 Found 2552 hits with Last Name = 'wang' and Initial = 'tc'
Found 2552 hits with Last Name = 'wang' and Initial = 'tc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218305

(3-chloro-N-((1S,2R)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1[C@@H](Cc2ccccc12)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C30H23ClN4O3/c31-24-17-32-25-16-20(10-13-23(24)25)30(38)34-28-22-6-2-1-5-19(22)15-26(28)33-29(37)18-8-11-21(12-9-18)35-14-4-3-7-27(35)36/h1-14,16-17,26,28,32H,15H2,(H,33,37)(H,34,38)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218298
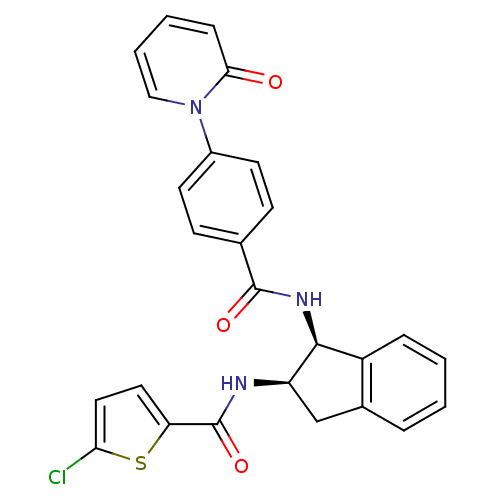
(5-chloro-N-((1S,2R)-1-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1Cc2ccccc2[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H20ClN3O3S/c27-22-13-12-21(34-22)26(33)28-20-15-17-5-1-2-6-19(17)24(20)29-25(32)16-8-10-18(11-9-16)30-14-4-3-7-23(30)31/h1-14,20,24H,15H2,(H,28,33)(H,29,32)/t20-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 2.80 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 49: 7596-9 (2006)
Article DOI: 10.1021/jm061101w
BindingDB Entry DOI: 10.7270/Q2862DQ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218280

(5-chloro-N-((3R,4S)-1-(2-methoxyacetyl)-4-(4-(2-ox...)Show SMILES COCC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-34-14-22(31)28-12-17(18(13-28)27-24(33)19-9-10-20(25)35-19)26-23(32)15-5-7-16(8-6-15)29-11-3-2-4-21(29)30/h2-11,17-18H,12-14H2,1H3,(H,26,32)(H,27,33)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218315

((3R,4S)-methyl 3-(2-chlorothiophene-5-carboxamido)...)Show SMILES COC(=O)C1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O |w:4.3| Show InChI InChI=1S/C24H22ClN3O5S/c1-33-24(32)15-12-17(18(13-15)27-23(31)19-9-10-20(25)34-19)26-22(30)14-5-7-16(8-6-14)28-11-3-2-4-21(28)29/h2-11,15,17-18H,12-13H2,1H3,(H,26,30)(H,27,31)/t15?,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216554
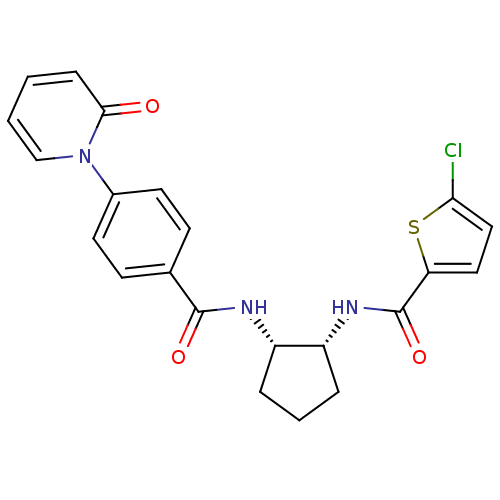
(5-CHLORO-N-((1R,2S)-2-(4-(2-OXOPYRIDIN-1(2H)-YL)BE...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C22H20ClN3O3S/c23-19-12-11-18(30-19)22(29)25-17-5-3-4-16(17)24-21(28)14-7-9-15(10-8-14)26-13-2-1-6-20(26)27/h1-2,6-13,16-17H,3-5H2,(H,24,28)(H,25,29)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216554

(5-CHLORO-N-((1R,2S)-2-(4-(2-OXOPYRIDIN-1(2H)-YL)BE...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C22H20ClN3O3S/c23-19-12-11-18(30-19)22(29)25-17-5-3-4-16(17)24-21(28)14-7-9-15(10-8-14)26-13-2-1-6-20(26)27/h1-2,6-13,16-17H,3-5H2,(H,24,28)(H,25,29)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218308
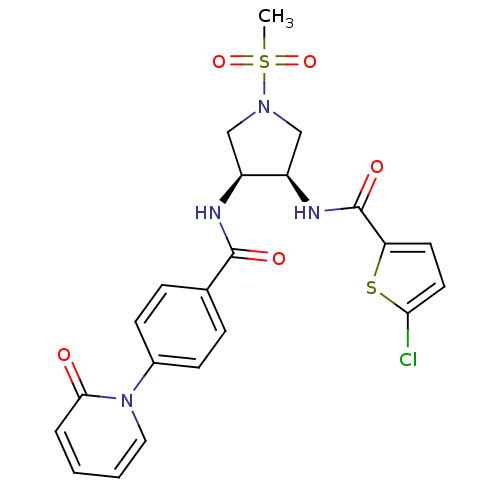
(5-chloro-N-((3R,4S)-1-(methylsulfonyl)-4-(4-(2-oxo...)Show SMILES CS(=O)(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C22H21ClN4O5S2/c1-34(31,32)26-12-16(17(13-26)25-22(30)18-9-10-19(23)33-18)24-21(29)14-5-7-15(8-6-14)27-11-3-2-4-20(27)28/h2-11,16-17H,12-13H2,1H3,(H,24,29)(H,25,30)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216584
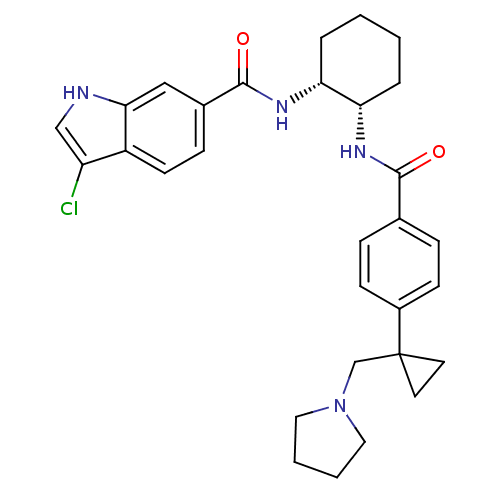
(3-chloro-N-((1R,2S)-2-(4-(1-(pyrrolidin-1-ylmethyl...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)C1(CN2CCCC2)CC1 Show InChI InChI=1S/C30H35ClN4O2/c31-24-18-32-27-17-21(9-12-23(24)27)29(37)34-26-6-2-1-5-25(26)33-28(36)20-7-10-22(11-8-20)30(13-14-30)19-35-15-3-4-16-35/h7-12,17-18,25-26,32H,1-6,13-16,19H2,(H,33,36)(H,34,37)/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218282
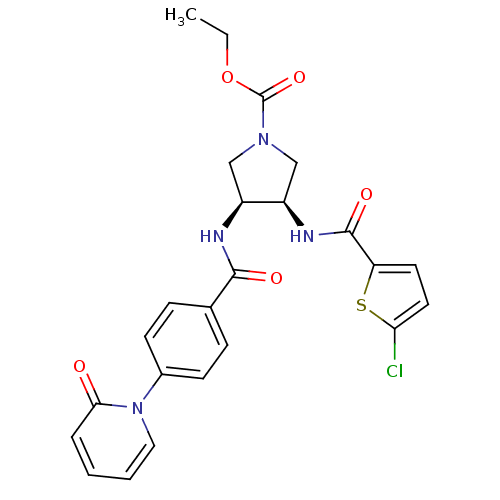
((3R,4S)-ethyl 3-(2-chlorothiophene-5-carboxamido)-...)Show SMILES CCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-2-34-24(33)28-13-17(18(14-28)27-23(32)19-10-11-20(25)35-19)26-22(31)15-6-8-16(9-7-15)29-12-4-3-5-21(29)30/h3-12,17-18H,2,13-14H2,1H3,(H,26,31)(H,27,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216572
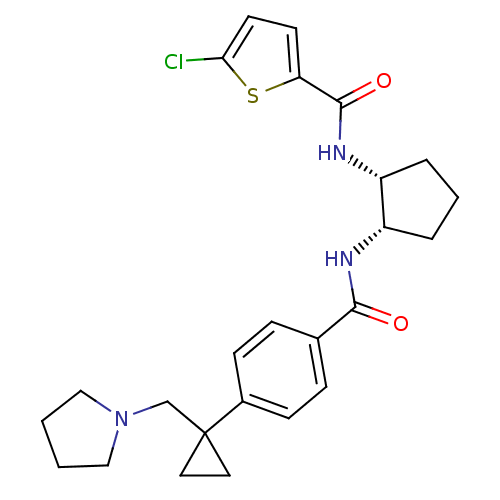
(5-chloro-N-((1R,2S)-2-(4-(1-(pyrrolidin-1-ylmethyl...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)C1(CN2CCCC2)CC1 Show InChI InChI=1S/C25H30ClN3O2S/c26-22-11-10-21(32-22)24(31)28-20-5-3-4-19(20)27-23(30)17-6-8-18(9-7-17)25(12-13-25)16-29-14-1-2-15-29/h6-11,19-20H,1-5,12-16H2,(H,27,30)(H,28,31)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218312
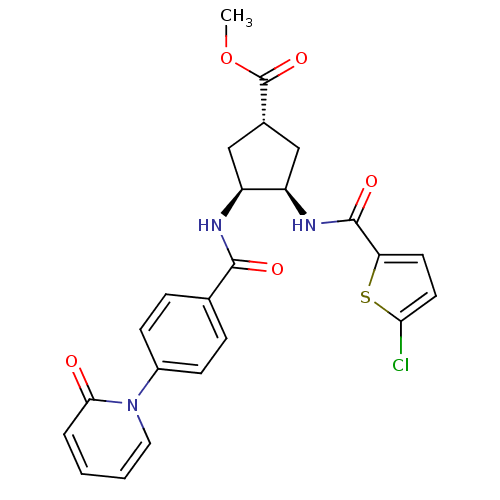
((1S,3R,4S)-methyl 3-(2-chlorothiophene-5-carboxami...)Show SMILES COC(=O)[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H22ClN3O5S/c1-33-24(32)15-12-17(18(13-15)27-23(31)19-9-10-20(25)34-19)26-22(30)14-5-7-16(8-6-14)28-11-3-2-4-21(28)29/h2-11,15,17-18H,12-13H2,1H3,(H,26,30)(H,27,31)/t15-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218290
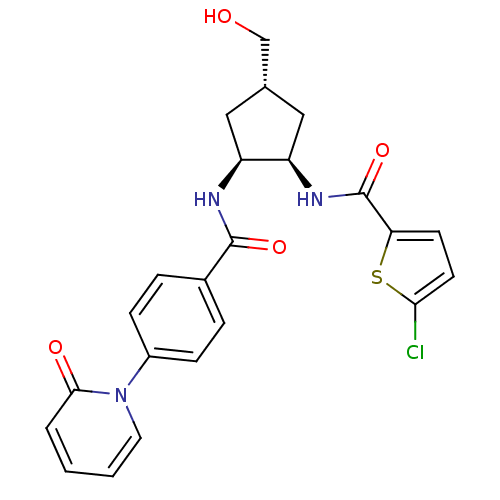
(5-chloro-N-((1R,2S,4S)-4-(hydroxymethyl)-2-(4-(2-o...)Show SMILES OC[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H22ClN3O4S/c24-20-9-8-19(32-20)23(31)26-18-12-14(13-28)11-17(18)25-22(30)15-4-6-16(7-5-15)27-10-2-1-3-21(27)29/h1-10,14,17-18,28H,11-13H2,(H,25,30)(H,26,31)/t14-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218300
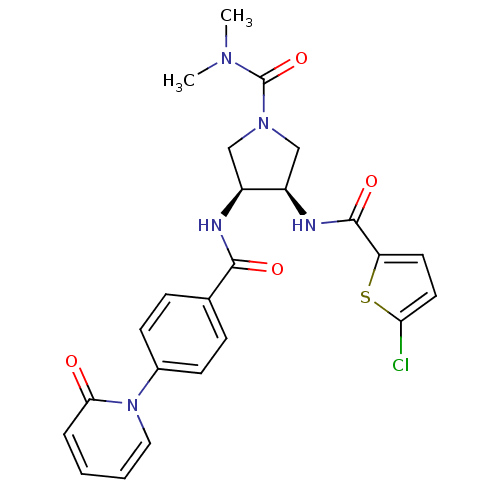
((3R,4S)-3-(2-chlorothiophene-5-carboxamido)-N,N-di...)Show SMILES CN(C)C(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H24ClN5O4S/c1-28(2)24(34)29-13-17(18(14-29)27-23(33)19-10-11-20(25)35-19)26-22(32)15-6-8-16(9-7-15)30-12-4-3-5-21(30)31/h3-12,17-18H,13-14H2,1-2H3,(H,26,32)(H,27,33)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218291
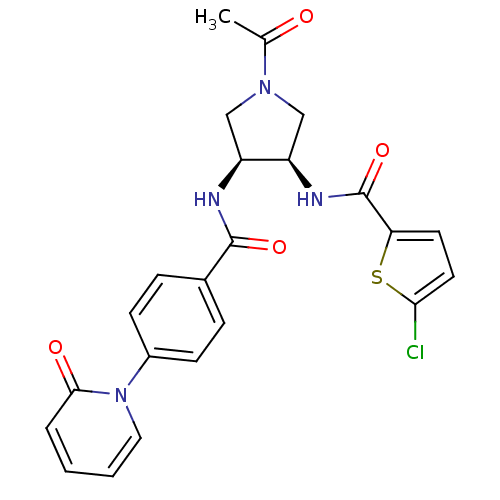
(CHEMBL394129 | N-((3R,4S)-1-acetyl-4-(4-(2-oxopyri...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H21ClN4O4S/c1-14(29)27-12-17(18(13-27)26-23(32)19-9-10-20(24)33-19)25-22(31)15-5-7-16(8-6-15)28-11-3-2-4-21(28)30/h2-11,17-18H,12-13H2,1H3,(H,25,31)(H,26,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218283
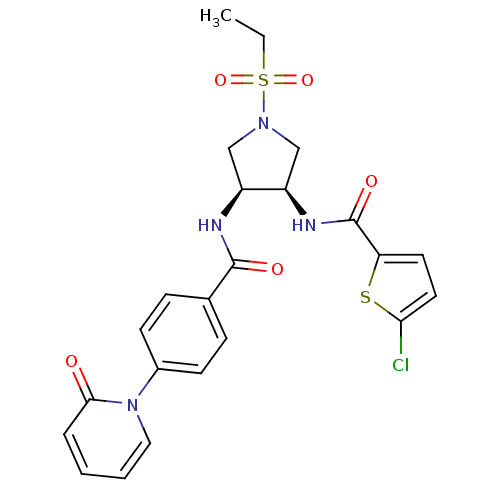
(5-chloro-N-((3R,4S)-1-(ethylsulfonyl)-4-(4-(2-oxop...)Show SMILES CCS(=O)(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H23ClN4O5S2/c1-2-35(32,33)27-13-17(18(14-27)26-23(31)19-10-11-20(24)34-19)25-22(30)15-6-8-16(9-7-15)28-12-4-3-5-21(28)29/h3-12,17-18H,2,13-14H2,1H3,(H,25,30)(H,26,31)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216556
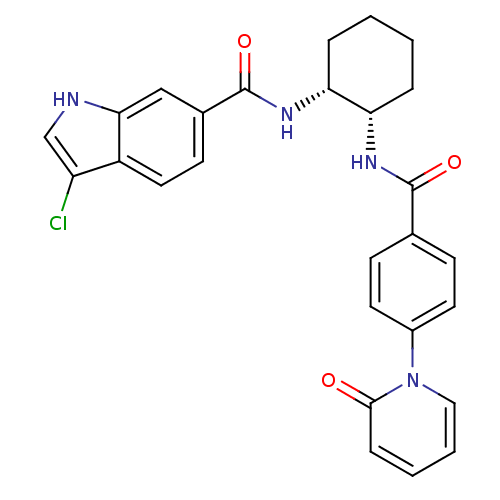
(3-CHLORO-N-((1R,2S) -2-(4-(2-OXOPYRIDIN-1(2H)-YL)B...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H25ClN4O3/c28-21-16-29-24-15-18(10-13-20(21)24)27(35)31-23-6-2-1-5-22(23)30-26(34)17-8-11-19(12-9-17)32-14-4-3-7-25(32)33/h3-4,7-16,22-23,29H,1-2,5-6H2,(H,30,34)(H,31,35)/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216604

(3-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H23ClN4O3/c27-20-15-28-23-14-17(9-12-19(20)23)26(34)30-22-5-3-4-21(22)29-25(33)16-7-10-18(11-8-16)31-13-2-1-6-24(31)32/h1-2,6-15,21-22,28H,3-5H2,(H,29,33)(H,30,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216579
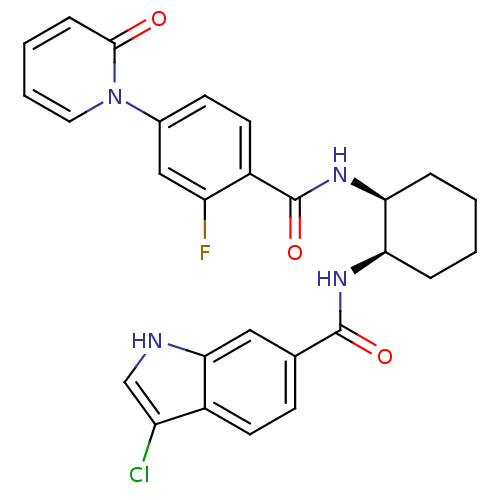
(CHEMBL401024 | cis-3-chloro-N-(2-(2-fluoro-4-(2-ox...)Show SMILES Fc1cc(ccc1C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc2c(Cl)c[nH]c2c1)-n1ccccc1=O Show InChI InChI=1S/C27H24ClFN4O3/c28-20-15-30-24-13-16(8-10-18(20)24)26(35)31-22-5-1-2-6-23(22)32-27(36)19-11-9-17(14-21(19)29)33-12-4-3-7-25(33)34/h3-4,7-15,22-23,30H,1-2,5-6H2,(H,31,35)(H,32,36)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218282

((3R,4S)-ethyl 3-(2-chlorothiophene-5-carboxamido)-...)Show SMILES CCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-2-34-24(33)28-13-17(18(14-28)27-23(32)19-10-11-20(25)35-19)26-22(31)15-6-8-16(9-7-15)29-12-4-3-5-21(29)30/h3-12,17-18H,2,13-14H2,1H3,(H,26,31)(H,27,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a in presence of tripeptide substrate at 25 degC |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218306
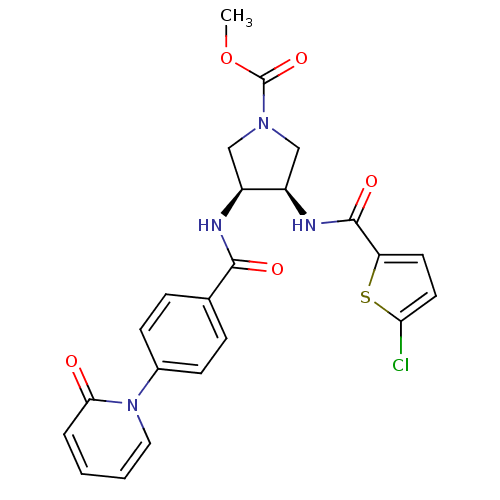
((3R,4S)-methyl 3-(2-chlorothiophene-5-carboxamido)...)Show SMILES COC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H21ClN4O5S/c1-33-23(32)27-12-16(17(13-27)26-22(31)18-9-10-19(24)34-18)25-21(30)14-5-7-15(8-6-14)28-11-3-2-4-20(28)29/h2-11,16-17H,12-13H2,1H3,(H,25,30)(H,26,31)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
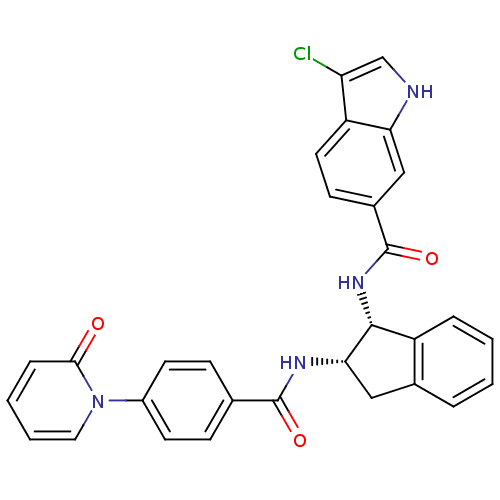
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218319
(3-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@H]1[C@H](Cc2ccccc12)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C30H23ClN4O3/c31-24-17-32-25-16-20(10-13-23(24)25)30(38)34-28-22-6-2-1-5-19(22)15-26(28)33-29(37)18-8-11-21(12-9-18)35-14-4-3-7-27(35)36/h1-14,16-17,26,28,32H,15H2,(H,33,37)(H,34,38)/t26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218294

((3R,4S)-2-methoxyethyl 3-(2-chlorothiophene-5-carb...)Show SMILES COCCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H25ClN4O6S/c1-35-12-13-36-25(34)29-14-18(19(15-29)28-24(33)20-9-10-21(26)37-20)27-23(32)16-5-7-17(8-6-16)30-11-3-2-4-22(30)31/h2-11,18-19H,12-15H2,1H3,(H,27,32)(H,28,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
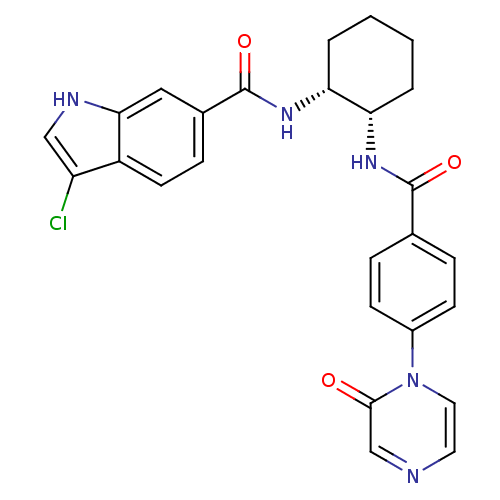
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216583
(3-chloro-N-((1R,2S)-2-(4-(2-oxopyrazin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccncc1=O Show InChI InChI=1S/C26H24ClN5O3/c27-20-14-29-23-13-17(7-10-19(20)23)26(35)31-22-4-2-1-3-21(22)30-25(34)16-5-8-18(9-6-16)32-12-11-28-15-24(32)33/h5-15,21-22,29H,1-4H2,(H,30,34)(H,31,35)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216620
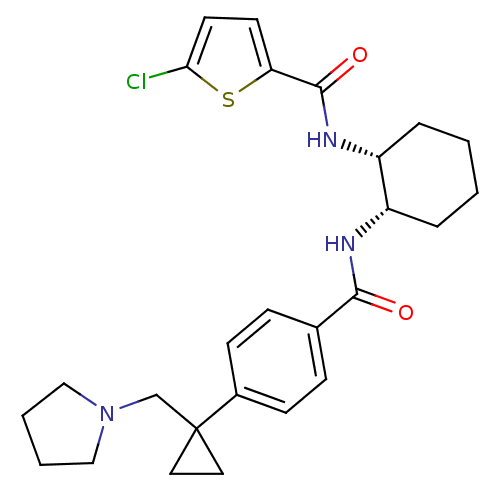
(5-chloro-N-((1R,2S)-2-(4-(1-(pyrrolidin-1-ylmethyl...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)C1(CN2CCCC2)CC1 Show InChI InChI=1S/C26H32ClN3O2S/c27-23-12-11-22(33-23)25(32)29-21-6-2-1-5-20(21)28-24(31)18-7-9-19(10-8-18)26(13-14-26)17-30-15-3-4-16-30/h7-12,20-21H,1-6,13-17H2,(H,28,31)(H,29,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216598
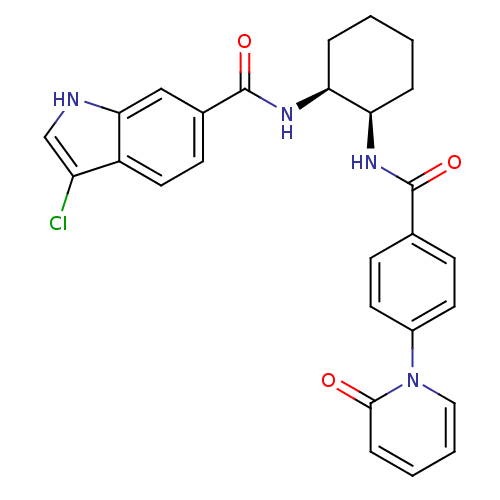
(3-chloro-N-((1S,2R)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H25ClN4O3/c28-21-16-29-24-15-18(10-13-20(21)24)27(35)31-23-6-2-1-5-22(23)30-26(34)17-8-11-19(12-9-17)32-14-4-3-7-25(32)33/h3-4,7-16,22-23,29H,1-2,5-6H2,(H,30,34)(H,31,35)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216598

(3-chloro-N-((1S,2R)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@H]1CCCC[C@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H25ClN4O3/c28-21-16-29-24-15-18(10-13-20(21)24)27(35)31-23-6-2-1-5-22(23)30-26(34)17-8-11-19(12-9-17)32-14-4-3-7-25(32)33/h3-4,7-16,22-23,29H,1-2,5-6H2,(H,30,34)(H,31,35)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218301
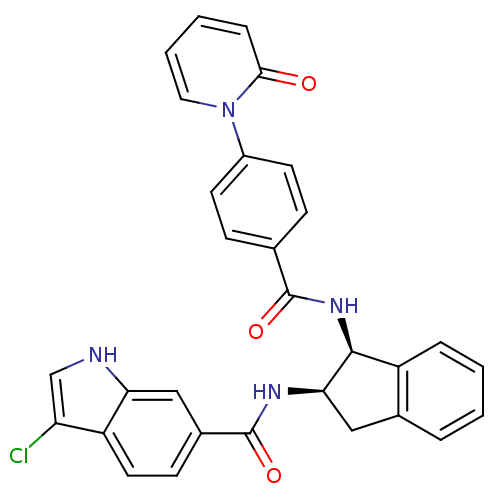
(3-chloro-N-((1S,2R)-1-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1Cc2ccccc2[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C30H23ClN4O3/c31-24-17-32-25-16-20(10-13-23(24)25)30(38)33-26-15-19-5-1-2-6-22(19)28(26)34-29(37)18-8-11-21(12-9-18)35-14-4-3-7-27(35)36/h1-14,16-17,26,28,32H,15H2,(H,33,38)(H,34,37)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216604

(3-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H23ClN4O3/c27-20-15-28-23-14-17(9-12-19(20)23)26(34)30-22-5-3-4-21(22)29-25(33)16-7-10-18(11-8-16)31-13-2-1-6-24(31)32/h1-2,6-15,21-22,28H,3-5H2,(H,29,33)(H,30,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18162
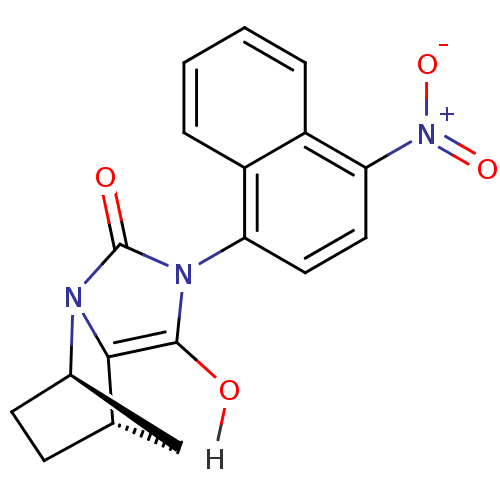
((1R,6R,7S)-4-(4-nitronaphthalen-1-yl)-2,4-diazatri...)Show SMILES Oc1c2[C@H]3CC[C@H](C3)n2c(=O)n1-c1ccc([N+]([O-])=O)c2ccccc12 |r,wU:6.6,3.7,(12.2,-7.28,;12.6,-8.77,;11.56,-9.96,;10.02,-9.96,;9.25,-11.29,;10.02,-12.62,;11.56,-12.62,;10.02,-11.73,;12.33,-11.29,;13.87,-10.92,;15.03,-11.93,;14,-9.4,;15.49,-9,;16.07,-7.57,;17.59,-7.36,;18.54,-8.57,;20.06,-8.36,;20.83,-9.69,;20.64,-6.93,;17.96,-10,;18.91,-11.21,;18.34,-12.64,;16.81,-12.86,;15.86,-11.64,;16.44,-10.22,)| Show InChI InChI=1S/C18H15N3O4/c22-17-16-10-5-6-11(9-10)19(16)18(23)20(17)14-7-8-15(21(24)25)13-4-2-1-3-12(13)14/h1-4,7-8,10-11,22H,5-6,9H2/t10-,11+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | 385 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 49: 7596-9 (2006)
Article DOI: 10.1021/jm061101w
BindingDB Entry DOI: 10.7270/Q2862DQ9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218286
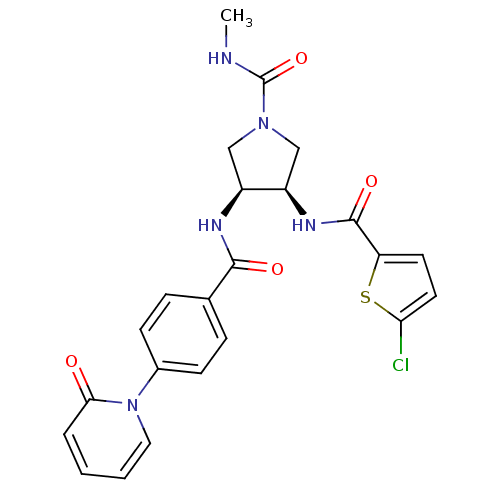
((3R,4S)-3-(2-chlorothiophene-5-carboxamido)-N-meth...)Show SMILES CNC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H22ClN5O4S/c1-25-23(33)28-12-16(17(13-28)27-22(32)18-9-10-19(24)34-18)26-21(31)14-5-7-15(8-6-14)29-11-3-2-4-20(29)30/h2-11,16-17H,12-13H2,1H3,(H,25,33)(H,26,31)(H,27,32)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216566
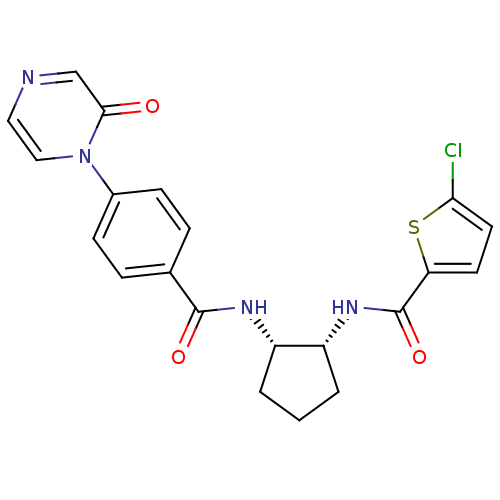
(5-chloro-N-((1R,2S)-2-(4-(2-oxopyrazin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccncc1=O Show InChI InChI=1S/C21H19ClN4O3S/c22-18-9-8-17(30-18)21(29)25-16-3-1-2-15(16)24-20(28)13-4-6-14(7-5-13)26-11-10-23-12-19(26)27/h4-12,15-16H,1-3H2,(H,24,28)(H,25,29)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM245383

(US9428504, 133)Show SMILES CC(C)(C)CN1CCC2(CN(c3c2c(ccc3O)-c2nc3cc(Cl)ccc3s2)c2ccccc2NC(=O)Nc2nc3ccc(F)cc3s2)CC1 Show InChI InChI=1S/C38H36ClFN6O2S2/c1-37(2,3)20-45-16-14-38(15-17-45)21-46(33-29(47)12-10-24(32(33)38)34-41-27-18-22(39)8-13-30(27)49-34)28-7-5-4-6-25(28)42-35(48)44-36-43-26-11-9-23(40)19-31(26)50-36/h4-13,18-19,47H,14-17,20-21H2,1-3H3,(H2,42,43,44,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company
US Patent
| Assay Description
A SPA membrane binding assay was used to identify inhibitors of [33P] 2MeS-ADP binding to cloned human P2Y1 receptors (The P2Y1 receptor membranes we... |
US Patent US9428504 (2016)
BindingDB Entry DOI: 10.7270/Q24748SD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216606
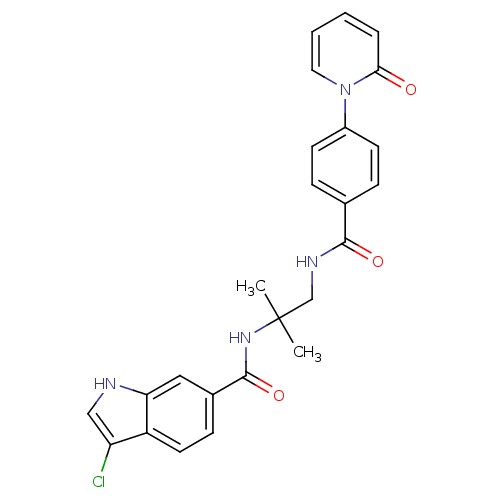
(3-chloro-N-(2-methyl-1-(4-(2-oxopyridin-1(2H)-yl)b...)Show SMILES CC(C)(CNC(=O)c1ccc(cc1)-n1ccccc1=O)NC(=O)c1ccc2c(Cl)c[nH]c2c1 Show InChI InChI=1S/C25H23ClN4O3/c1-25(2,29-24(33)17-8-11-19-20(26)14-27-21(19)13-17)15-28-23(32)16-6-9-18(10-7-16)30-12-4-3-5-22(30)31/h3-14,27H,15H2,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218295
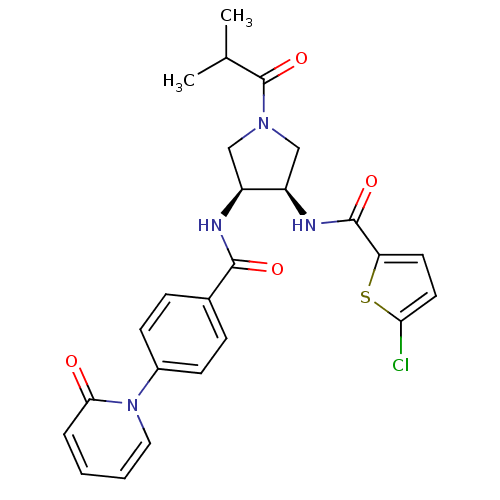
(5-chloro-N-((3R,4S)-1-isobutyryl-4-(4-(2-oxopyridi...)Show SMILES CC(C)C(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H25ClN4O4S/c1-15(2)25(34)29-13-18(19(14-29)28-24(33)20-10-11-21(26)35-20)27-23(32)16-6-8-17(9-7-16)30-12-4-3-5-22(30)31/h3-12,15,18-19H,13-14H2,1-2H3,(H,27,32)(H,28,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216576
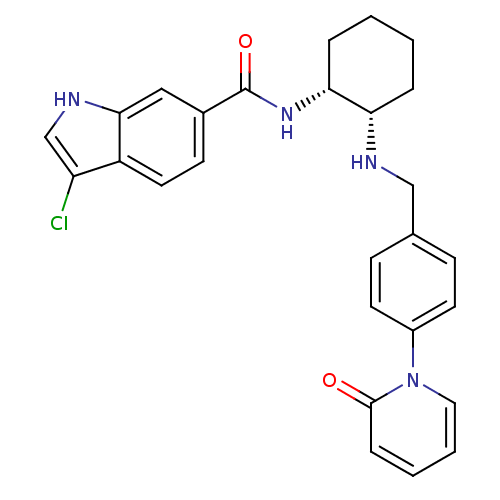
(CHEMBL231940 | cis-N-(2-(4-(2-oxopyridin-1(2H)-yl)...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C27H27ClN4O2/c28-22-17-30-25-15-19(10-13-21(22)25)27(34)31-24-6-2-1-5-23(24)29-16-18-8-11-20(12-9-18)32-14-4-3-7-26(32)33/h3-4,7-15,17,23-24,29-30H,1-2,5-6,16H2,(H,31,34)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18169
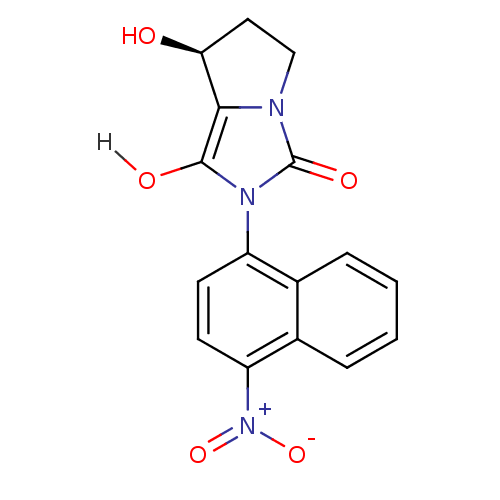
((7S,7aR)-7-hydroxy-2-(4-nitronaphthalen-1-yl)-hexa...)Show SMILES O[C@H]1CCn2c1c(O)n(-c1ccc([N+]([O-])=O)c3ccccc13)c2=O |r,wD:1.0,(-7.31,4.33,;-6.4,3.09,;-6.88,1.62,;-5.63,.72,;-4.39,1.62,;-4.86,3.09,;-3.62,3.99,;-3.62,5.53,;-2.37,3.09,;-.83,3.09,;.03,4.36,;1.57,4.25,;2.24,2.87,;3.78,2.76,;4.45,1.37,;4.64,4.03,;1.38,1.59,;2.05,.21,;1.19,-1.07,;-.35,-.96,;-1.02,.43,;-.16,1.7,;-2.85,1.62,;-1.94,.38,)| Show InChI InChI=1S/C16H13N3O5/c20-13-7-8-17-14(13)15(21)18(16(17)22)11-5-6-12(19(23)24)10-4-2-1-3-9(10)11/h1-6,13,20-21H,7-8H2/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | -49.9 | n/a | n/a | 281 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 49: 7596-9 (2006)
Article DOI: 10.1021/jm061101w
BindingDB Entry DOI: 10.7270/Q2862DQ9 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18165
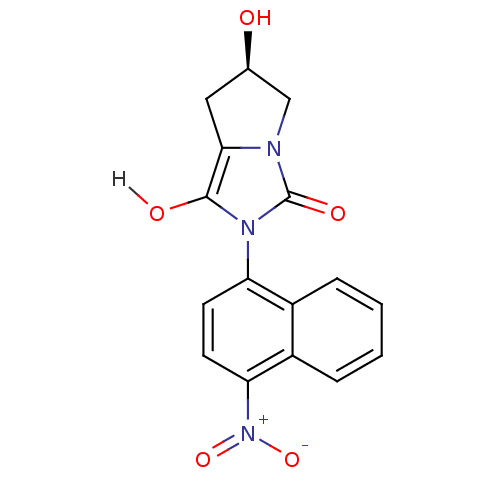
((6R)-6-hydroxy-2-(4-nitronaphthalen-1-yl)-hexahydr...)Show SMILES O[C@@H]1Cc2c(O)n(-c3ccc([N+]([O-])=O)c4ccccc34)c(=O)n2C1 |r,wD:1.0,(-8.34,1.15,;-6.88,1.62,;-6.4,3.09,;-4.86,3.09,;-3.62,3.99,;-3.62,5.53,;-2.37,3.09,;-.83,3.09,;.03,4.36,;1.57,4.25,;2.24,2.87,;3.78,2.76,;4.45,1.37,;4.64,4.03,;1.38,1.59,;2.05,.21,;1.19,-1.07,;-.35,-.96,;-1.02,.43,;-.16,1.7,;-2.85,1.62,;-1.94,.38,;-4.39,1.62,;-5.63,.72,)| Show InChI InChI=1S/C16H13N3O5/c20-9-7-14-15(21)18(16(22)17(14)8-9)12-5-6-13(19(23)24)11-4-2-1-3-10(11)12/h1-6,9,20-21H,7-8H2/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | -49.9 | n/a | n/a | 320 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 49: 7596-9 (2006)
Article DOI: 10.1021/jm061101w
BindingDB Entry DOI: 10.7270/Q2862DQ9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216562
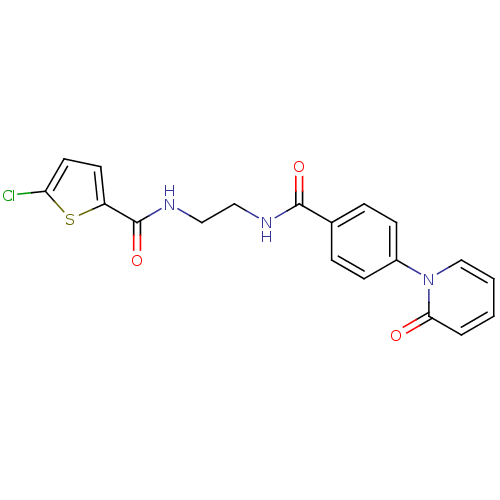
(5-CHLORO-N-(2-(4-(2-OXOPYRIDIN-1(2H)-YL)BENZAMIDO)...)Show SMILES Clc1ccc(s1)C(=O)NCCNC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C19H16ClN3O3S/c20-16-9-8-15(27-16)19(26)22-11-10-21-18(25)13-4-6-14(7-5-13)23-12-2-1-3-17(23)24/h1-9,12H,10-11H2,(H,21,25)(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216557
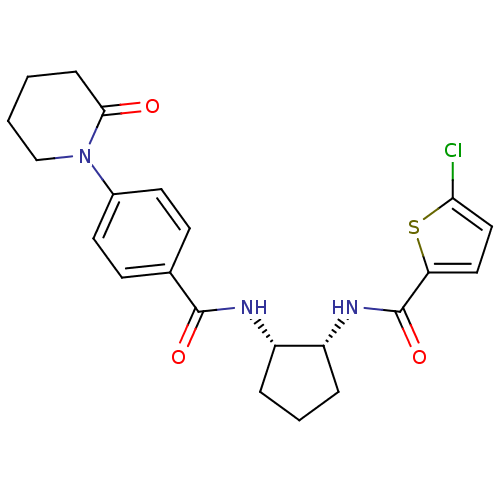
(5-chloro-N-((1R,2S)-2-(4-(2-oxopiperidin-1-yl)benz...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C22H24ClN3O3S/c23-19-12-11-18(30-19)22(29)25-17-5-3-4-16(17)24-21(28)14-7-9-15(10-8-14)26-13-2-1-6-20(26)27/h7-12,16-17H,1-6,13H2,(H,24,28)(H,25,29)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
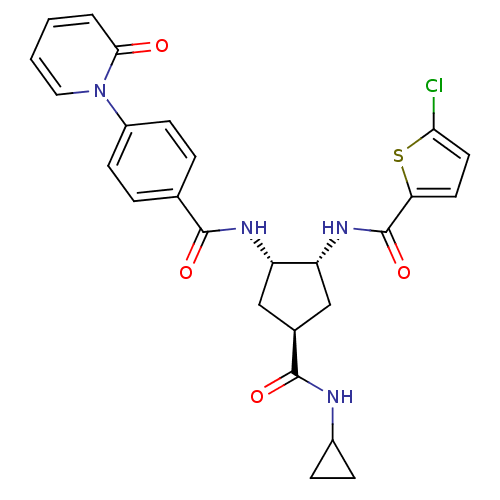
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218284
(5-chloro-N-((1R,2S,4S)-4-(cyclopropylcarbamoyl)-2-...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1C[C@H](C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)NC1CC1 Show InChI InChI=1S/C26H25ClN4O4S/c27-22-11-10-21(36-22)26(35)30-20-14-16(25(34)28-17-6-7-17)13-19(20)29-24(33)15-4-8-18(9-5-15)31-12-2-1-3-23(31)32/h1-5,8-12,16-17,19-20H,6-7,13-14H2,(H,28,34)(H,29,33)(H,30,35)/t16-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
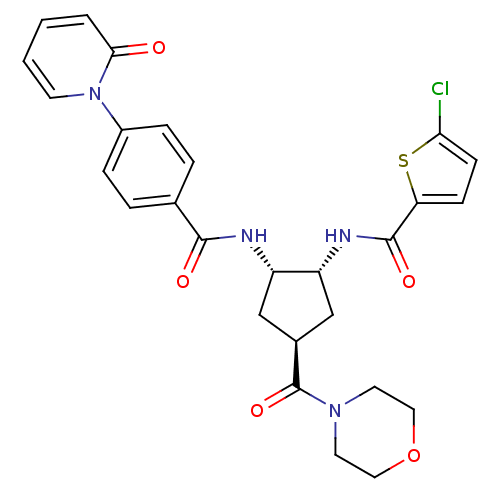
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218314
(5-chloro-N-((1R,2S,4S)-4-(morpholine-4-carbonyl)-2...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1C[C@H](C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)N1CCOCC1 Show InChI InChI=1S/C27H27ClN4O5S/c28-23-9-8-22(38-23)26(35)30-21-16-18(27(36)31-11-13-37-14-12-31)15-20(21)29-25(34)17-4-6-19(7-5-17)32-10-2-1-3-24(32)33/h1-10,18,20-21H,11-16H2,(H,29,34)(H,30,35)/t18-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
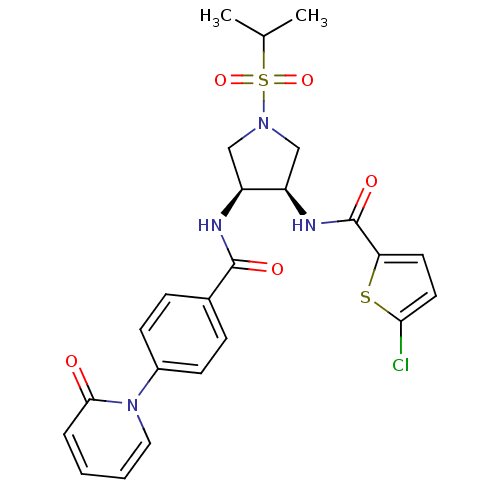
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218304
(5-chloro-N-((3R,4S)-1-(isopropylsulfonyl)-4-(4-(2-...)Show SMILES CC(C)S(=O)(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H25ClN4O5S2/c1-15(2)36(33,34)28-13-18(19(14-28)27-24(32)20-10-11-21(25)35-20)26-23(31)16-6-8-17(9-7-16)29-12-4-3-5-22(29)30/h3-12,15,18-19H,13-14H2,1-2H3,(H,26,31)(H,27,32)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218281
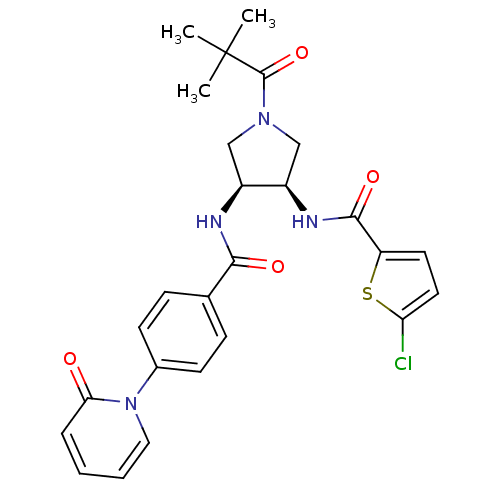
(5-chloro-N-((3R,4S)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES CC(C)(C)C(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H27ClN4O4S/c1-26(2,3)25(35)30-14-18(19(15-30)29-24(34)20-11-12-21(27)36-20)28-23(33)16-7-9-17(10-8-16)31-13-5-4-6-22(31)32/h4-13,18-19H,14-15H2,1-3H3,(H,28,33)(H,29,34)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18164
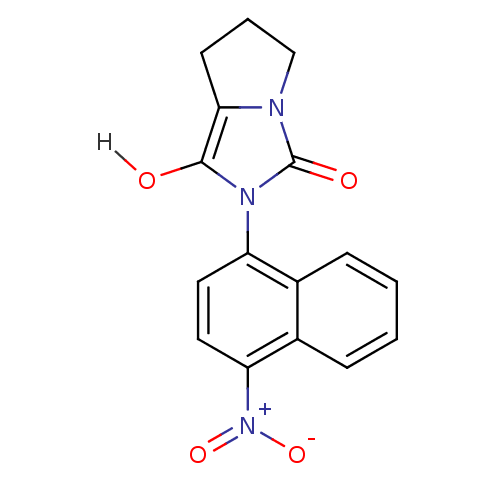
(2-(4-nitronaphthalen-1-yl)-hexahydro-1H-pyrrolo[1,...)Show SMILES Oc1c2CCCn2c(=O)n1-c1ccc([N+]([O-])=O)c2ccccc12 |(-3.62,5.53,;-3.62,3.99,;-4.86,3.09,;-6.4,3.09,;-6.88,1.62,;-5.63,.72,;-4.39,1.62,;-2.85,1.62,;-1.94,.38,;-2.37,3.09,;-.83,3.09,;.03,4.36,;1.57,4.25,;2.24,2.87,;3.78,2.76,;4.45,1.37,;4.64,4.03,;1.38,1.59,;2.05,.21,;1.19,-1.07,;-.35,-.96,;-1.02,.43,;-.16,1.7,)| Show InChI InChI=1S/C16H13N3O4/c20-15-14-6-3-9-17(14)16(21)18(15)12-7-8-13(19(22)23)11-5-2-1-4-10(11)12/h1-2,4-5,7-8,20H,3,6,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | -49.6 | n/a | n/a | 270 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 49: 7596-9 (2006)
Article DOI: 10.1021/jm061101w
BindingDB Entry DOI: 10.7270/Q2862DQ9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218296
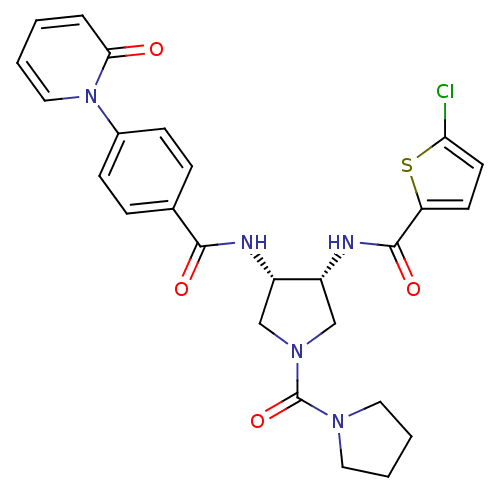
(5-chloro-N-((3R,4S)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CN(C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)N1CCCC1 Show InChI InChI=1S/C26H26ClN5O4S/c27-22-11-10-21(37-22)25(35)29-20-16-31(26(36)30-12-3-4-13-30)15-19(20)28-24(34)17-6-8-18(9-7-17)32-14-2-1-5-23(32)33/h1-2,5-11,14,19-20H,3-4,12-13,15-16H2,(H,28,34)(H,29,35)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216594
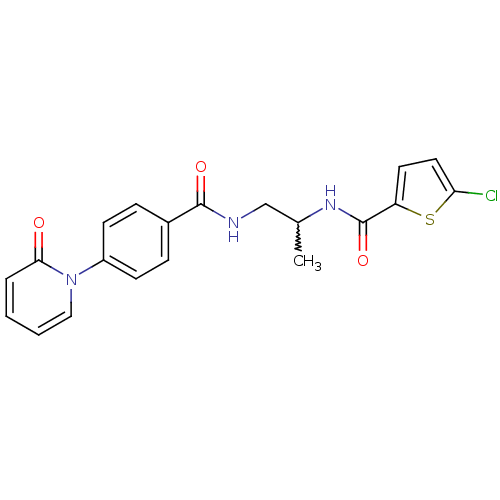
(5-chloro-N-(1-(4-(2-oxopyridin-1(2H)-yl)benzamido)...)Show SMILES CC(CNC(=O)c1ccc(cc1)-n1ccccc1=O)NC(=O)c1ccc(Cl)s1 |w:1.0| Show InChI InChI=1S/C20H18ClN3O3S/c1-13(23-20(27)16-9-10-17(21)28-16)12-22-19(26)14-5-7-15(8-6-14)24-11-3-2-4-18(24)25/h2-11,13H,12H2,1H3,(H,22,26)(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216578
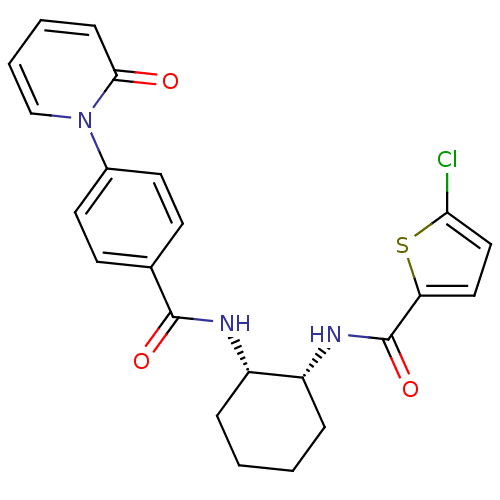
(5-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H22ClN3O3S/c24-20-13-12-19(31-20)23(30)26-18-6-2-1-5-17(18)25-22(29)15-8-10-16(11-9-15)27-14-4-3-7-21(27)28/h3-4,7-14,17-18H,1-2,5-6H2,(H,25,29)(H,26,30)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 4419-27 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.029
BindingDB Entry DOI: 10.7270/Q2416XVV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data