.077
BindingDB Entry DOI: 10.7270/Q2M61MHT |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50418161
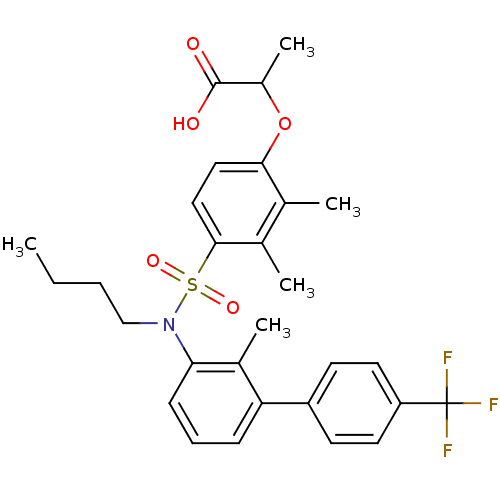
(CHEMBL1760424)Show SMILES CCCCN(c1cccc(c1C)-c1ccc(cc1)C(F)(F)F)S(=O)(=O)c1ccc(OC(C)C(O)=O)c(C)c1C Show InChI InChI=1S/C29H32F3NO5S/c1-6-7-17-33(39(36,37)27-16-15-26(18(2)19(27)3)38-21(5)28(34)35)25-10-8-9-24(20(25)4)22-11-13-23(14-12-22)29(30,31)32/h8-16,21H,6-7,17H2,1-5H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2345-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.077
BindingDB Entry DOI: 10.7270/Q2M61MHT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502510

(CHEMBL4474610)Show InChI InChI=1S/C16H10F3N5/c17-16(18,19)14-11(7-21-8-22-14)15-23-12-4-1-9(6-20)5-13(12)24(15)10-2-3-10/h1,4-5,7-8,10H,2-3H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50418123
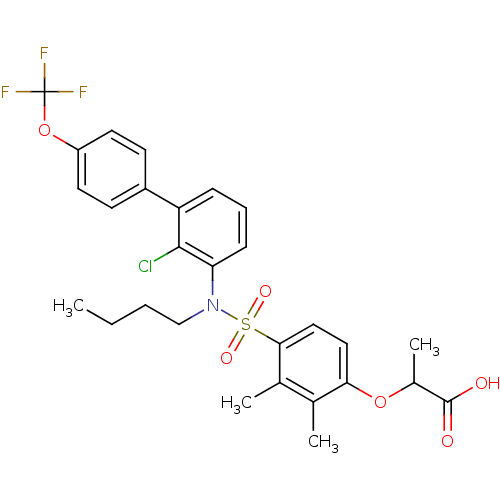
(CHEMBL1760005)Show SMILES CCCCN(c1cccc(-c2ccc(OC(F)(F)F)cc2)c1Cl)S(=O)(=O)c1ccc(OC(C)C(O)=O)c(C)c1C Show InChI InChI=1S/C28H29ClF3NO6S/c1-5-6-16-33(40(36,37)25-15-14-24(17(2)18(25)3)38-19(4)27(34)35)23-9-7-8-22(26(23)29)20-10-12-21(13-11-20)39-28(30,31)32/h7-15,19H,5-6,16H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]GW 2433 from human PPARdelta by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2345-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.077
BindingDB Entry DOI: 10.7270/Q2M61MHT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502504
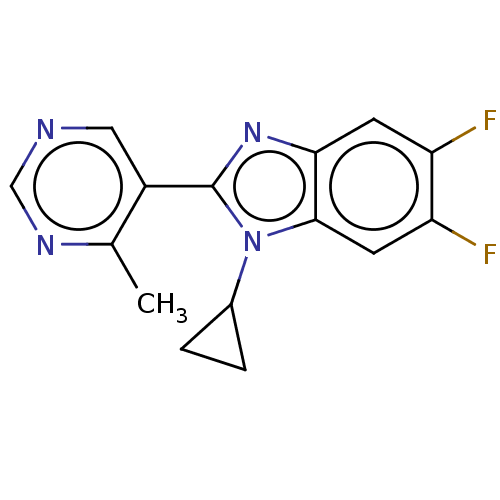
(CHEMBL4582349)Show InChI InChI=1S/C15H12F2N4/c1-8-10(6-18-7-19-8)15-20-13-4-11(16)12(17)5-14(13)21(15)9-2-3-9/h4-7,9H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256667
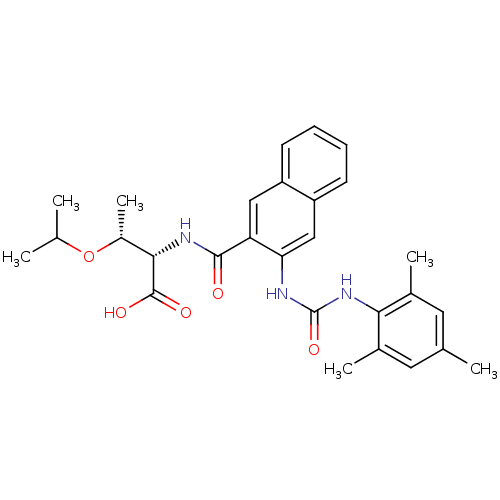
((2S,3R)-3-isopropoxy-2-(3-(3-mesitylureido)-2-naph...)Show SMILES CC(C)O[C@H](C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C28H33N3O5/c1-15(2)36-19(6)25(27(33)34)30-26(32)22-13-20-9-7-8-10-21(20)14-23(22)29-28(35)31-24-17(4)11-16(3)12-18(24)5/h7-15,19,25H,1-6H3,(H,30,32)(H,33,34)(H2,29,31,35)/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256668
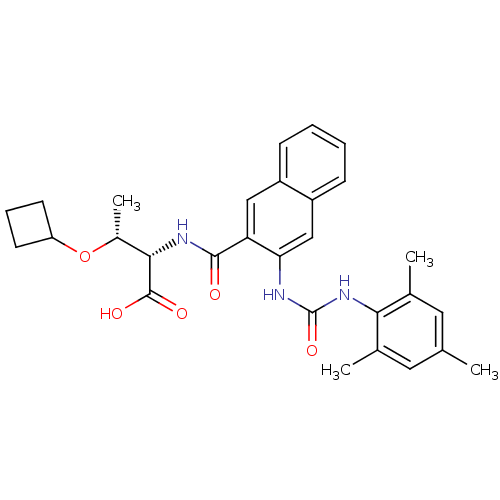
((2S,3R)-3-cyclobutoxy-2-(3-(3-mesitylureido)-2-nap...)Show SMILES C[C@@H](OC1CCC1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C29H33N3O5/c1-16-12-17(2)25(18(3)13-16)32-29(36)30-24-15-21-9-6-5-8-20(21)14-23(24)27(33)31-26(28(34)35)19(4)37-22-10-7-11-22/h5-6,8-9,12-15,19,22,26H,7,10-11H2,1-4H3,(H,31,33)(H,34,35)(H2,30,32,36)/t19-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50502508

(CHEMBL4476620)Show InChI InChI=1S/C15H10F4N4/c16-9-3-11-12(4-10(9)17)23(7-1-2-7)15(22-11)8-5-20-6-21-13(8)14(18)19/h3-7,14H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Selenity Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP11B2 using deoxycorticosterone as substrate incubated for 90 mins by cell-based HPLC analysis |
ACS Med Chem Lett 10: 1056-1060 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00152
BindingDB Entry DOI: 10.7270/Q2639T0V |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27739
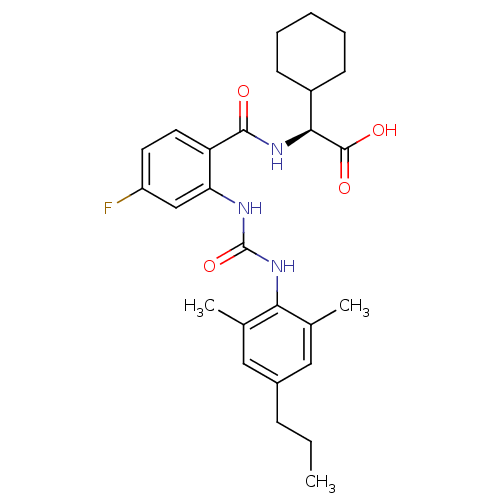
((2S)-2-cyclohexyl-2-[(2-{[(2,6-dimethyl-4-propylph...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(F)ccc2C(=O)N[C@@H](C2CCCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C27H34FN3O4/c1-4-8-18-13-16(2)23(17(3)14-18)31-27(35)29-22-15-20(28)11-12-21(22)25(32)30-24(26(33)34)19-9-6-5-7-10-19/h11-15,19,24H,4-10H2,1-3H3,(H,30,32)(H,33,34)(H2,29,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data