 Found 11453 hits with Last Name = 'zheng' and Initial = 'x'
Found 11453 hits with Last Name = 'zheng' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50608295
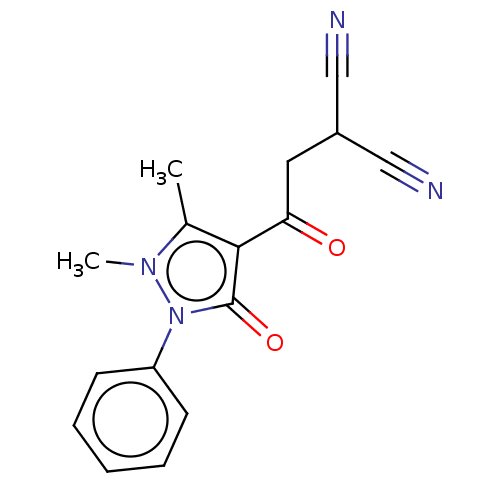
(CHEMBL5271774) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50266003
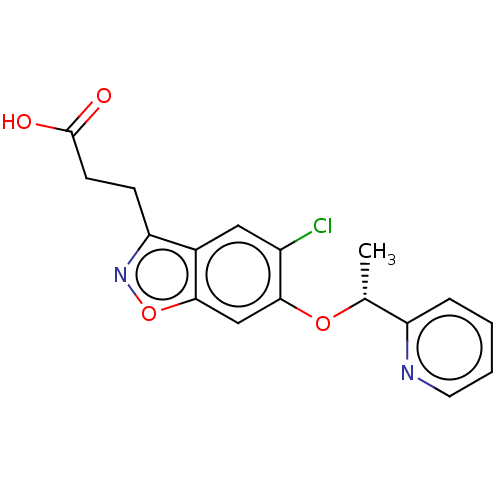
(CHEMBL4091152)Show SMILES C[C@@H](Oc1cc2onc(CCC(O)=O)c2cc1Cl)c1ccccn1 |r| Show InChI InChI=1S/C17H15ClN2O4/c1-10(13-4-2-3-7-19-13)23-16-9-15-11(8-12(16)18)14(20-24-15)5-6-17(21)22/h2-4,7-10H,5-6H2,1H3,(H,21,22)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate ... |
J Med Chem 60: 3383-3404 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00055
BindingDB Entry DOI: 10.7270/Q26112SB |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50266041

(CHEMBL4070212)Show SMILES C[C@@H](Oc1cc2oc(=O)n(CCC(O)=O)c2cc1Cl)c1ccccn1 |r| Show InChI InChI=1S/C17H15ClN2O5/c1-10(12-4-2-3-6-19-12)24-14-9-15-13(8-11(14)18)20(17(23)25-15)7-5-16(21)22/h2-4,6,8-10H,5,7H2,1H3,(H,21,22)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate measured aft... |
J Med Chem 60: 3383-3404 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00055
BindingDB Entry DOI: 10.7270/Q26112SB |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50067447
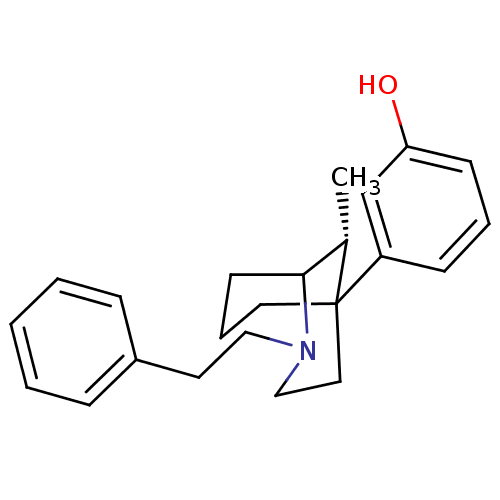
(3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...)Show InChI InChI=1S/C23H29NO/c1-18-22-11-6-13-23(18,20-9-5-10-21(25)17-20)14-16-24(22)15-12-19-7-3-2-4-8-19/h2-5,7-10,17-18,22,25H,6,11-16H2,1H3/t18-,22?,23?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by DAMGO (Opioid receptor mu 1) |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50608296

(CHEMBL5270286) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM60212
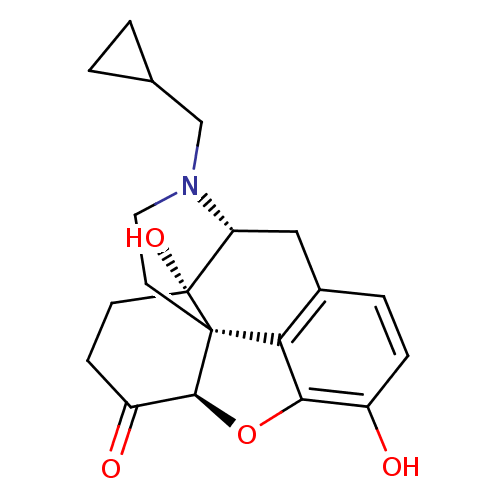
((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 using [3H]naloxone as radioligand. |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50064786
((S)-1-(2-(4-(4-methoxyphenyl)piperazin-1-yl)ethyl)...)Show SMILES CNC(=O)c1ccc2[C@H](CCN3CCN(CC3)c3ccc(OC)cc3)OCCc2c1 |r| Show InChI InChI=1S/C24H31N3O3/c1-25-24(28)19-3-8-22-18(17-19)10-16-30-23(22)9-11-26-12-14-27(15-13-26)20-4-6-21(29-2)7-5-20/h3-8,17,23H,9-16H2,1-2H3,(H,25,28)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by DAMGO (Opioid receptor mu 1) |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50266064
(CHEMBL4104310)Show InChI InChI=1S/C16H13ClN2O5/c17-11-7-12-14(24-16(22)19(12)6-4-15(20)21)8-13(11)23-9-10-3-1-2-5-18-10/h1-3,5,7-8H,4,6,9H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate measured aft... |
J Med Chem 60: 3383-3404 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00055
BindingDB Entry DOI: 10.7270/Q26112SB |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50108659
(1'-benzyl-3-methoxy-3H-spiro[2-benzofuran-1,4'-pip...)Show InChI InChI=1S/C20H23NO2/c1-22-19-17-9-5-6-10-18(17)20(23-19)11-13-21(14-12-20)15-16-7-3-2-4-8-16/h2-10,19H,11-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50067447

(3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...)Show InChI InChI=1S/C23H29NO/c1-18-22-11-6-13-23(18,20-9-5-10-21(25)17-20)14-16-24(22)15-12-19-7-3-2-4-8-19/h2-5,7-10,17-18,22,25H,6,11-16H2,1H3/t18-,22?,23?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by U69,593 in Opioid receptor kappa 1 |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50108653
(1'-benzyl-3-methoxyspiro[3,4-dihydro-1H-isochromen...)Show InChI InChI=1S/C21H25NO2/c1-23-20-15-18-9-5-6-10-19(18)21(24-20)11-13-22(14-12-21)16-17-7-3-2-4-8-17/h2-10,20H,11-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding activity against Opioid receptor mu 1 using [3H]-DAMGO as radioligand in rat brain membranes. |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50045767
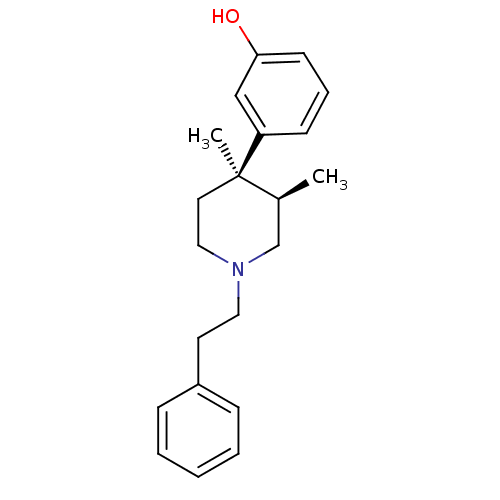
((+)-N-phenethyl trans-3(R),4(R)-dimethyl-4-(3-hydr...)Show SMILES C[C@H]1CN(CCc2ccccc2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C21H27NO/c1-17-16-22(13-11-18-7-4-3-5-8-18)14-12-21(17,2)19-9-6-10-20(23)15-19/h3-10,15,17,23H,11-14,16H2,1-2H3/t17-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 using [3H]-Naloxone as radioligand. |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50120472
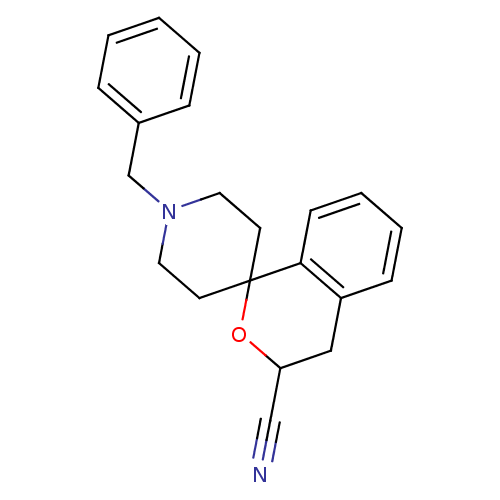
(1'-benzylspiro[3,4-dihydro-1H-isochromene-1,4'-(he...)Show InChI InChI=1S/C21H22N2O/c22-15-19-14-18-8-4-5-9-20(18)21(24-19)10-12-23(13-11-21)16-17-6-2-1-3-7-17/h1-9,19H,10-14,16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50108669
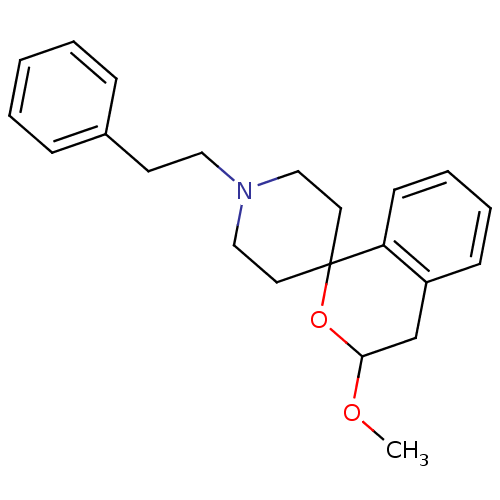
(1-[3-methoxyspiro[3,4-dihydro-1H-isochromene-1,4'-...)Show InChI InChI=1S/C22H27NO2/c1-24-21-17-19-9-5-6-10-20(19)22(25-21)12-15-23(16-13-22)14-11-18-7-3-2-4-8-18/h2-10,21H,11-17H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by U69,593 in Opioid receptor kappa 1 |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50067447

(3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...)Show InChI InChI=1S/C23H29NO/c1-18-22-11-6-13-23(18,20-9-5-10-21(25)17-20)14-16-24(22)15-12-19-7-3-2-4-8-19/h2-5,7-10,17-18,22,25H,6,11-16H2,1H3/t18-,22?,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding activity against Opioid receptor mu 1 using [3H]-DAMGO as radioligand in rat brain membranes. |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Sus scrofa) | BDBM50033116
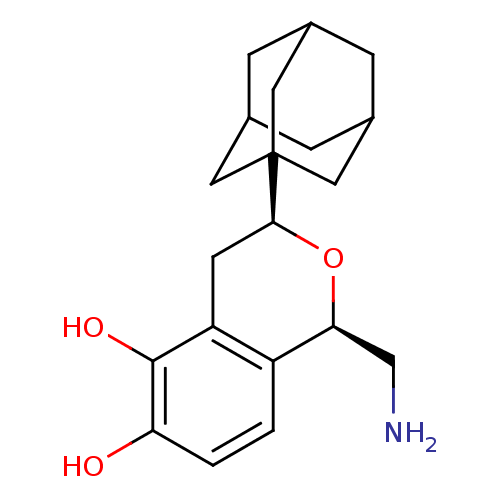
((1R,3S)-3-Adamantan-1-yl-1-aminomethyl-isochroman-...)Show SMILES NC[C@@H]1O[C@@H](Cc2c(O)c(O)ccc12)C12CC3CC(CC(C3)C1)C2 |TLB:21:16:23:22.20.19,21:20:23:15.16.17,THB:19:18:15:22.20.21,19:20:15:23.18.17| Show InChI InChI=1S/C20H27NO3/c21-10-17-14-1-2-16(22)19(23)15(14)6-18(24-17)20-7-11-3-12(8-20)5-13(4-11)9-20/h1-2,11-13,17-18,22-23H,3-10,21H2/t11?,12?,13?,17-,18-,20?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113073
BindingDB Entry DOI: 10.7270/Q2FT8R1H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 using [3H]ethylketocyclazocine as radioligand. |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24198
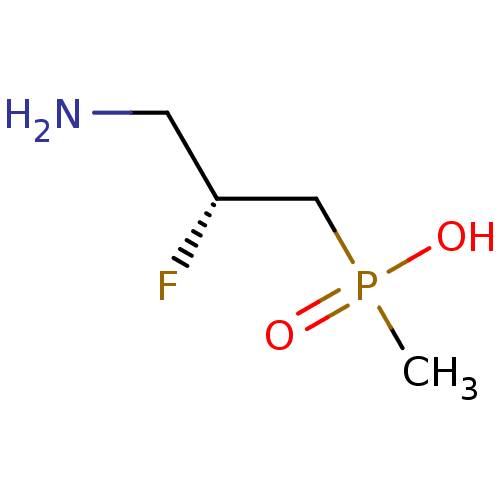
(3-aminopropylphosphinic derivative, (R)-8 | [(2R)-...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8)/t4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | -47.3 | n/a | n/a | 14 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1
(Sus scrofa) | BDBM50137938

(CHEMBL177469 | {(S)-1-[2-(N'-Benzyl-hydrazinocarbo...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NNCc1ccccc1 Show InChI InChI=1S/C31H36N4O5/c1-22(2)18-27(34-31(39)40-21-25-16-10-5-11-17-25)29(37)33-26(19-23-12-6-3-7-13-23)28(36)30(38)35-32-20-24-14-8-4-9-15-24/h3-17,22,26-27,32H,18-21H2,1-2H3,(H,33,37)(H,34,39)(H,35,38)/t26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against porcine erythrocyte calpain I was determined |
J Med Chem 47: 72-9 (2003)
Article DOI: 10.1021/jm0301336
BindingDB Entry DOI: 10.7270/Q2ZP45KD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding activity against Opioid receptor kappa 1 using [3H]U69,593 as radioligand in guinea pig membranes . |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24195
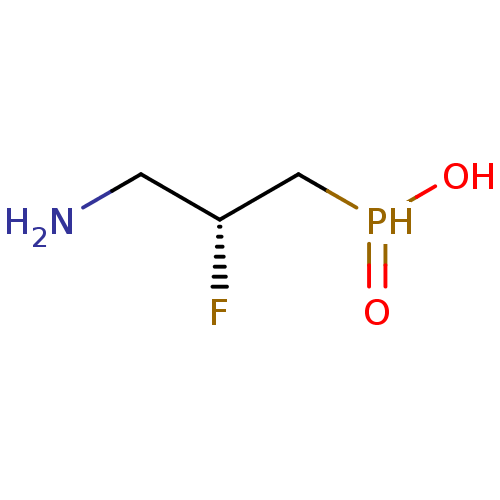
(3-aminopropylphosphinic derivative, (R)-7 | AZD335...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7)/t3-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | -46.9 | n/a | n/a | 8.64 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24193
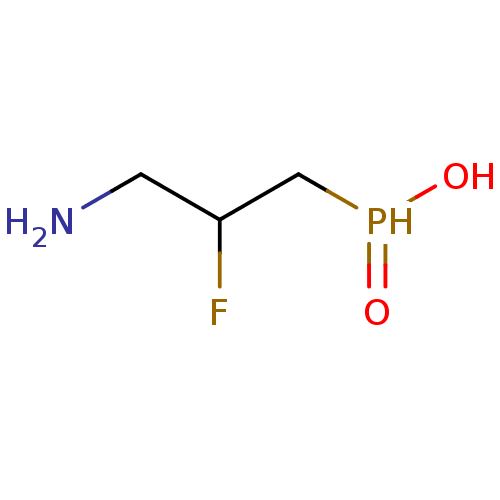
((3-amino-2-fluoropropyl)phosphinic acid | 3-aminop...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | -45.2 | n/a | n/a | 15 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50067447

(3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...)Show InChI InChI=1S/C23H29NO/c1-18-22-11-6-13-23(18,20-9-5-10-21(25)17-20)14-16-24(22)15-12-19-7-3-2-4-8-19/h2-5,7-10,17-18,22,25H,6,11-16H2,1H3/t18-,22?,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by SNC80 (Opioid receptor delta 1) |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24196
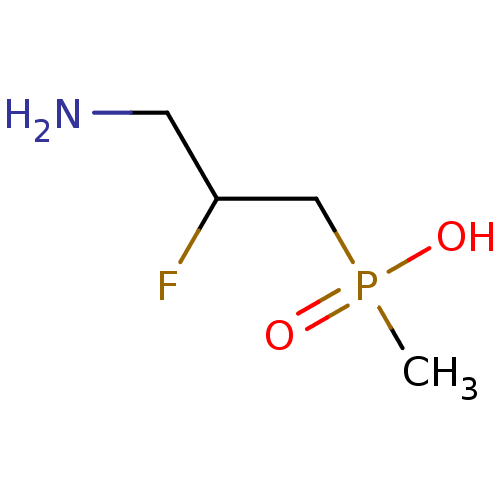
((3-amino-2-fluoropropyl)(methyl)phosphinic acid | ...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | -44.4 | n/a | n/a | 23 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24184
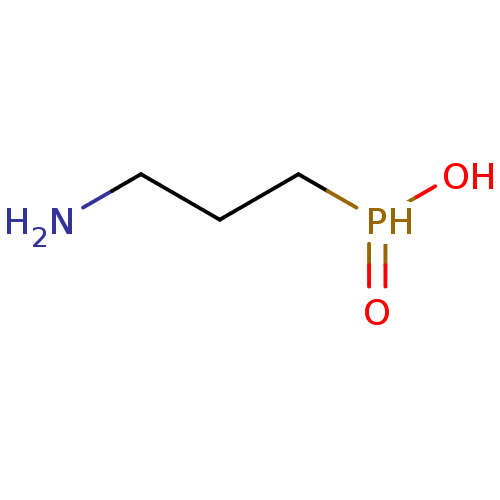
((3-aminopropyl)phosphinic acid | 3-aminopropylphos...)Show InChI InChI=1S/C3H10NO2P/c4-2-1-3-7(5)6/h7H,1-4H2,(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | -44.2 | n/a | n/a | 19 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50067447

(3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...)Show InChI InChI=1S/C23H29NO/c1-18-22-11-6-13-23(18,20-9-5-10-21(25)17-20)14-16-24(22)15-12-19-7-3-2-4-8-19/h2-5,7-10,17-18,22,25H,6,11-16H2,1H3/t18-,22?,23?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding activity against kOpioid receptor kappa 1 using [3H]U69,593 as radioligand in guinea pig membranes . |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM60212

((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by SNC80 (Opioid receptor delta 1) |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50067446
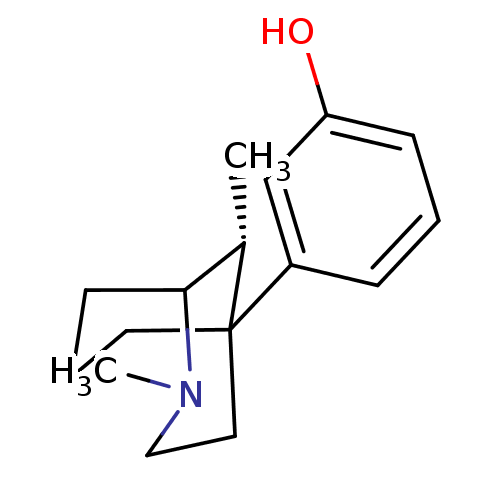
(3-((R)-2,9-Dimethyl-2-aza-bicyclo[3.3.1]non-5-yl)-...)Show InChI InChI=1S/C16H23NO/c1-12-15-7-4-8-16(12,9-10-17(15)2)13-5-3-6-14(18)11-13/h3,5-6,11-12,15,18H,4,7-10H2,1-2H3/t12-,15?,16?/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by DAMGO (Opioid receptor mu 1) |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184807
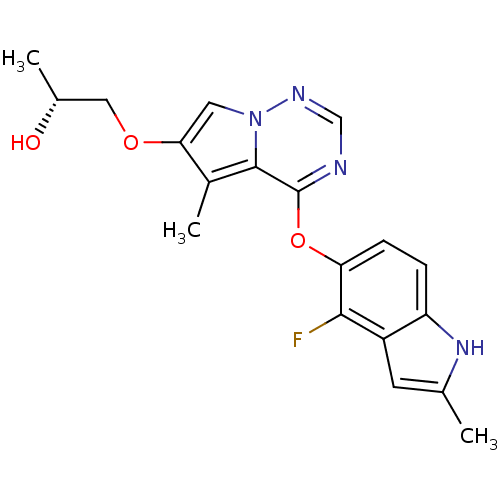
((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR2 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Flk1 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24185
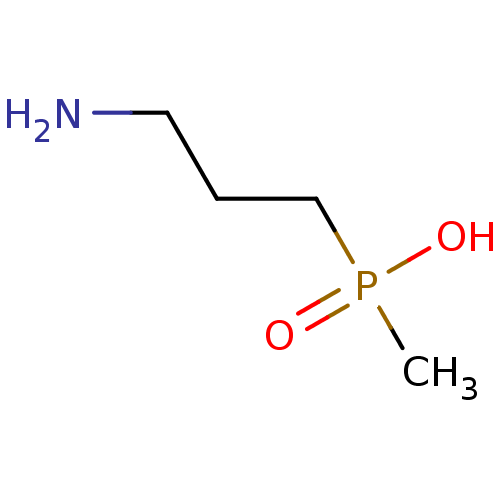
((3-aminopropyl)(methyl)phosphinic acid | 3-Apmpa |...)Show InChI InChI=1S/C4H12NO2P/c1-8(6,7)4-2-3-5/h2-5H2,1H3,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 33 | -42.3 | n/a | n/a | 41 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50349984
(CHEMBL1813048)Show SMILES CC(=O)Nc1cccc(Nc2ncnc(n2)N2CCC(CC2)OCc2ccc(OC(F)(F)F)cc2)c1C Show InChI InChI=1S/C25H27F3N6O3/c1-16-21(31-17(2)35)4-3-5-22(16)32-23-29-15-30-24(33-23)34-12-10-19(11-13-34)36-14-18-6-8-20(9-7-18)37-25(26,27)28/h3-9,15,19H,10-14H2,1-2H3,(H,31,35)(H,29,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to inactivated human sodium channel Nav1.7 expressed in human HEK293 cells by patch-clamp electrophysiological assay |
J Med Chem 54: 4427-45 (2011)
Article DOI: 10.1021/jm200018k
BindingDB Entry DOI: 10.7270/Q2N016W1 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Rattus norvegicus) | BDBM50266003

(CHEMBL4091152)Show SMILES C[C@@H](Oc1cc2onc(CCC(O)=O)c2cc1Cl)c1ccccn1 |r| Show InChI InChI=1S/C17H15ClN2O4/c1-10(13-4-2-3-7-19-13)23-16-9-15-11(8-12(16)18)14(20-24-15)5-6-17(21)22/h2-4,7-10H,5-6H2,1H3,(H,21,22)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat KMO expressed in HEK293 cells using kynurenine as substrate measured after 20 hrs by LC-MS/MS analysis |
J Med Chem 60: 3383-3404 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00055
BindingDB Entry DOI: 10.7270/Q26112SB |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1
(Sus scrofa) | BDBM50101321
(CHEMBL299750 | [(S)-1-((S)-1-Benzyl-2-benzyloxycar...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NOCc1ccccc1 Show InChI InChI=1S/C31H35N3O6/c1-22(2)18-27(33-31(38)39-20-24-14-8-4-9-15-24)29(36)32-26(19-23-12-6-3-7-13-23)28(35)30(37)34-40-21-25-16-10-5-11-17-25/h3-17,22,26-27H,18-21H2,1-2H3,(H,32,36)(H,33,38)(H,34,37)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was evaluated against porcine erythrocyte Calpain 1 at the S1 subsite of the enzyme |
Bioorg Med Chem Lett 11: 1753-5 (2001)
BindingDB Entry DOI: 10.7270/Q26972V8 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24191

((3-amino-2-oxopropyl)phosphinic acid | (3-amino-2-...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h8H,1-2,4H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | -41.4 | n/a | n/a | 81 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24186
((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h6-7H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | -41.3 | n/a | n/a | 130 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50045767

((+)-N-phenethyl trans-3(R),4(R)-dimethyl-4-(3-hydr...)Show SMILES C[C@H]1CN(CCc2ccccc2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C21H27NO/c1-17-16-22(13-11-18-7-4-3-5-8-18)14-12-21(17,2)19-9-6-10-20(23)15-19/h3-10,15,17,23H,11-14,16H2,1-2H3/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 using [3H]-Ethylketocyclazocine as radioligand. |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1
(Sus scrofa) | BDBM50137939

(CHEMBL366793 | {(S)-3-Methyl-1-[2-oxo-2-phenethylc...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C32H37N3O5/c1-23(2)20-28(35-32(39)40-22-26-16-10-5-11-17-26)30(37)34-27(21-25-14-8-4-9-15-25)29(36)31(38)33-19-18-24-12-6-3-7-13-24/h3-17,23,27-28H,18-22H2,1-2H3,(H,33,38)(H,34,37)(H,35,39)/t27-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against porcine erythrocyte calpain I was determined |
J Med Chem 47: 72-9 (2003)
Article DOI: 10.1021/jm0301336
BindingDB Entry DOI: 10.7270/Q2ZP45KD |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50184807

((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against VEGFR1 |
J Med Chem 49: 2143-6 (2006)
Article DOI: 10.1021/jm051106d
BindingDB Entry DOI: 10.7270/Q2WW7H7N |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1
(Sus scrofa) | BDBM50101317
(CHEMBL301598 | [(S)-1-((S)-1-Benzyl-2-ethoxycarbam...)Show SMILES CCONC(=O)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C26H33N3O6/c1-4-35-29-25(32)23(30)21(16-19-11-7-5-8-12-19)27-24(31)22(15-18(2)3)28-26(33)34-17-20-13-9-6-10-14-20/h5-14,18,21-22H,4,15-17H2,1-3H3,(H,27,31)(H,28,33)(H,29,32)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was evaluated against porcine erythrocyte Calpain 1 at the S1 subsite of the enzyme |
Bioorg Med Chem Lett 11: 1753-5 (2001)
BindingDB Entry DOI: 10.7270/Q26972V8 |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1
(Sus scrofa) | BDBM50093805

(CHEMBL416281 | [(S)-1-((S)-1-Benzyl-2-oxo-2-phenet...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C32H37N3O5/c1-23(2)20-28(35-32(39)40-22-26-16-10-5-11-17-26)30(37)34-27(21-25-14-8-4-9-15-25)29(36)31(38)33-19-18-24-12-6-3-7-13-24/h3-17,23,27-28H,18-22H2,1-2H3,(H,33,38)(H,34,37)(H,35,39)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of Calpain 1 from porcine erythrocytes |
Bioorg Med Chem Lett 10: 2497-500 (2001)
BindingDB Entry DOI: 10.7270/Q2QF8S4Z |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24194

(3-aminopropylphosphinic derivative, (S)-7 | [(2S)-...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | -40.4 | n/a | n/a | 250 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1
(Sus scrofa) | BDBM50093805

(CHEMBL416281 | [(S)-1-((S)-1-Benzyl-2-oxo-2-phenet...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C32H37N3O5/c1-23(2)20-28(35-32(39)40-22-26-16-10-5-11-17-26)30(37)34-27(21-25-14-8-4-9-15-25)29(36)31(38)33-19-18-24-12-6-3-7-13-24/h3-17,23,27-28H,18-22H2,1-2H3,(H,33,38)(H,34,37)(H,35,39)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tennessee
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was evaluated against porcine erythrocyte Calpain 1 at the S1 subsite of the enzyme |
Bioorg Med Chem Lett 11: 1753-5 (2001)
BindingDB Entry DOI: 10.7270/Q26972V8 |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1
(Sus scrofa) | BDBM50137932

(CHEMBL177592 | {(S)-1-[2-((R)-1-Hydrazinomethyl-2-...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)C(=O)N[C@@H](CNN)Cc1ccccc1 Show InChI InChI=1S/C33H41N5O5/c1-23(2)18-29(38-33(42)43-22-26-16-10-5-11-17-26)31(40)37-28(20-25-14-8-4-9-15-25)30(39)32(41)36-27(21-35-34)19-24-12-6-3-7-13-24/h3-17,23,27-29,35H,18-22,34H2,1-2H3,(H,36,41)(H,37,40)(H,38,42)/t27-,28+,29?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against porcine erythrocyte calpain I was determined |
J Med Chem 47: 72-9 (2003)
Article DOI: 10.1021/jm0301336
BindingDB Entry DOI: 10.7270/Q2ZP45KD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50045772
(3-((3R,4R)-1,3,4-Trimethyl-piperidin-4-yl)-phenol ...)Show InChI InChI=1S/C14H21NO/c1-11-10-15(3)8-7-14(11,2)12-5-4-6-13(16)9-12/h4-6,9,11,16H,7-8,10H2,1-3H3/t11-,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 using [3H]-Naloxone as radioligand. |
J Med Chem 41: 4143-9 (1998)
Article DOI: 10.1021/jm980290i
BindingDB Entry DOI: 10.7270/Q2RB73Q6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032720

(CHEMBL3354696)Show SMILES NC1=N[C@]2(CO1)c1cc(OCC3CCCCC3)ccc1Oc1ccc(cc21)-c1cncnc1 |r,t:1| Show InChI InChI=1S/C26H26N4O3/c27-25-30-26(15-32-25)21-10-18(19-12-28-16-29-13-19)6-8-23(21)33-24-9-7-20(11-22(24)26)31-14-17-4-2-1-3-5-17/h6-13,16-17H,1-5,14-15H2,(H2,27,30)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data