

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18793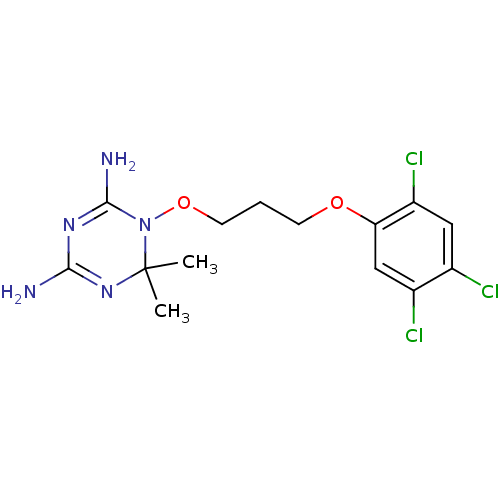 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program Curated by ChEMBL | Assay Description Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay | Antimicrob Agents Chemother 54: 2603-10 (2010) Article DOI: 10.1128/AAC.01526-09 BindingDB Entry DOI: 10.7270/Q2VX0GQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166735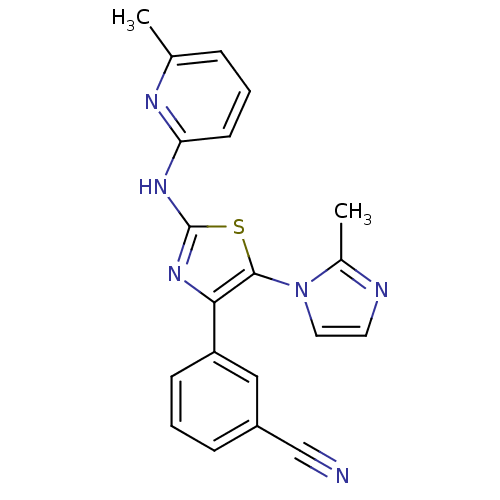 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50035483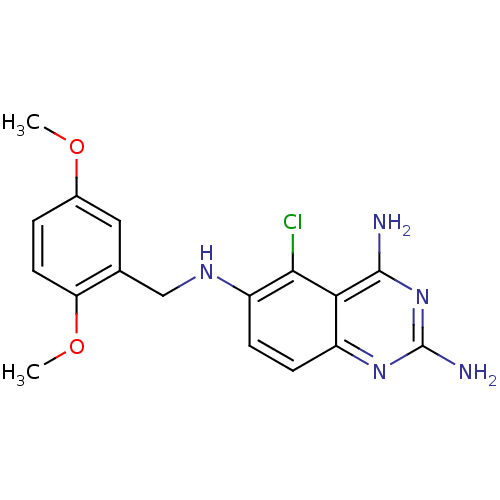 (5-Chloro-N*6*-(2,5-dimethoxy-benzyl)-quinazoline-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program Curated by ChEMBL | Assay Description Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay | Antimicrob Agents Chemother 54: 2603-10 (2010) Article DOI: 10.1128/AAC.01526-09 BindingDB Entry DOI: 10.7270/Q2VX0GQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166742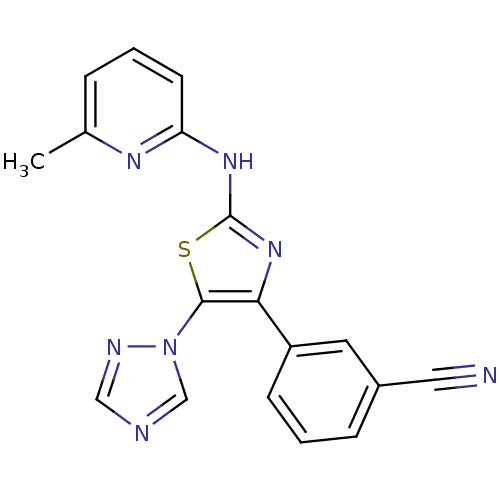 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166739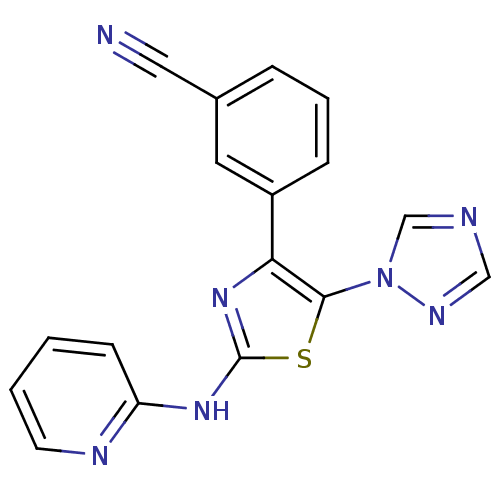 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 30.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program Curated by ChEMBL | Assay Description Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay | Antimicrob Agents Chemother 54: 2603-10 (2010) Article DOI: 10.1128/AAC.01526-09 BindingDB Entry DOI: 10.7270/Q2VX0GQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166742 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175864 (Bz-Nle-Lys-Arg-Arg-B(OH)2 | CHEMBL199845) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against CF40.NS3pro from Dengue virus type 2 | Bioorg Med Chem Lett 16: 36-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.062 BindingDB Entry DOI: 10.7270/Q2639P97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166739 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18792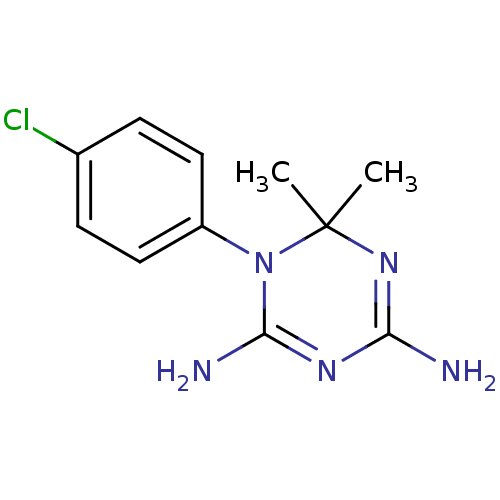 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 55.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kenya Medical Research Institute/Wellcome Trust Collaborative Research Program Curated by ChEMBL | Assay Description Binding affinity to human recombinant DHFR expressed in Escherichia coli BL21(DE3) by competitive binding assay | Antimicrob Agents Chemother 54: 2603-10 (2010) Article DOI: 10.1128/AAC.01526-09 BindingDB Entry DOI: 10.7270/Q2VX0GQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166745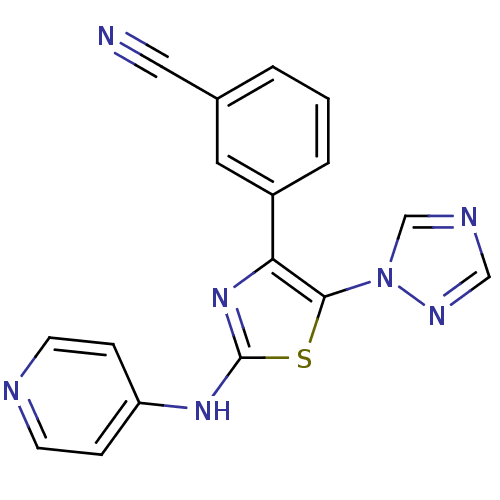 (3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166737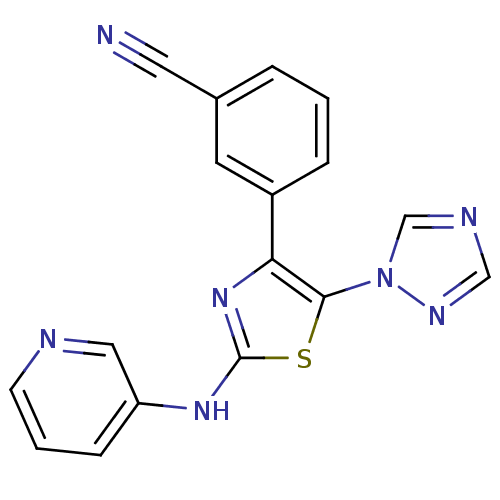 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166743 (3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166743 (3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166741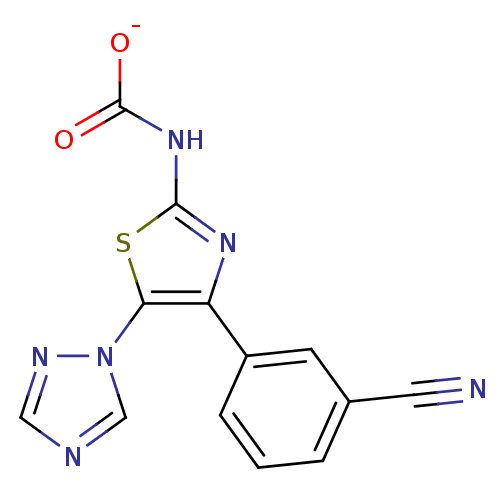 (4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166735 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166735 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166745 (3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50166741 (4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of luciferase production elicited by NECA by compound in CHO cells transfected with human adenosine A2b receptor and a luciferase expressi... | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166735 (3-[5-(2-Methyl-imidazol-1-yl)-2-(pyrazin-2-ylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 419 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427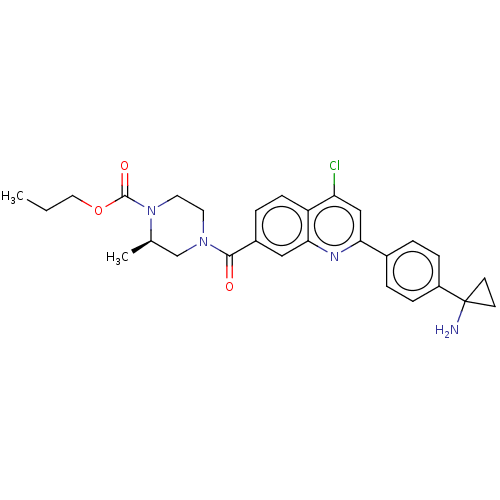 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166743 (3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 698 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-AB-MECA from human adenosine A3 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175863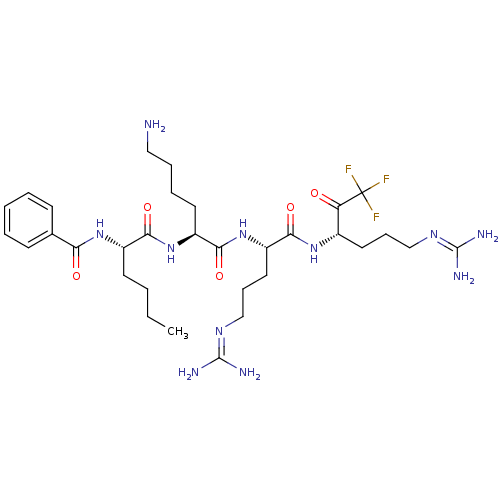 (Bz-Nle-Lys-Arg-Arg-CF3 | CHEMBL200294) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against CF40.NS3pro from Dengue virus type 2 | Bioorg Med Chem Lett 16: 36-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.062 BindingDB Entry DOI: 10.7270/Q2639P97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175982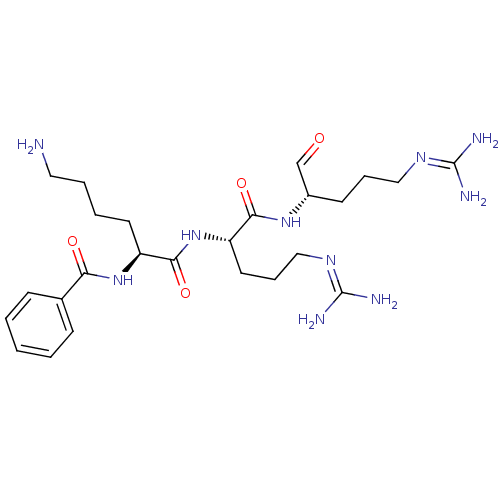 (Bz-Lys-Arg-Arg-H | CHEMBL199510) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166739 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166739 (3-[2-(Pyridin-2-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50207120 (3-isobutyl-8-((6-methoxyisoquinolin-4-yl)methyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of adenosine A1 receptor | Bioorg Med Chem Lett 17: 2376-9 (2007) Article DOI: 10.1016/j.bmcl.2006.11.019 BindingDB Entry DOI: 10.7270/Q2X63NSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166737 (3-[2-(Pyridin-3-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166743 (3-[5-Imidazol-1-yl-2-(pyridin-3-ylamino)-thiazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175984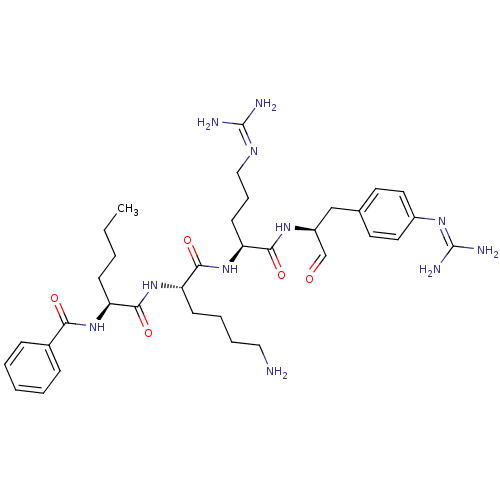 (BZ-Nle-Lys-Arg-(4-guanidinyl)-Phe-H | Bz-Nle-Lys-A...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166741 (4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433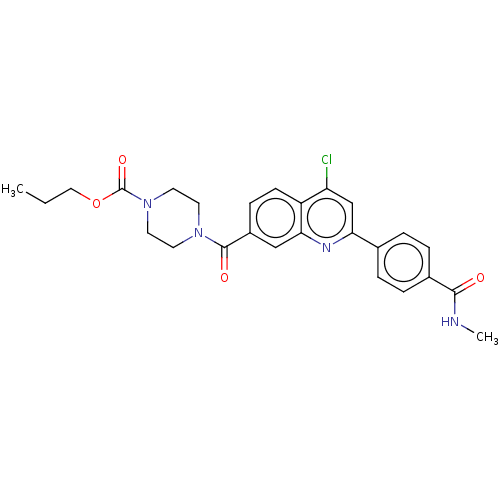 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166745 (3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434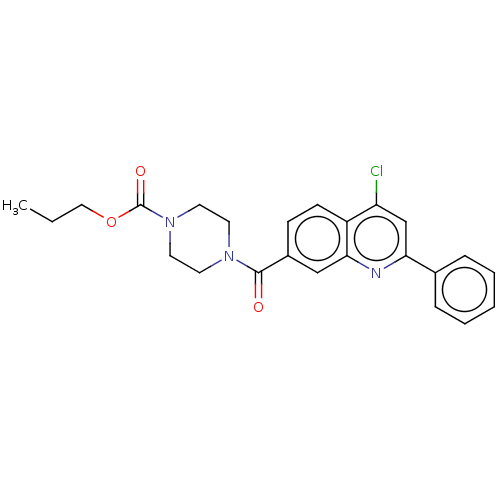 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166745 (3-[2-(Pyridin-4-ylamino)-5-[1,2,4]triazol-1-yl-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175976 (Bz-Ala-Lys-Arg-Arg-H | CHEMBL197765) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166741 (4-(3-cyanophenyl)-5-(1H-1,2,4-triazol-1-yl)-1,3-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM33259 (Bz-NKRR-H | Bz-Nle-Lys-Arg-Arg-H | CHEMBL256877) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM33259 (Bz-NKRR-H | Bz-Nle-Lys-Arg-Arg-H | CHEMBL256877) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against CF40.NS3pro from Dengue virus type 2 | Bioorg Med Chem Lett 16: 36-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.062 BindingDB Entry DOI: 10.7270/Q2639P97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175988 (Bz-Nle-Lys-Arg-(p-Me)Phe-H | CHEMBL199396) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175987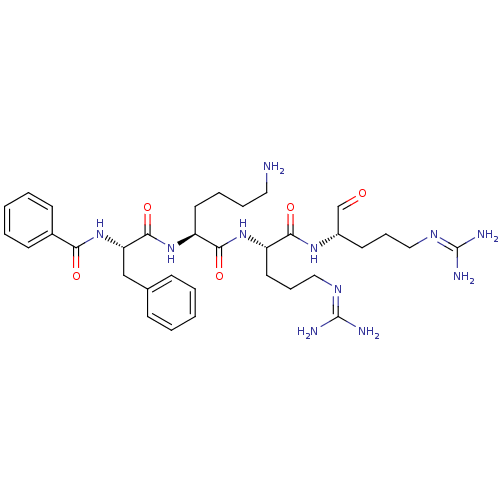 (Bz-Phe-Lys-Arg-Arg-H | CHEMBL199736) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175974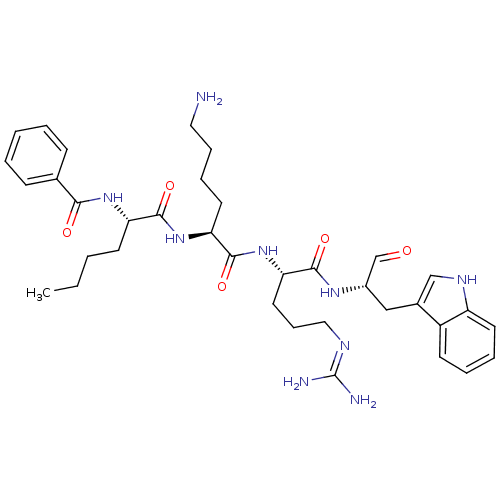 (Bz-Nle-Lys-Arg-Trp-H | CHEMBL433993) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175993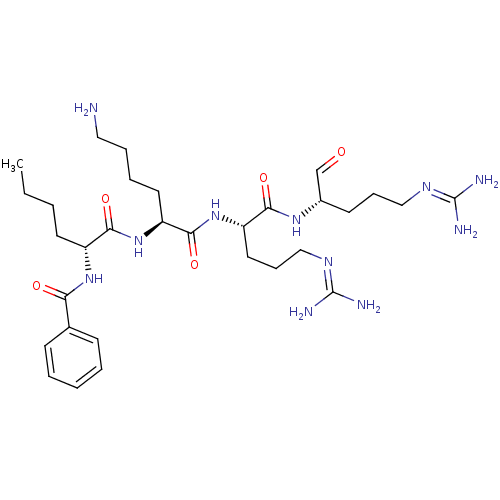 (Bz-D-Nle-Lys-Arg-Arg-H | CHEMBL201775) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50166742 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptors transfected in CHO cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50166742 (3-[2-(3-Methyl-pyridin-2-ylamino)-5-[1,2,4]triazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]ZM-241,385 from human adenosine A2a receptors transfected in HEK 293 cells | Bioorg Med Chem Lett 15: 3081-5 (2005) Article DOI: 10.1016/j.bmcl.2005.04.021 BindingDB Entry DOI: 10.7270/Q2X63NQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175978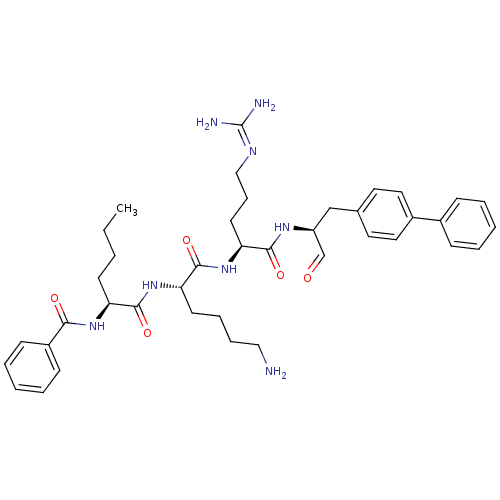 (Bz-Nle-Lys-Arg-(p-Ph)Phe-H | CHEMBL381854) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175981 (Bz-Arg-Arg-H | CHEMBL197766) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Dengue virus 2) | BDBM50175985 (Bz-Nle-Phe-Arg-Arg-H | CHEMBL199582) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibitory activity against dengue 2 NS3 protease fused via a linker to region of NS2B | Bioorg Med Chem Lett 16: 40-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.049 BindingDB Entry DOI: 10.7270/Q2J965X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1078 total ) | Next | Last >> |