

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194104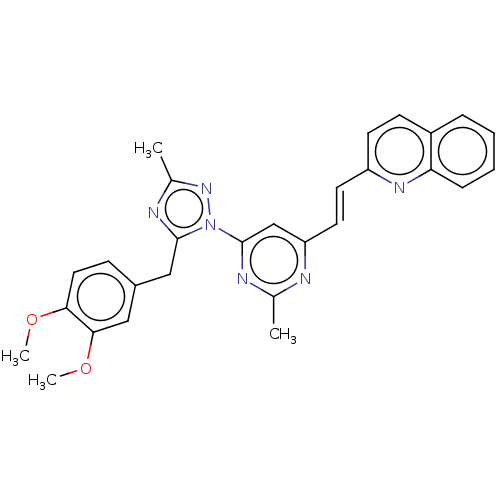 (US9200001, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194105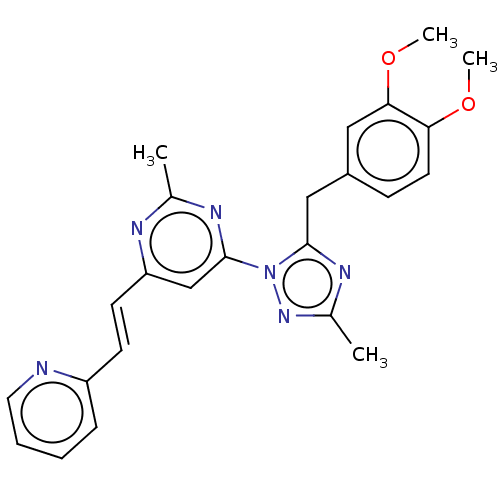 (US9200001, 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194113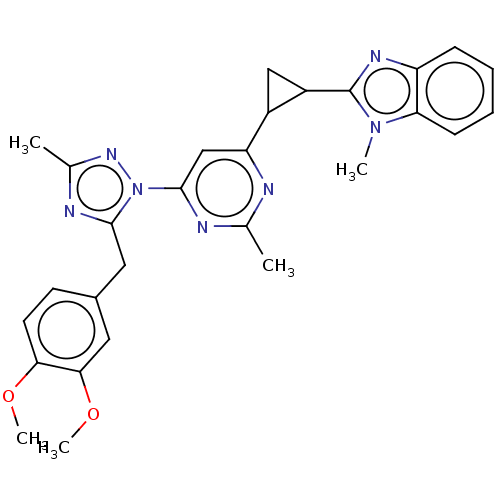 (US9200001, 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194090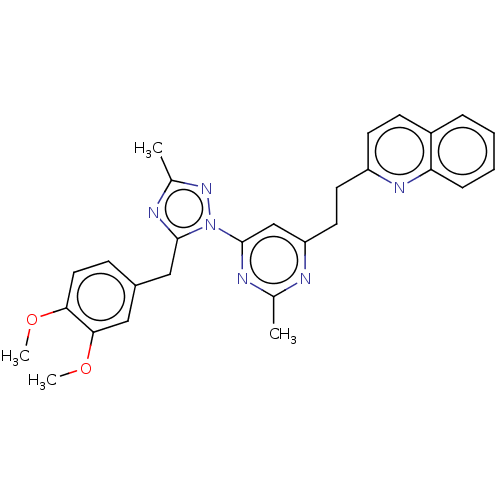 (US9200001, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194122 (US9200001, 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194223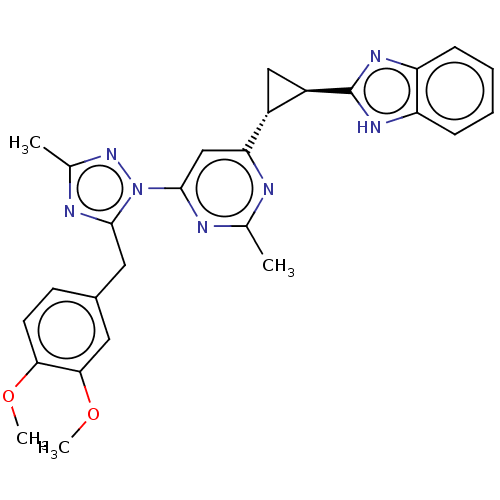 (US9200001, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194099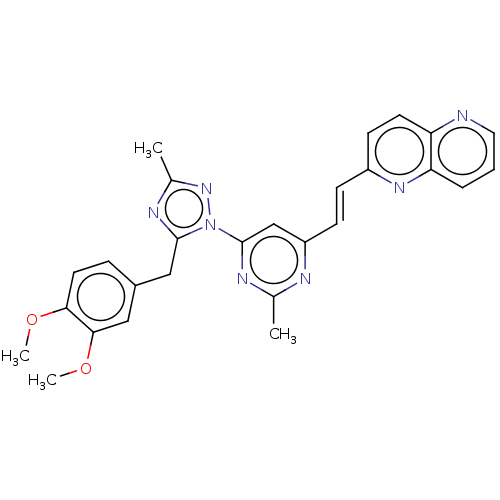 (US9200001, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163726 (US9062059, 2-27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194089 (US9200001, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194111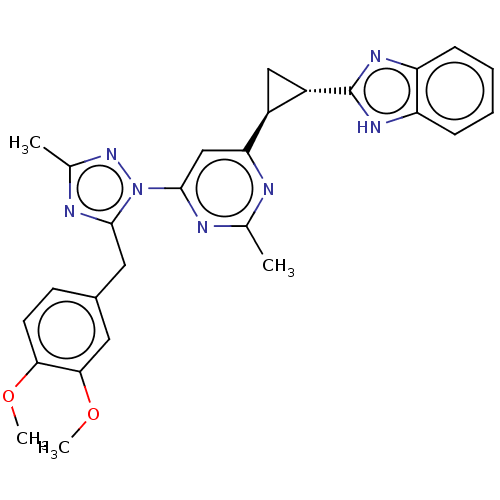 (US9200001, 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194102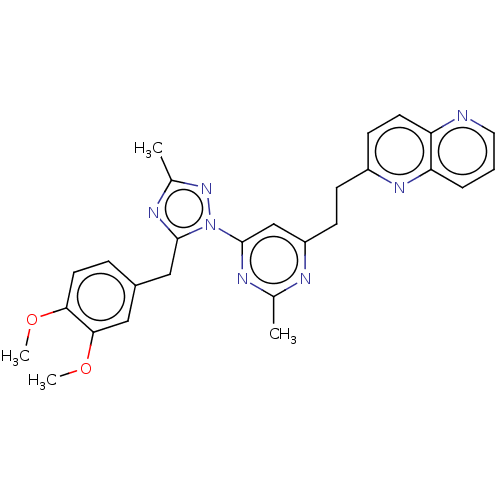 (US9200001, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194095 (US9200001, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163722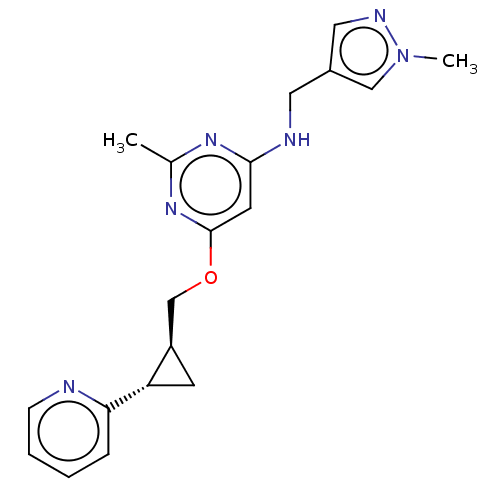 (US9062059, 1-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163741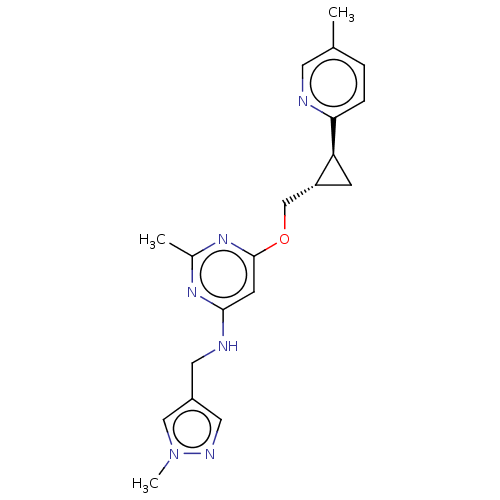 (US9062059, 2-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163729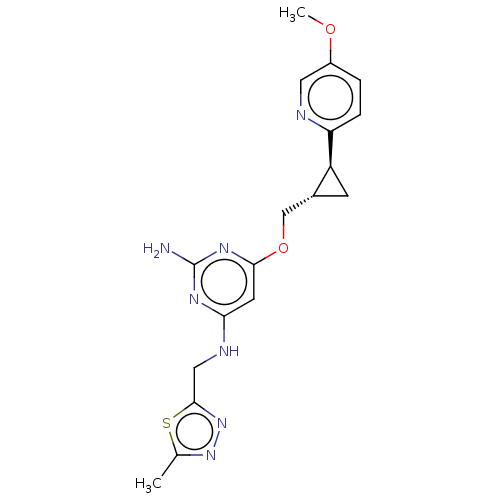 (US9062059, 2-53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194101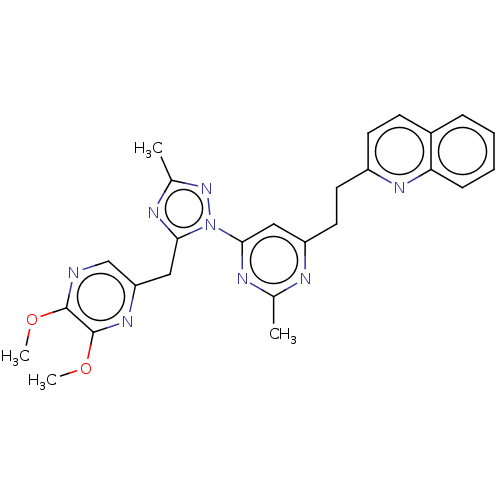 (US9200001, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244119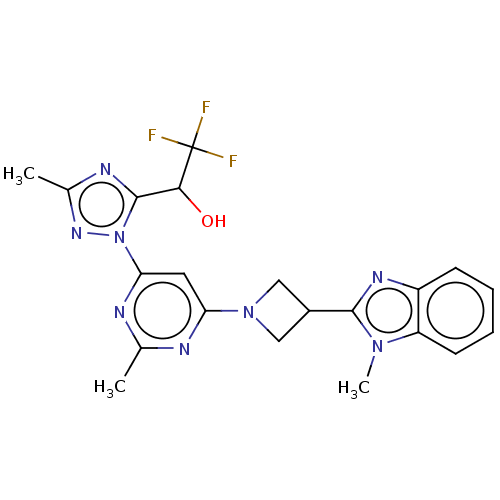 (US9428492, 3 | US9428492, 3a | US9428492, 3b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | -58.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50240829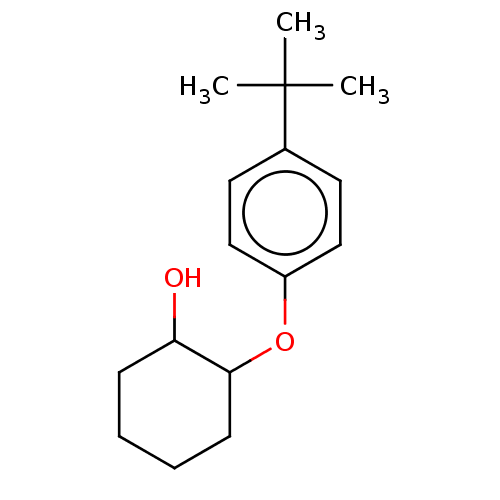 (CHEMBL3559801) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leipzig University Curated by ChEMBL | Assay Description Effect on pancreatic polypeptide-mediated displacement of 125I-pancreatic polypeptide from human C-terminal eYFP-tagged NY4 receptor expressed in HEK... | J Med Chem 60: 7605-7612 (2017) Article DOI: 10.1021/acs.jmedchem.7b00976 BindingDB Entry DOI: 10.7270/Q22B915R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194119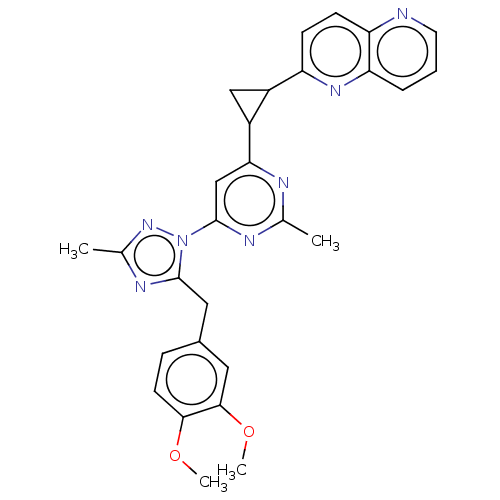 (US9200001, 34) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194094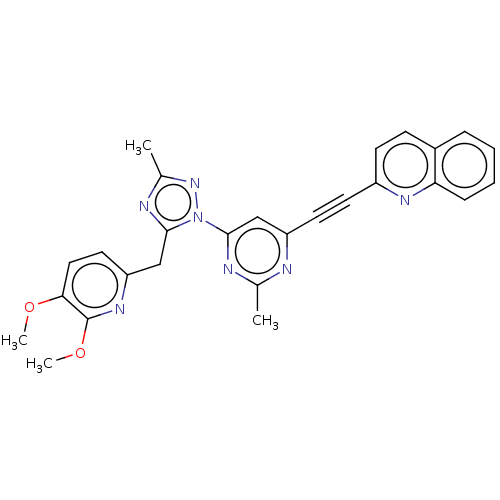 (US9200001, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163737 (US9062059, 9-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194145 (US9200001, 61) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163735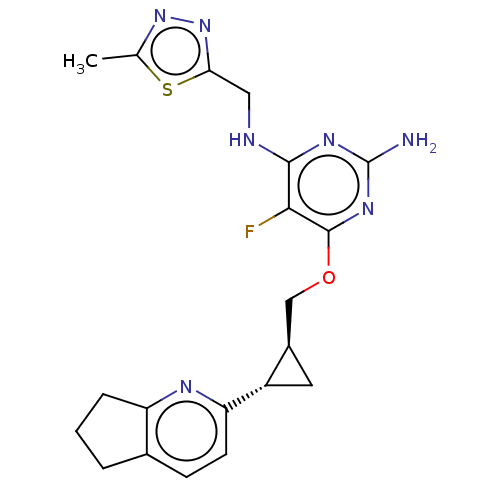 (US9062059, 3-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244145 (US9428492, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | -57.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244119 (US9428492, 3 | US9428492, 3a | US9428492, 3b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335985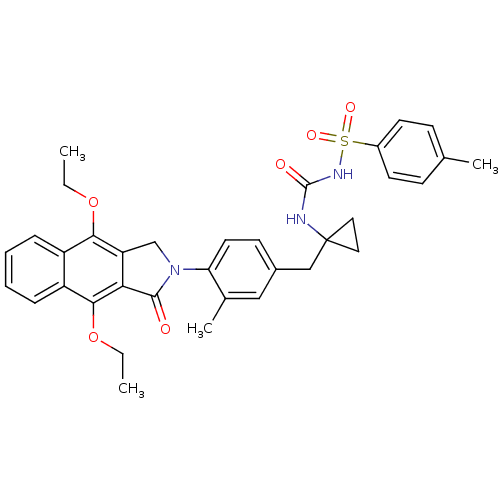 (CHEMBL1669013 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194106 (US9200001, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244137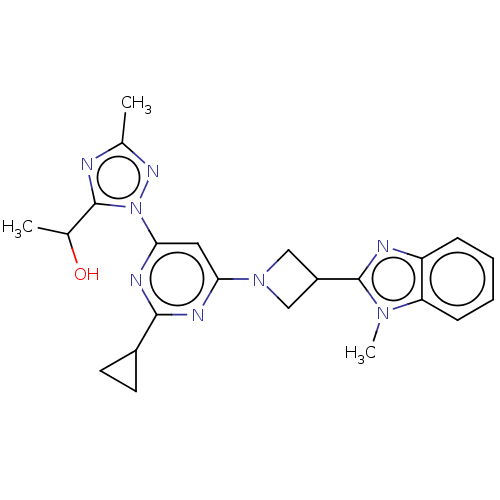 (US9428492, 10 | US9428492, 10a | US9428492, 10b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194134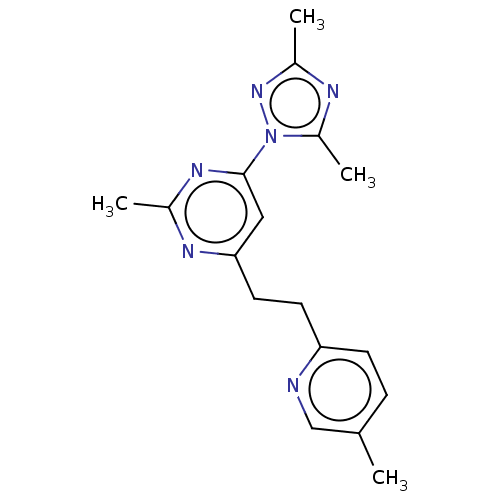 (US9200001, 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194092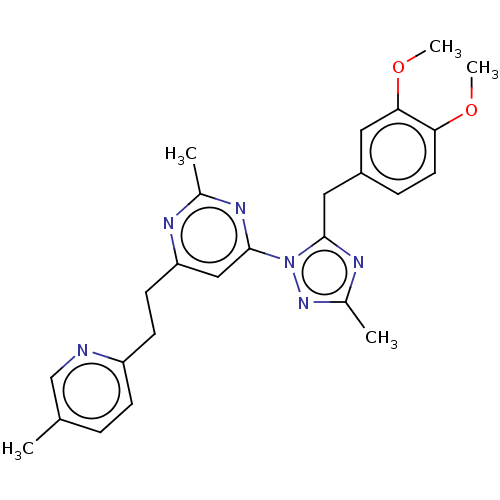 (US9200001, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335980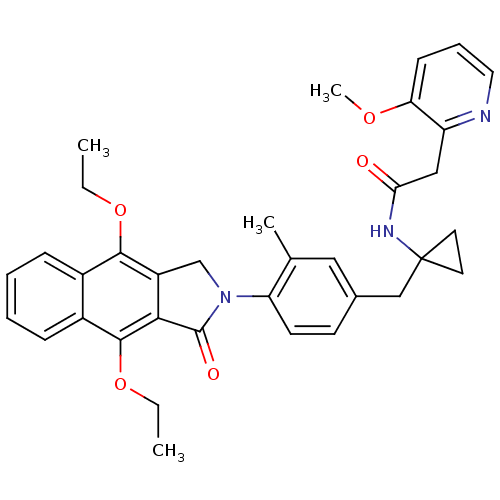 (CHEMBL1669017 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335986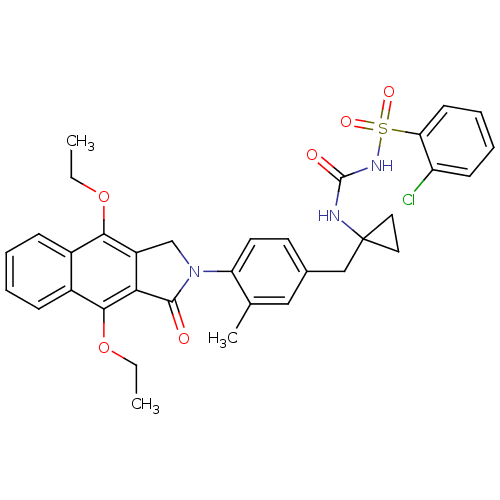 (2-chloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335989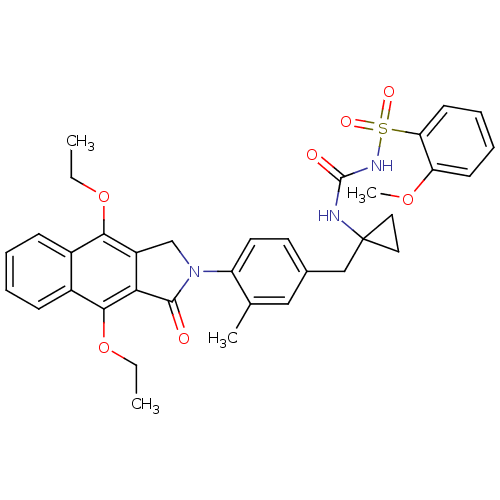 (CHEMBL1669009 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244127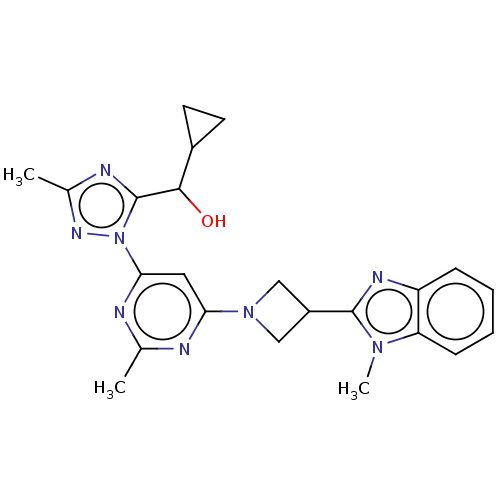 (US9428492, 6 | US9428492, 6a | US9428492, 6b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335981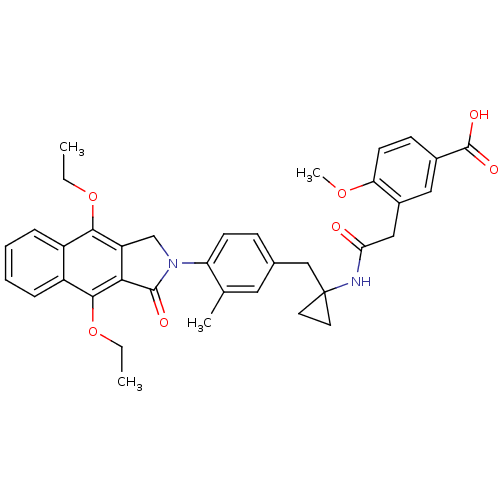 (3-(2-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]isoindol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335984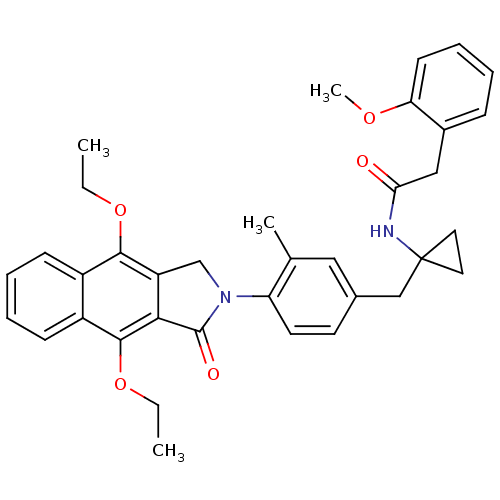 (CHEMBL1669018 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01511 BindingDB Entry DOI: 10.7270/Q22Z19M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194133 (US9200001, 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244137 (US9428492, 10 | US9428492, 10a | US9428492, 10b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194120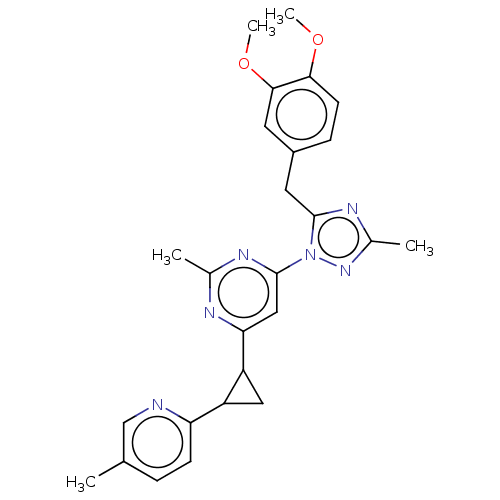 (US9200001, 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244117 (US9428492, 1 | US9428492, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319837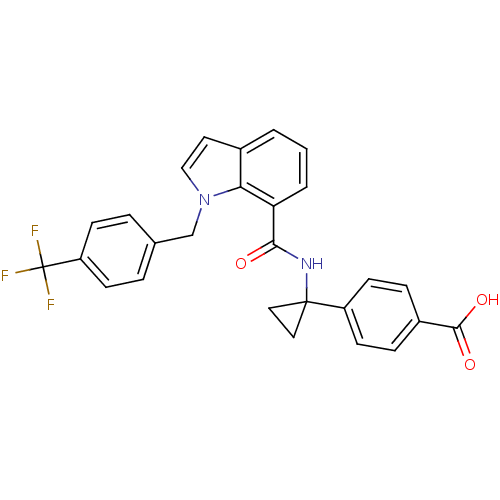 (4-(1-(1-(4-(trifluoromethyl)benzyl)-1H-indole-7-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in HEK293-EBNA cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335988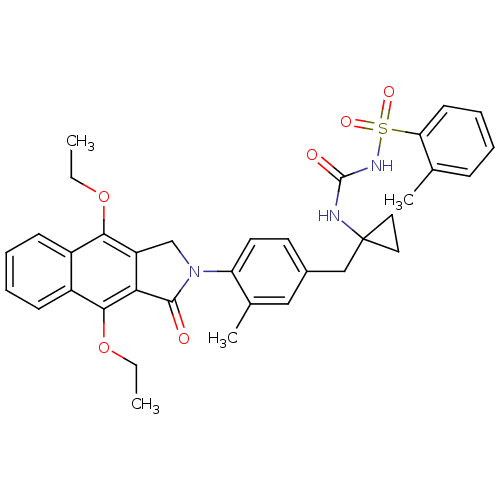 (CHEMBL1669010 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335990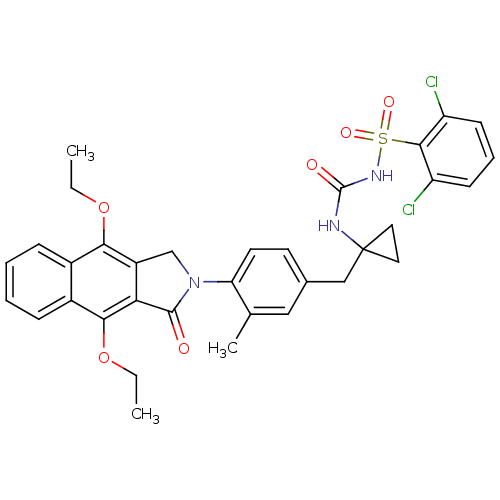 (2,6-dichloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50594944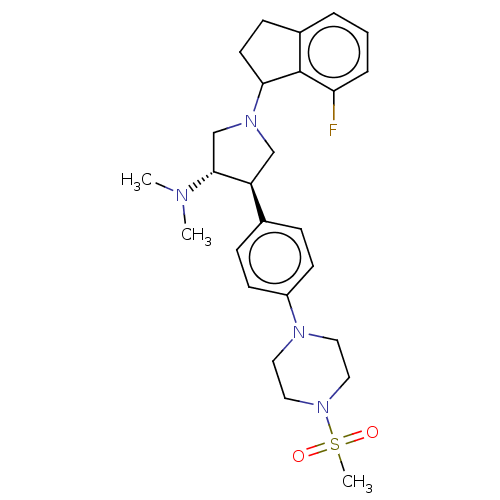 (CHEMBL5181703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114144 BindingDB Entry DOI: 10.7270/Q2DN4921 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50319836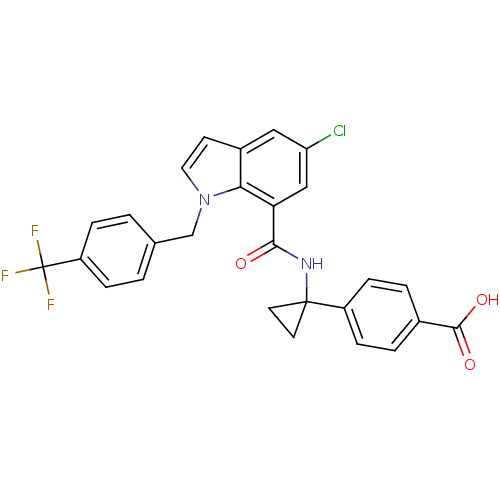 (4-(1-(5-chloro-1-(4-(trifluoromethyl)benzyl)-1H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Ltd Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in HEK293-EBNA cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 3760-3 (2010) Article DOI: 10.1016/j.bmcl.2010.04.065 BindingDB Entry DOI: 10.7270/Q20P106K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50336003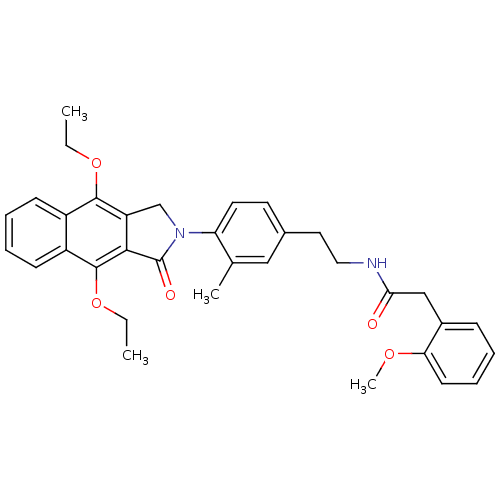 (CHEMBL1669023 | N-(4-(4,9-diethoxy-1-oxo-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335982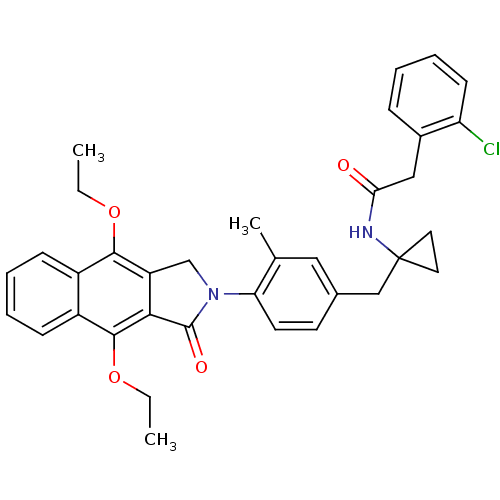 (2-(2-chlorophenyl)-N-(1-(4-(4,9-diethoxy-1-oxo-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244124 (US9428492, 5 | US9428492, 5a | US9428492, 5b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM244142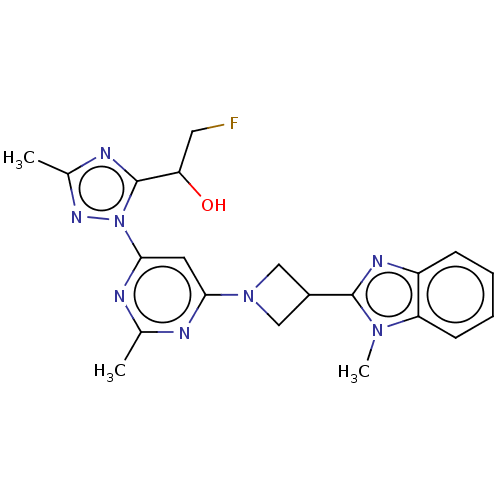 (US9428492, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.340 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, S... | US Patent US9428492 (2016) BindingDB Entry DOI: 10.7270/Q2HD7TKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5367 total ) | Next | Last >> |