

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209097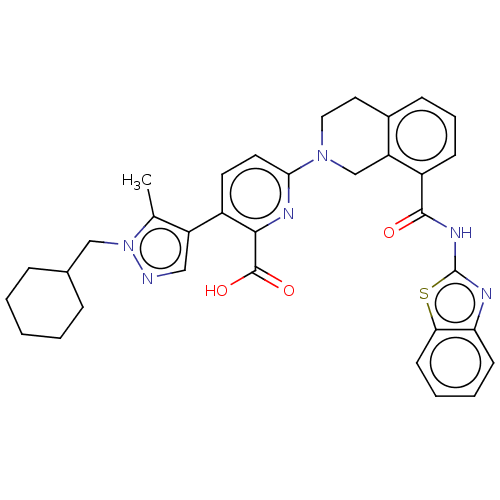 (US9266877, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50561528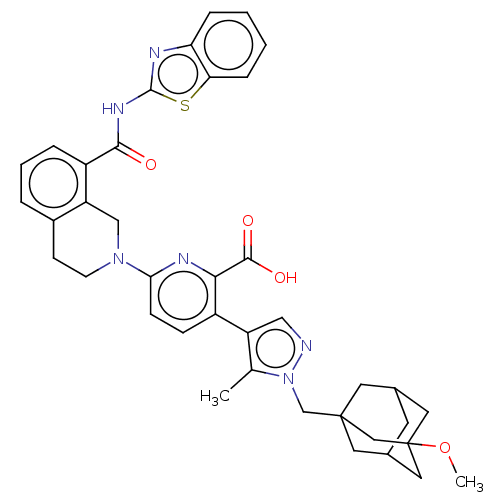 (CHEMBL4762875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50162797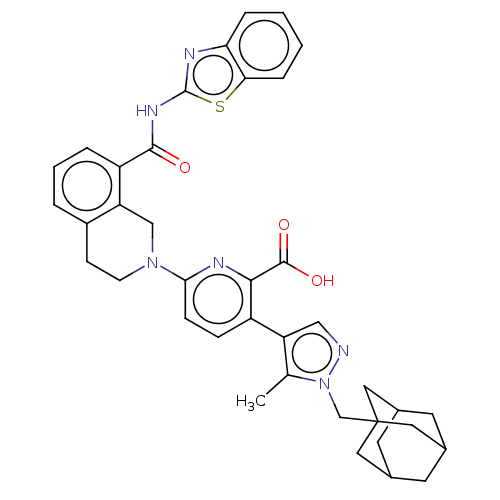 (CHEMBL3793424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209074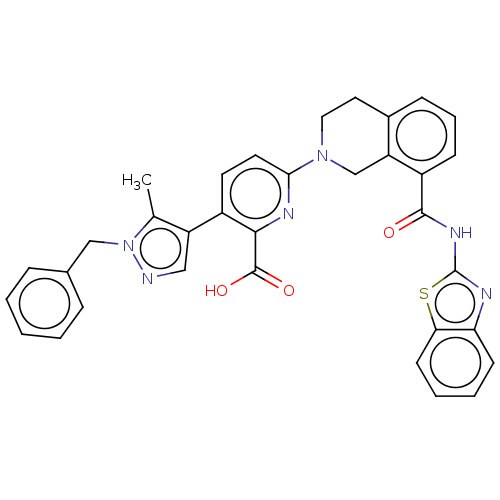 (US9266877, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209066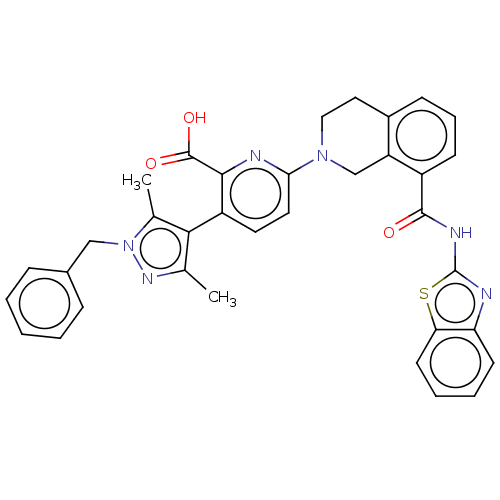 (US9266877, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209055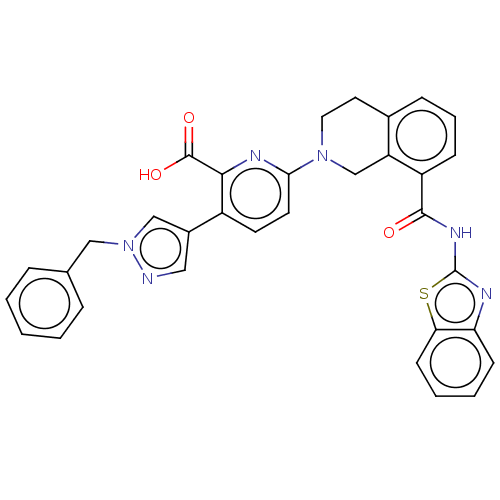 (US9266877, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209179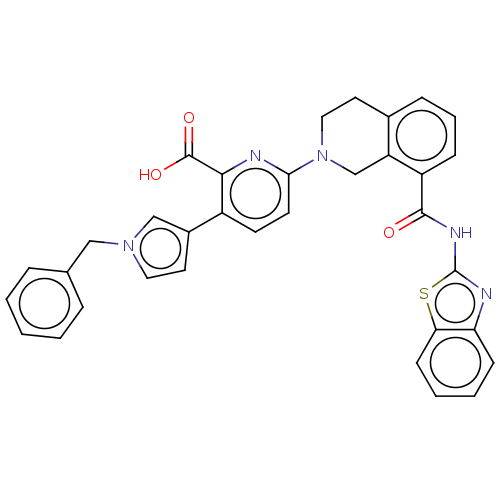 (US9266877, 125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987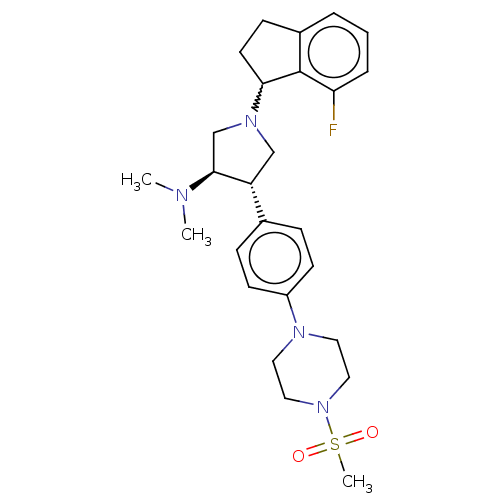 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223987 (A-395 (5) | rac-(3R,4S)-1-(7-fluoro-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209073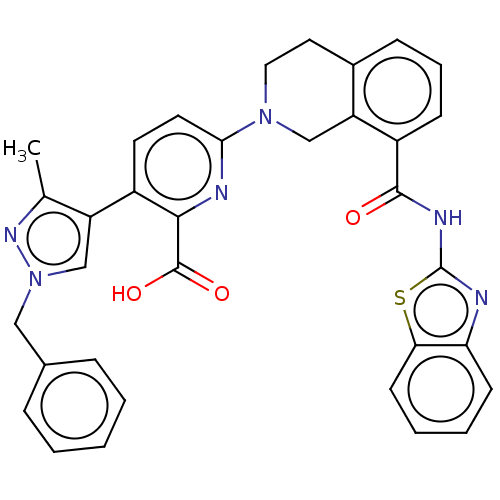 (US9266877, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223986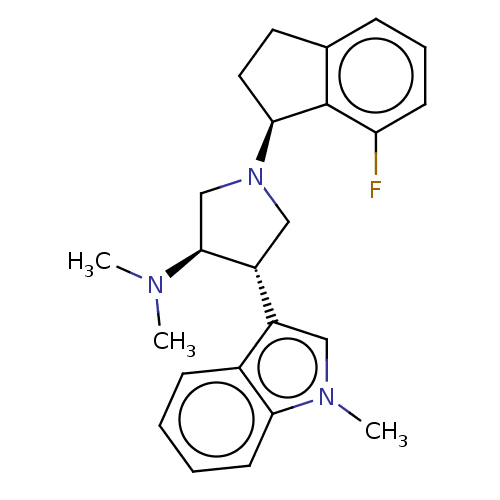 ((3R,4S)-1-[(1S)-7-fluoroindan-1-yl]-N,N-dimethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM220447 (US10633379, Compound X | US9296741, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD2 BD1 to BD2 (G73 to A560 residues) (unknown origin) | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220432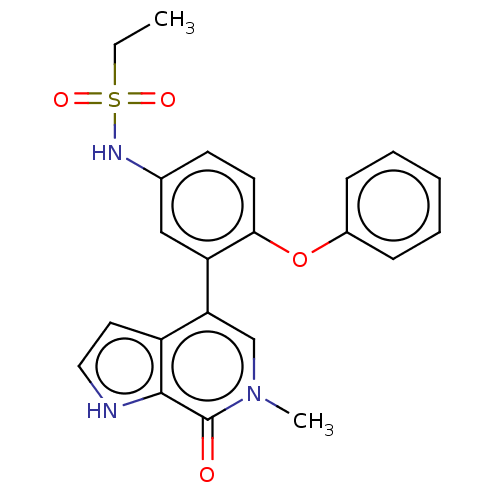 (US9296741, 21) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human placental aldose reductase | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220447 (US10633379, Compound X | US9296741, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM220447 (US10633379, Compound X | US9296741, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220415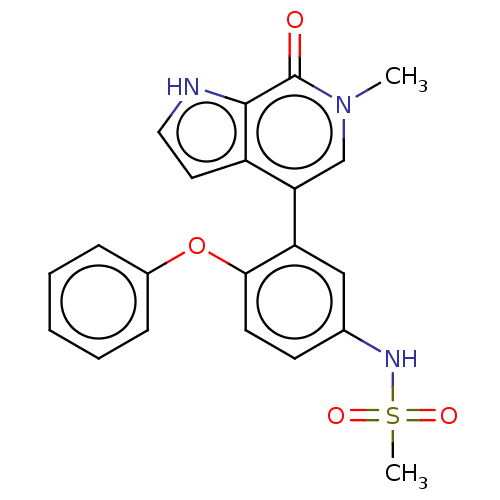 (US9296741, 4) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of ADP-induced platelet aggregation in human platelet-rich plasma | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220433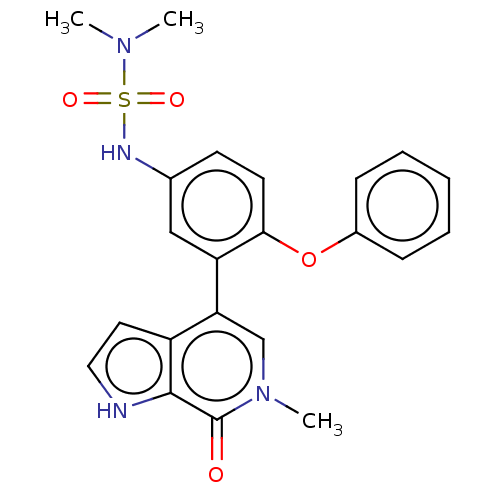 (US9296741, 22) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31202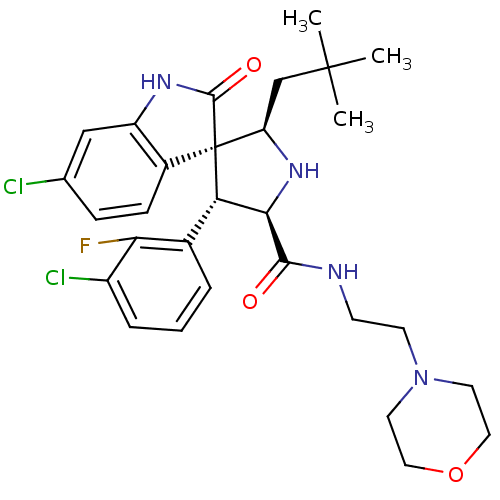 (JMC493432 Compound 8 | MI-63) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
University of Michigan | Assay Description The dose-dependent binding experiments were carried out with serial dilutions of the tested compounds in DMSO. A 5 ul sample of the tested samples an... | J Med Chem 49: 3432-5 (2006) Article DOI: 10.1021/jm051122a BindingDB Entry DOI: 10.7270/Q24F1P3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220445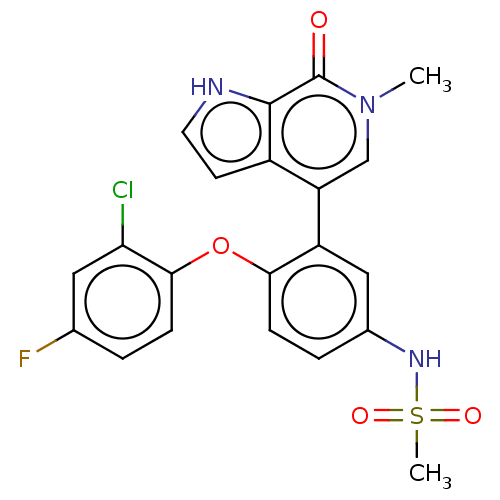 (US9296741, 34) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50241749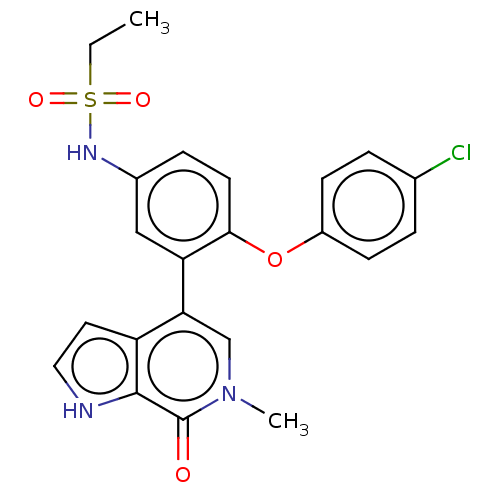 (CHEMBL4098055) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description The compound was tested for the inhibition of fibrinogen receptor | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220506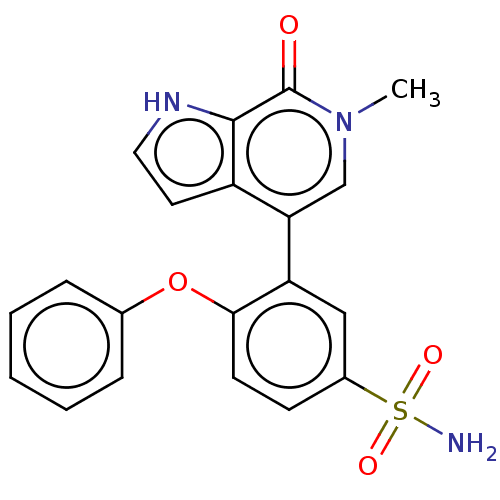 (US9296741, 95) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220468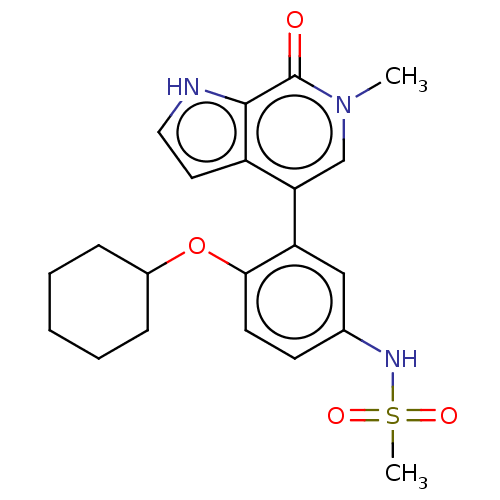 (US9296741, 57) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18665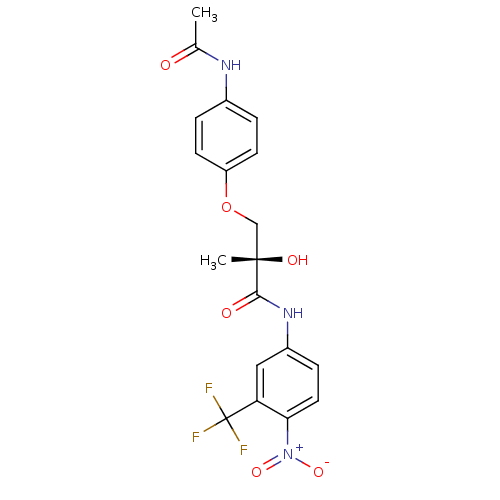 ((2S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.98 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223985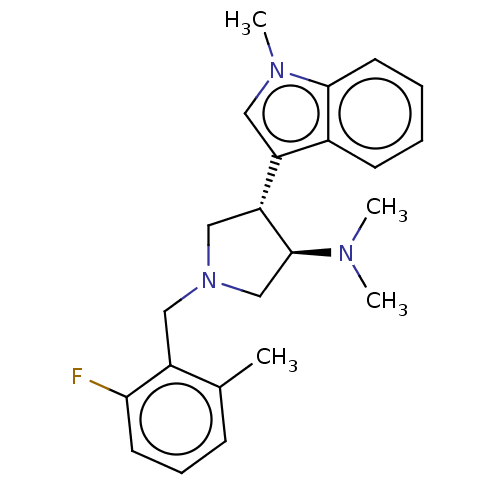 (rac-(3R,4S)-1-(2-fluoro-6-methylbenzyl)-N,N-dimeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
AbbVie Inc. | Assay Description For the assay, compounds were dispensed in assay-ready plates using a three-fold serial dilution from 50 μM to ~850 pM using an Echo 550 Acousti... | Nat Chem Biol 13: 389-395 (2017) Article DOI: 10.1038/nchembio.2306 BindingDB Entry DOI: 10.7270/Q2NG4PGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220434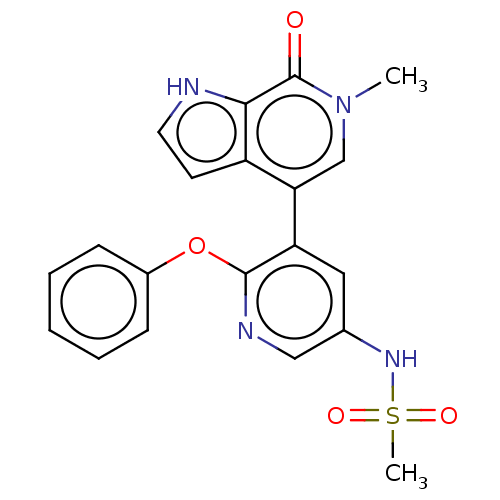 (US9296741, 23) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description The compound was tested for the inhibition of fibrinogen receptor | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220416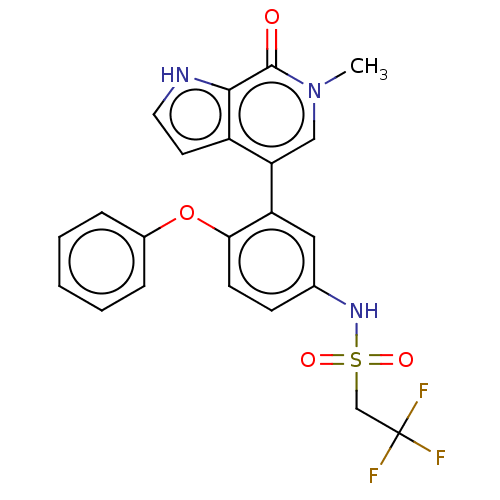 (US9296741, 5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220438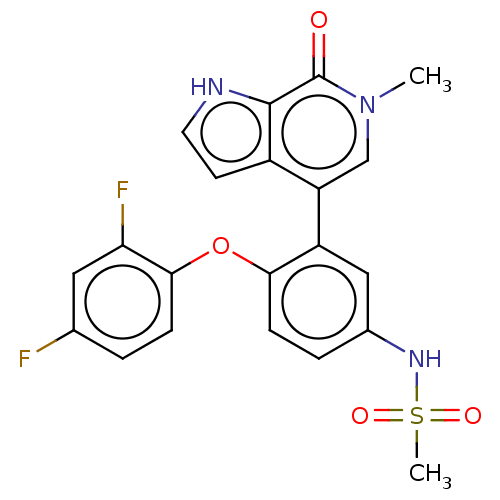 (US9296741, 27) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Tested in vitro for the inhibition of HMG-CoA reductase from partially purified microsomal preparations. | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18675 ((2S)-2-hydroxy-3-(4-isothiocyanatophenoxy)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.62 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18661 ((2R)-3-[(4-acetamidophenyl)sulfanyl]-2-hydroxy-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.90 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209056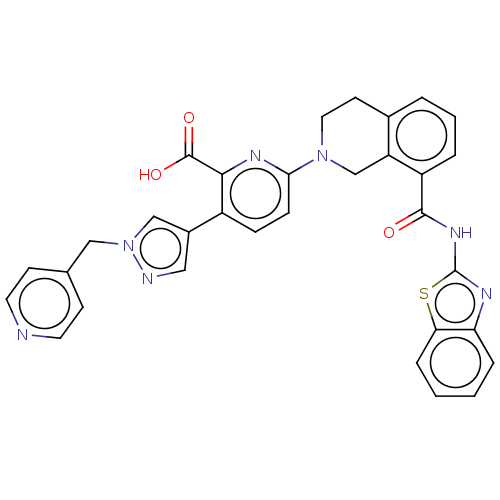 (US9266877, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220496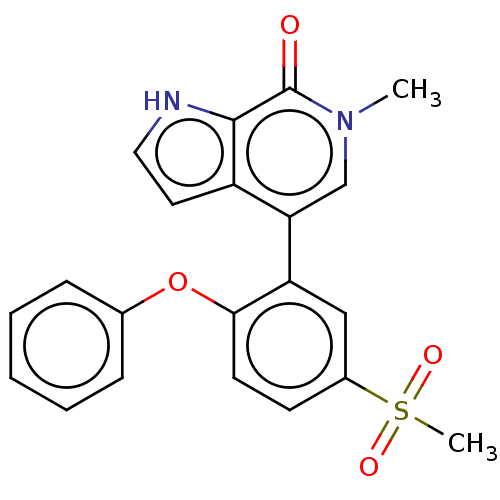 (US9296741, 85) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for its inhibitory activity against human placental aldose reductase | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209176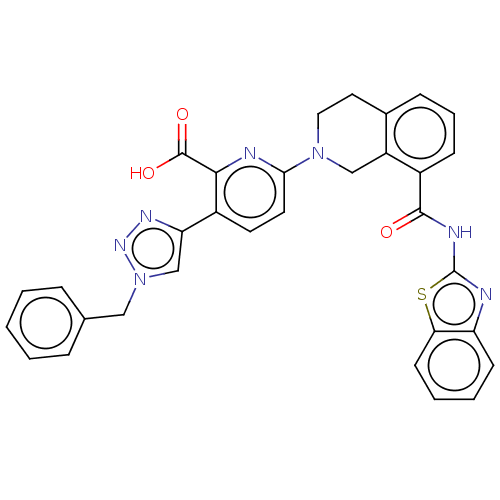 (US9266877, 122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18667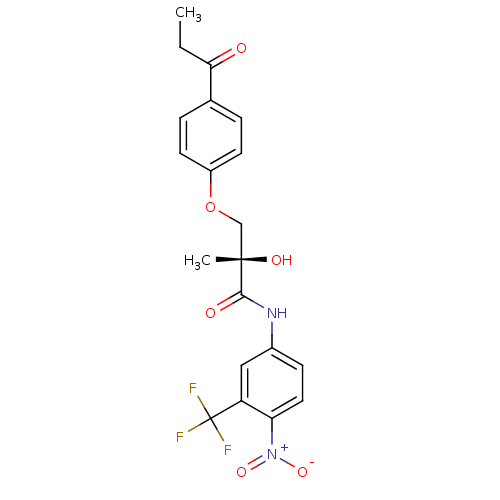 ((2S)-2-hydroxy-2-methyl-N-[4-nitro-3-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.07 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50162797 (CHEMBL3793424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) F-Bak(GQVGRQLAIIGDK(6-FAM)INR-amide) binding to BCL2 (unknown origin) incubated for 1 hr by TR-FR... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18663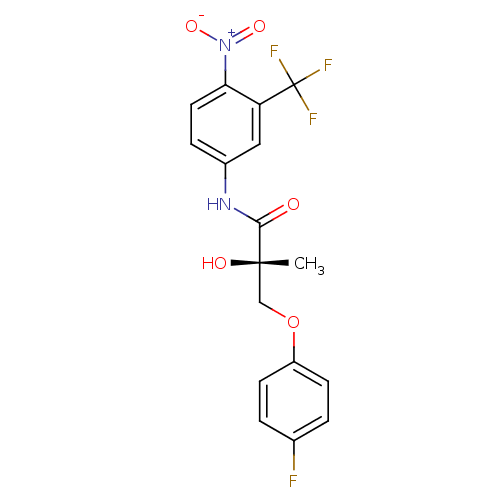 ((2S)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-[4-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.11 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220422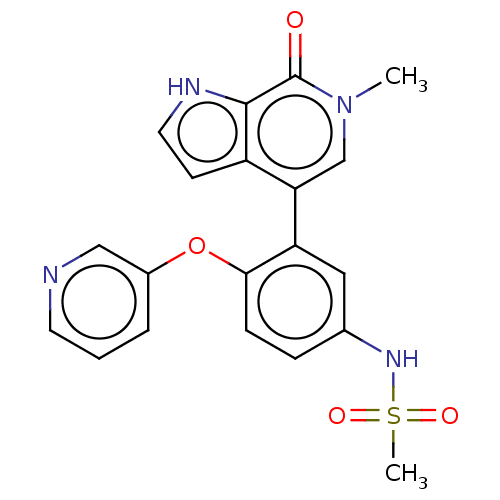 (US9296741, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50241744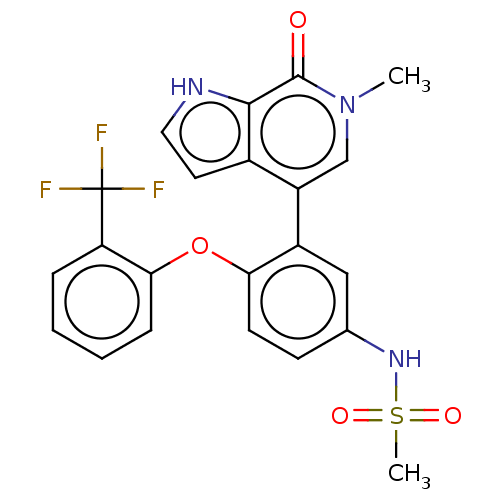 (CHEMBL4060619) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50196034 (CHEMBL410802 | QEDIIRNIARHLAQVGDSMDR) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of FAM-Bak from human Bcl-xL by FP assay | J Med Chem 49: 6139-42 (2006) Article DOI: 10.1021/jm060460o BindingDB Entry DOI: 10.7270/Q24M946T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18676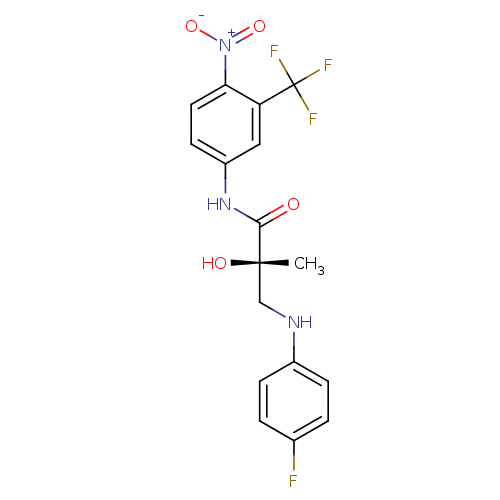 ((2S)-3-[(4-fluorophenyl)amino]-2-hydroxy-2-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.96 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50241743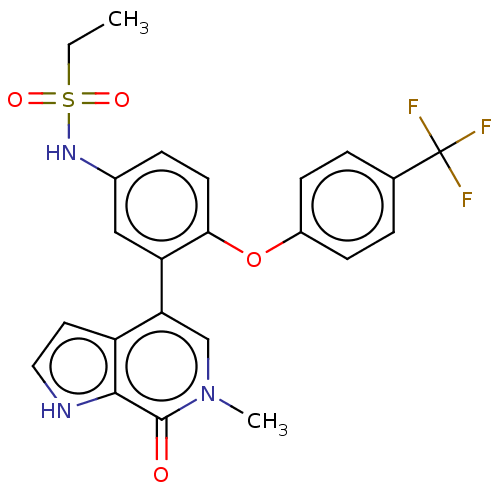 (CHEMBL4059602) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220501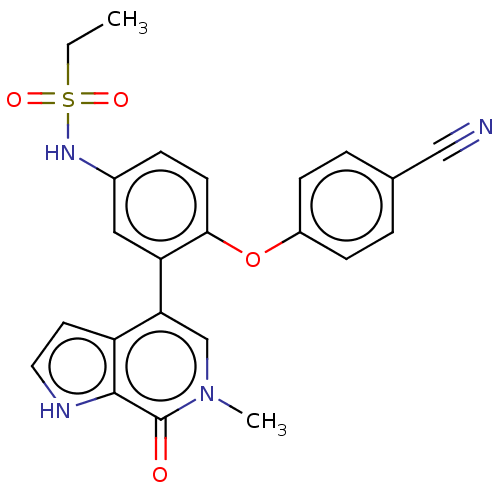 (US9296741, 90) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description The compound was tested for the inhibition of fibrinogen receptor | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18668 ((2S)-3-(4-chlorophenoxy)-2-hydroxy-2-methyl-N-[4-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 9.56 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220417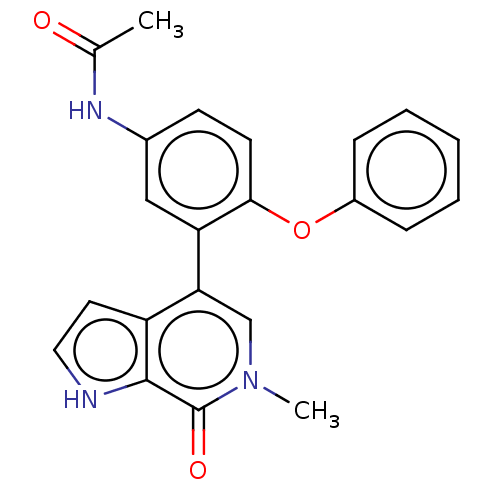 (US9296741, 6) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Compound was tested in vitro for its inhibitory activity against human placental aldose reductase | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50020933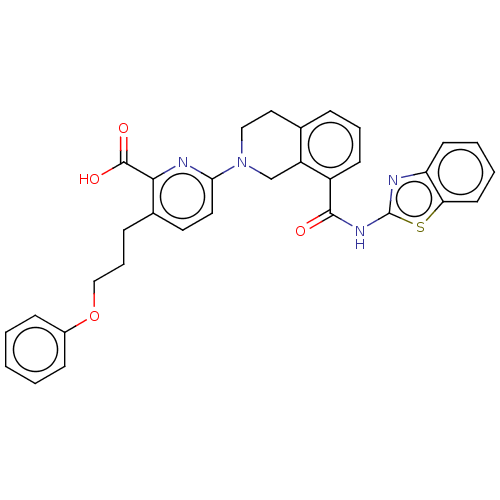 (CHEMBL3287296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50196034 (CHEMBL410802 | QEDIIRNIARHLAQVGDSMDR) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of FAM-Bid from human Bcl2 by FP assay | J Med Chem 49: 6139-42 (2006) Article DOI: 10.1021/jm060460o BindingDB Entry DOI: 10.7270/Q24M946T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18669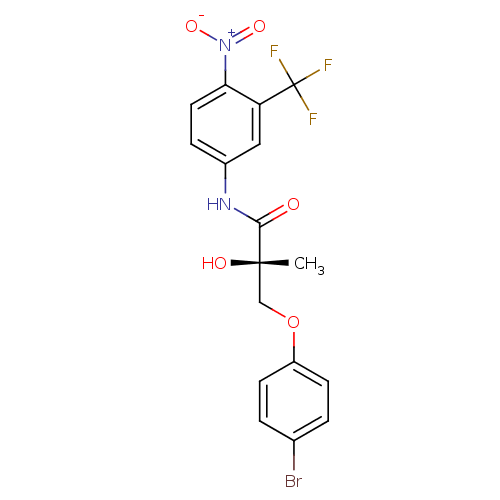 ((2S)-3-(4-bromophenoxy)-2-hydroxy-2-methyl-N-[4-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11.6 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee at Memphis | Assay Description The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... | J Med Chem 47: 993-8 (2004) Article DOI: 10.1021/jm030336u BindingDB Entry DOI: 10.7270/Q2JH3JFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM220473 (US9296741, 62) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to human N-terminal His6-tagged BRD4 BD1-BD2 (K57 to K550 residues) after 1 hr using alexa-647 conjugated probe by TR-FRET assay | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50196034 (CHEMBL410802 | QEDIIRNIARHLAQVGDSMDR) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of FAM-Bid from human Mcl1 by FP assay | J Med Chem 49: 6139-42 (2006) Article DOI: 10.1021/jm060460o BindingDB Entry DOI: 10.7270/Q24M946T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM220447 (US10633379, Compound X | US9296741, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of Fibrinogen binding to Fibrinogen receptor | J Med Chem 60: 8369-8384 (2017) Article DOI: 10.1021/acs.jmedchem.7b00746 BindingDB Entry DOI: 10.7270/Q2251MB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM31198 (spiro-oxindole, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
University of Michigan | Assay Description The dose-dependent binding experiments were carried out with serial dilutions of the tested compounds in DMSO. A 5 ul sample of the tested samples an... | J Med Chem 49: 3432-5 (2006) Article DOI: 10.1021/jm051122a BindingDB Entry DOI: 10.7270/Q24F1P3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1159 total ) | Next | Last >> |