 Found 1581 hits with Last Name = 'keller' and Initial = 't'
Found 1581 hits with Last Name = 'keller' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
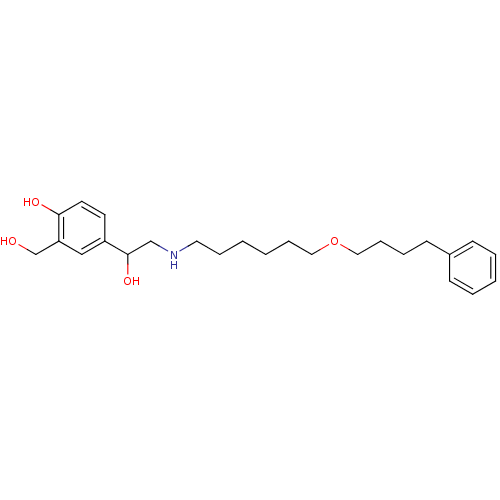
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390424
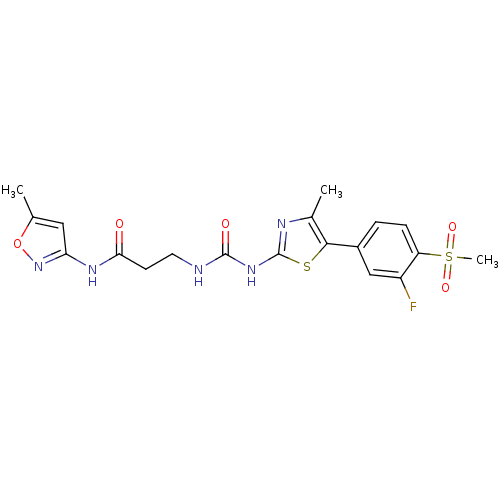
(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318156
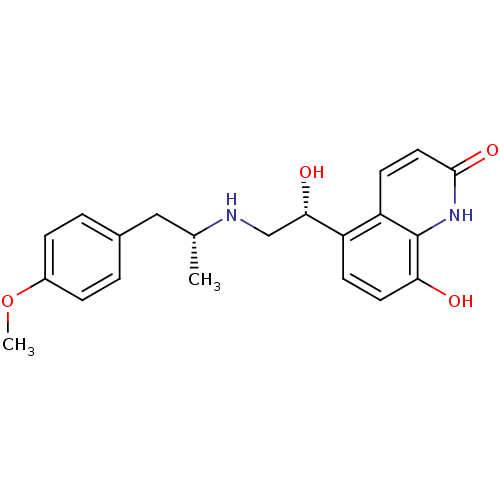
(CHEMBL1094785 | carmoterol)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C21H24N2O4/c1-13(11-14-3-5-15(27-2)6-4-14)22-12-19(25)16-7-9-18(24)21-17(16)8-10-20(26)23-21/h3-10,13,19,22,24-25H,11-12H2,1-2H3,(H,23,26)/t13-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase |
J Med Chem 31: 500-3 (1988)
BindingDB Entry DOI: 10.7270/Q28P61Q3 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006222

((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C(CO)[C@@H](O)[C@H]1O |r,t:13| Show InChI InChI=1S/C11H13N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h1,3-4,6,8-9,17-19H,2H2,(H2,12,13,14)/t6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50367250

(3-DEAZAARISTEROMYCIN A | CHEMBL268272)Show SMILES Nc1nccc2n(cnc12)[C@@H]1C[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C12H16N4O3/c13-12-9-7(1-2-14-12)16(5-15-9)8-3-6(4-17)10(18)11(8)19/h1-2,5-6,8,10-11,17-19H,3-4H2,(H2,13,14)/t6-,8-,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against S-adenosyl-homocysteine hydrolase |
J Med Chem 28: 471-7 (1985)
BindingDB Entry DOI: 10.7270/Q21C1XF3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390409
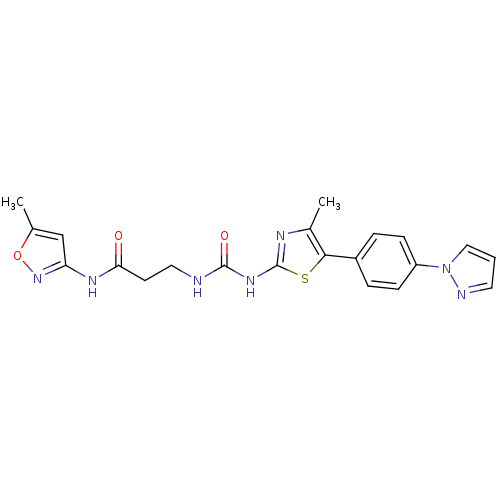
(CHEMBL1986603)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(cc2)-n2cccn2)no1 Show InChI InChI=1S/C21H21N7O3S/c1-13-12-17(27-31-13)25-18(29)8-10-22-20(30)26-21-24-14(2)19(32-21)15-4-6-16(7-5-15)28-11-3-9-23-28/h3-7,9,11-12H,8,10H2,1-2H3,(H,25,27,29)(H2,22,24,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390408
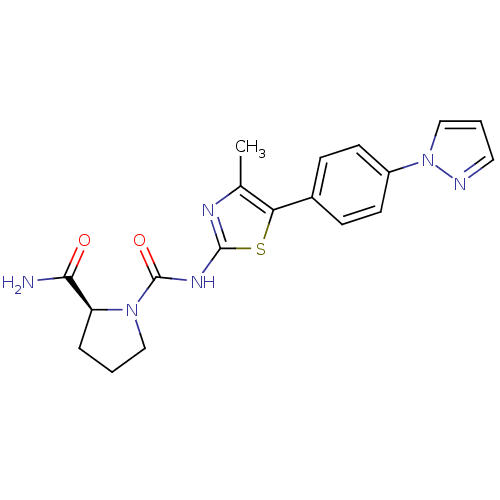
(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18793
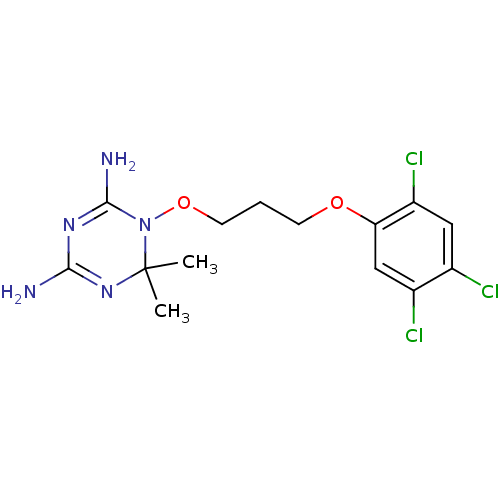
(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166735
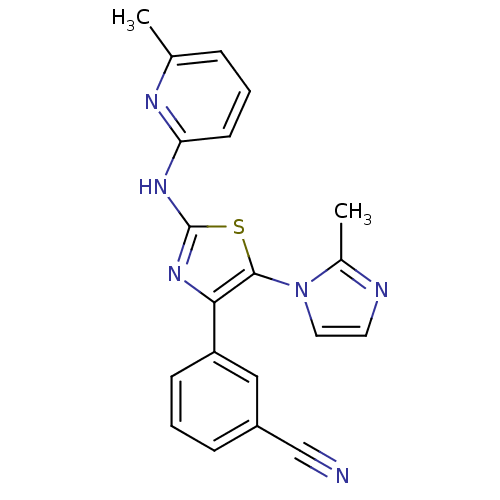
(3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...)Show SMILES Cc1nccn1-c1sc(Nc2cccc(C)n2)nc1-c1cccc(c1)C#N Show InChI InChI=1S/C20H16N6S/c1-13-5-3-8-17(23-13)24-20-25-18(16-7-4-6-15(11-16)12-21)19(27-20)26-10-9-22-14(26)2/h3-11H,1-2H3,(H,23,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50035483
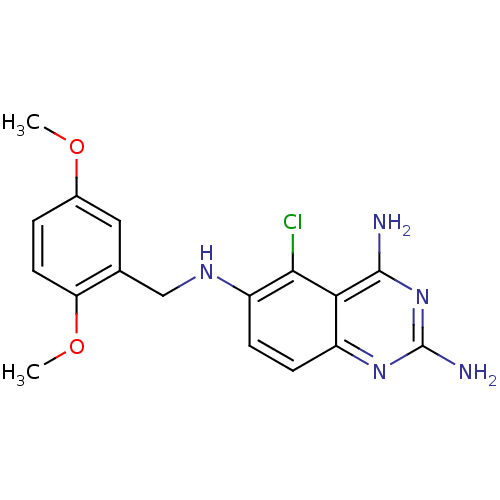
(5-Chloro-N*6*-(2,5-dimethoxy-benzyl)-quinazoline-2...)Show InChI InChI=1S/C17H18ClN5O2/c1-24-10-3-6-13(25-2)9(7-10)8-21-12-5-4-11-14(15(12)18)16(19)23-17(20)22-11/h3-7,21H,8H2,1-2H3,(H4,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390426
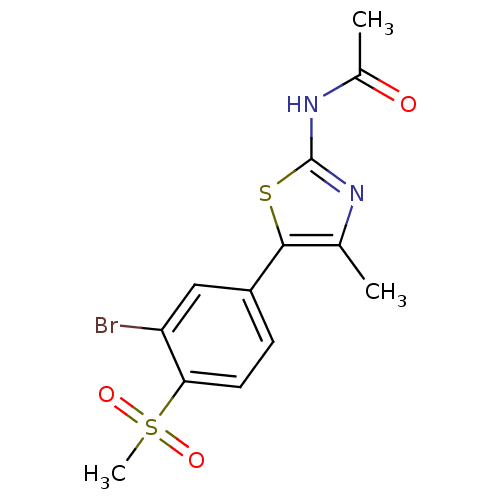
(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390419
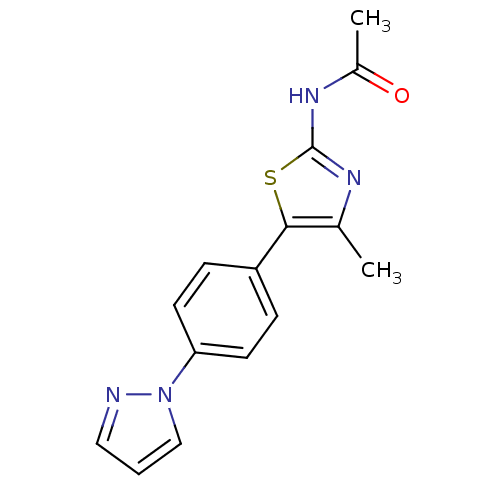
(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390423
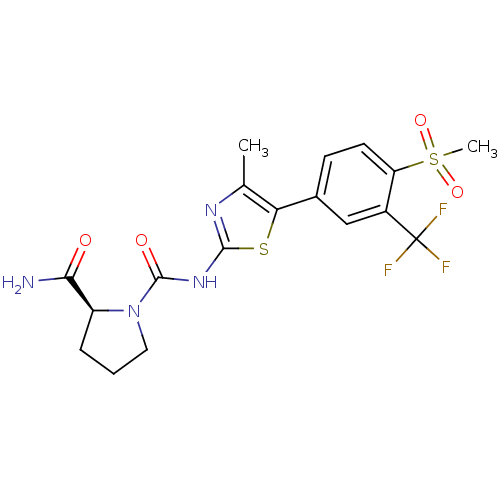
(CHEMBL2071337)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O |r| Show InChI InChI=1S/C18H19F3N4O4S2/c1-9-14(10-5-6-13(31(2,28)29)11(8-10)18(19,20)21)30-16(23-9)24-17(27)25-7-3-4-12(25)15(22)26/h5-6,8,12H,3-4,7H2,1-2H3,(H2,22,26)(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166742
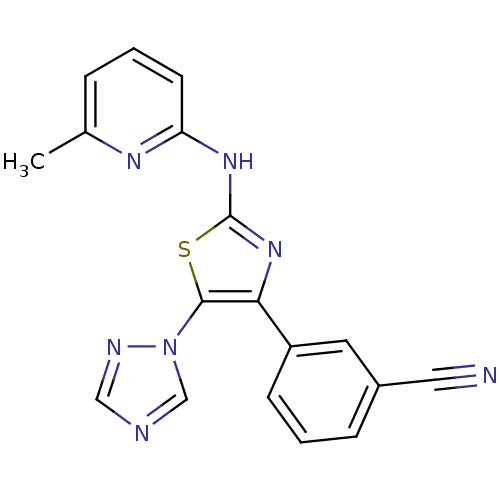
(3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...)Show SMILES Cc1cccc(Nc2nc(c(s2)-n2cncn2)-c2cccc(c2)C#N)n1 Show InChI InChI=1S/C18H13N7S/c1-12-4-2-7-15(22-12)23-18-24-16(14-6-3-5-13(8-14)9-19)17(26-18)25-11-20-10-21-25/h2-8,10-11H,1H3,(H,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390425
(CHEMBL2071341)Show SMILES Cc1nc(NC(=O)NCCC(=O)OC(C)(C)C)sc1-c1ccc(c(F)c1)S(C)(=O)=O Show InChI InChI=1S/C19H24FN3O5S2/c1-11-16(12-6-7-14(13(20)10-12)30(5,26)27)29-18(22-11)23-17(25)21-9-8-15(24)28-19(2,3)4/h6-7,10H,8-9H2,1-5H3,(H2,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151720
(ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c(NC=O)c2)cc1 |r| Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22)/t13-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166739
(3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-4-3-5-13(8-12)15-16(24-11-19-10-21-24)25-17(23-15)22-14-6-1-2-7-20-14/h1-8,10-11H,(H,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390418
(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390419

(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 30.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390426

(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50405655

(CHEMBL147260)Show SMILES Nc1nccc2n(cnc12)C1C=C[C@@H](O)[C@H]1O |c:13| Show InChI InChI=1S/C11H12N4O2/c12-11-9-6(3-4-13-11)15(5-14-9)7-1-2-8(16)10(7)17/h1-5,7-8,10,16-17H,(H2,12,13)/t7?,8-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase |
J Med Chem 31: 500-3 (1988)
BindingDB Entry DOI: 10.7270/Q28P61Q3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390415
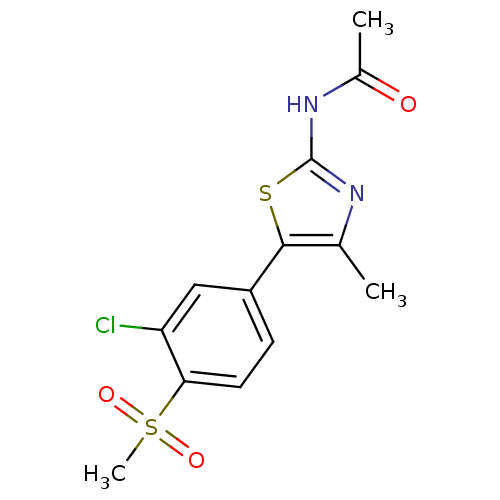
(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390411
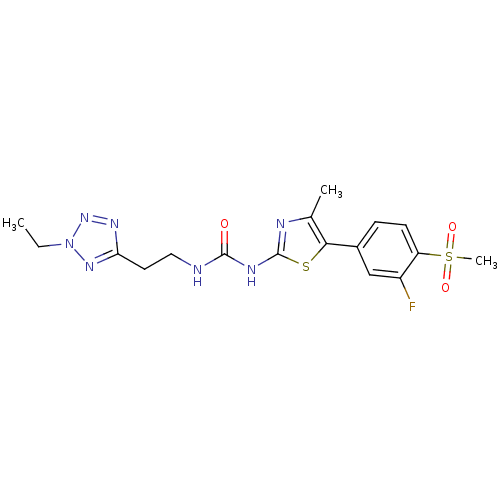
(CHEMBL2071342)Show SMILES CCn1nnc(CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)n1 Show InChI InChI=1S/C17H20FN7O3S2/c1-4-25-23-14(22-24-25)7-8-19-16(26)21-17-20-10(2)15(29-17)11-5-6-13(12(18)9-11)30(3,27)28/h5-6,9H,4,7-8H2,1-3H3,(H2,19,20,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50166742

(3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...)Show SMILES Cc1cccc(Nc2nc(c(s2)-n2cncn2)-c2cccc(c2)C#N)n1 Show InChI InChI=1S/C18H13N7S/c1-12-4-2-7-15(22-12)23-18-24-16(14-6-3-5-13(8-14)9-19)17(26-18)25-11-20-10-21-25/h2-8,10-11H,1H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50006215
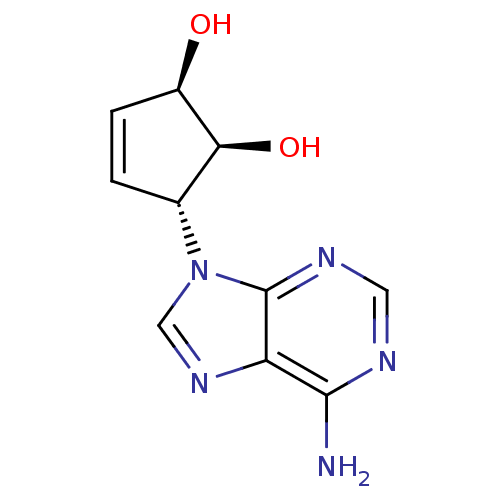
((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C=C[C@@H](O)[C@H]1O |c:13| Show InChI InChI=1S/C10H11N5O2/c11-9-7-10(13-3-12-9)15(4-14-7)5-1-2-6(16)8(5)17/h1-6,8,16-17H,(H2,11,12,13)/t5-,6-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase |
J Med Chem 31: 500-3 (1988)
BindingDB Entry DOI: 10.7270/Q28P61Q3 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Dengue virus 2) | BDBM50175864

(Bz-Nle-Lys-Arg-Arg-B(OH)2 | CHEMBL199845)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#5](-[#8])-[#8] Show InChI InChI=1S/C31H54BN11O7/c1-2-3-13-22(41-26(45)20-11-5-4-6-12-20)28(47)42-23(14-7-8-17-33)29(48)43-24(16-10-19-39-31(36)37)27(46)40-21(25(44)32(49)50)15-9-18-38-30(34)35/h4-6,11-12,21-24,49-50H,2-3,7-10,13-19,33H2,1H3,(H,40,46)(H,41,45)(H,42,47)(H,43,48)(H4,34,35,38)(H4,36,37,39)/t21-,22-,23-,24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases
Curated by ChEMBL
| Assay Description
Inhibitory activity against CF40.NS3pro from Dengue virus type 2 |
Bioorg Med Chem Lett 16: 36-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.062
BindingDB Entry DOI: 10.7270/Q2639P97 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390417
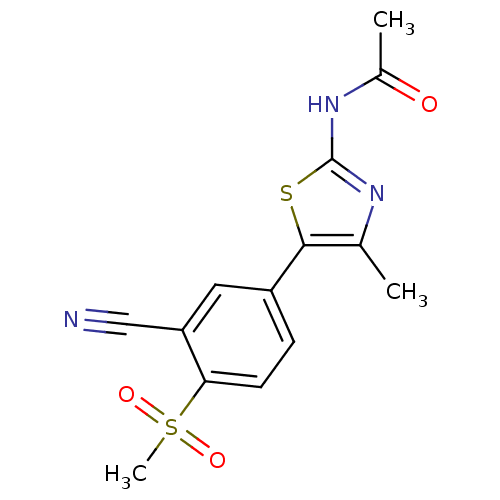
(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390422
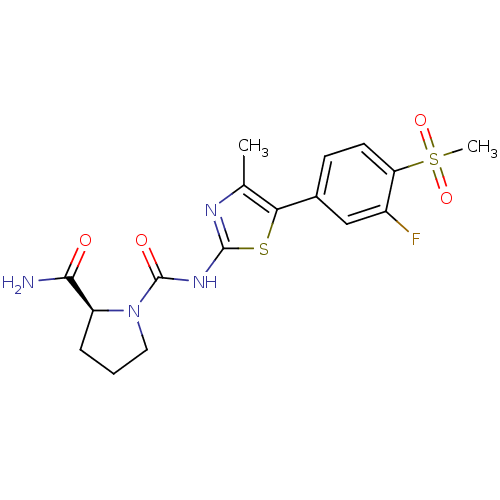
(CHEMBL2071336)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(c(F)c1)S(C)(=O)=O |r| Show InChI InChI=1S/C17H19FN4O4S2/c1-9-14(10-5-6-13(11(18)8-10)28(2,25)26)27-16(20-9)21-17(24)22-7-3-4-12(22)15(19)23/h5-6,8,12H,3-4,7H2,1-2H3,(H2,19,23)(H,20,21,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166739

(3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-4-3-5-13(8-12)15-16(24-11-19-10-21-24)25-17(23-15)22-14-6-1-2-7-20-14/h1-8,10-11H,(H,20,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18792
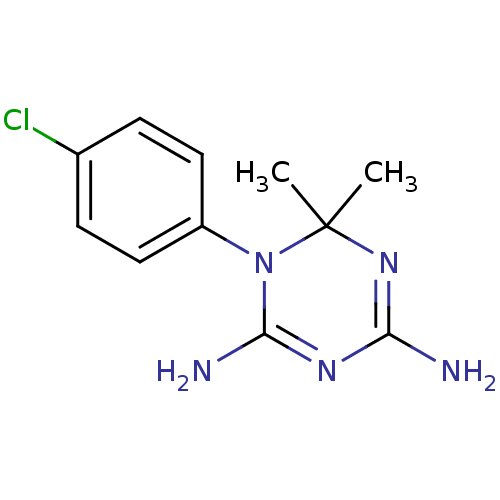
(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 55.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay |
Antimicrob Agents Chemother 54: 2603-10 (2010)
Article DOI: 10.1128/AAC.01526-09
BindingDB Entry DOI: 10.7270/Q2VX0GQW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390415

(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390426

(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390418

(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390417

(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166745
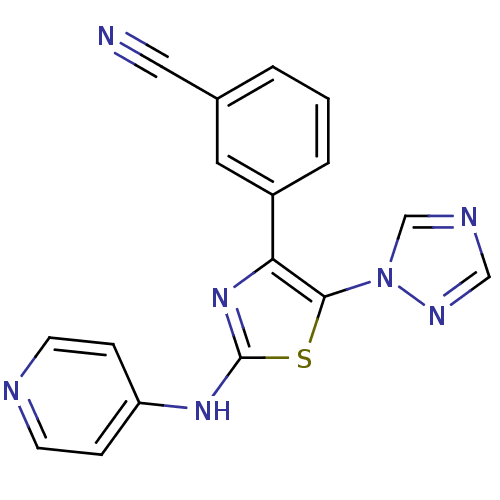
(3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-9-12-2-1-3-13(8-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-4-6-19-7-5-14/h1-8,10-11H,(H,19,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390418

(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318159
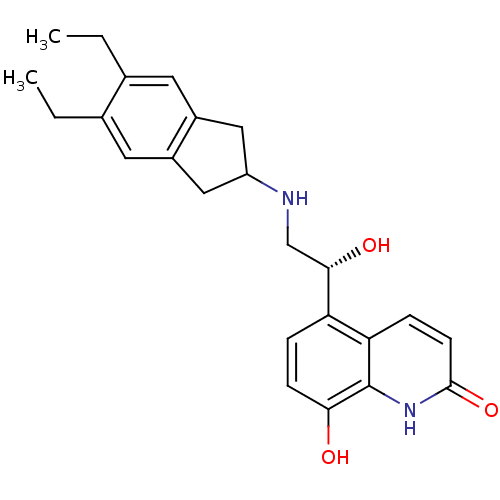
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390415

(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390417

(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166737
(3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...)Show InChI InChI=1S/C17H11N7S/c18-8-12-3-1-4-13(7-12)15-16(24-11-20-10-21-24)25-17(23-15)22-14-5-2-6-19-9-14/h1-7,9-11H,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390410
(CHEMBL2069328)Show SMILES CC(=O)c1ccc(cc1)-c1sc(NC(=O)NCCC(=O)NC(C)(C)C)nc1C Show InChI InChI=1S/C20H26N4O3S/c1-12-17(15-8-6-14(7-9-15)13(2)25)28-19(22-12)23-18(27)21-11-10-16(26)24-20(3,4)5/h6-9H,10-11H2,1-5H3,(H,24,26)(H2,21,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50390425

(CHEMBL2071341)Show SMILES Cc1nc(NC(=O)NCCC(=O)OC(C)(C)C)sc1-c1ccc(c(F)c1)S(C)(=O)=O Show InChI InChI=1S/C19H24FN3O5S2/c1-11-16(12-6-7-14(13(20)10-12)30(5,26)27)29-18(22-11)23-17(25)21-9-8-15(24)28-19(2,3)4/h6-7,10H,8-9H2,1-5H3,(H2,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110beta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023889
(5-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-pent-3-ene-...)Show InChI InChI=1S/C11H14N4O2/c12-11-10-9(3-4-13-11)15(7-14-10)5-1-2-8(17)6-16/h1-4,7-8,16-17H,5-6H2,(H2,12,13)/b2-1-/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50166743

(3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...)Show InChI InChI=1S/C18H12N6S/c19-10-13-3-1-4-14(9-13)16-17(24-8-7-21-12-24)25-18(23-16)22-15-5-2-6-20-11-15/h1-9,11-12H,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... |
Bioorg Med Chem Lett 15: 3081-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.021
BindingDB Entry DOI: 10.7270/Q2X63NQG |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50088426

((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C11H15N5O3/c12-10-7-11(14-3-13-10)16(4-15-7)6-1-5(2-17)8(18)9(6)19/h3-6,8-9,17-19H,1-2H2,(H2,12,13,14)/t5-,6-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against S-adenosyl-homocysteine hydrolase |
J Med Chem 28: 471-7 (1985)
BindingDB Entry DOI: 10.7270/Q21C1XF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390413
(CHEMBL2071326)Show InChI InChI=1S/C12H13N3O3S2/c1-7-11(19-12(14-7)15-8(2)16)9-3-5-10(6-4-9)20(13,17)18/h3-6H,1-2H3,(H2,13,17,18)(H,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390413

(CHEMBL2071326)Show InChI InChI=1S/C12H13N3O3S2/c1-7-11(19-12(14-7)15-8(2)16)9-3-5-10(6-4-9)20(13,17)18/h3-6H,1-2H3,(H2,13,17,18)(H,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50023879

(2-[2-(6-Amino-purin-9-yl)-ethylidene]-propane-1,3-...)Show SMILES [#7]-c1ncnc2n(-[#6]\[#6]=[#6](/[#6]-[#8])-[#6]-[#8])cnc12 Show InChI InChI=1S/C10H13N5O2/c11-9-8-10(13-5-12-9)15(6-14-8)2-1-7(3-16)4-17/h1,5-6,16-17H,2-4H2,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas
Curated by ChEMBL
| Assay Description
Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase |
J Med Chem 31: 1729-38 (1988)
BindingDB Entry DOI: 10.7270/Q2HM5920 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data