

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (GUINEA PIG) | BDBM50326659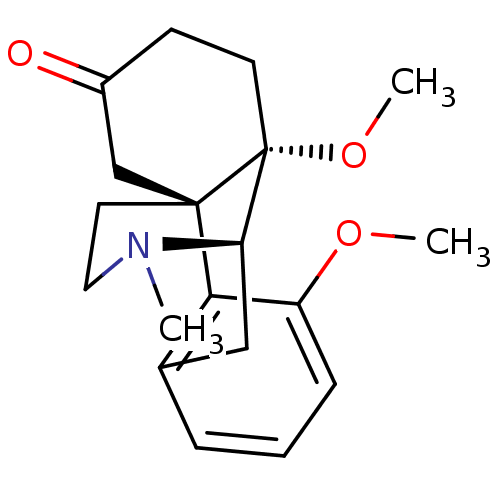 (3,10-dimethoxy-17-methyl-(10S)-17-azatetracyclo[7....) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]DAMGO from mu receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50285003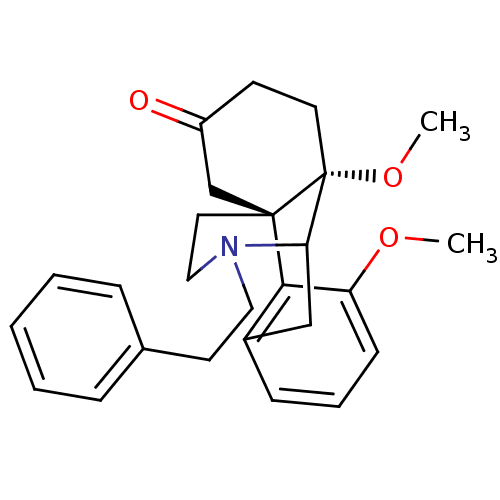 (3,10-dimethoxy-17-phenethyl-17-azatetracyclo[7.5.3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]DAMGO from mu receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50302828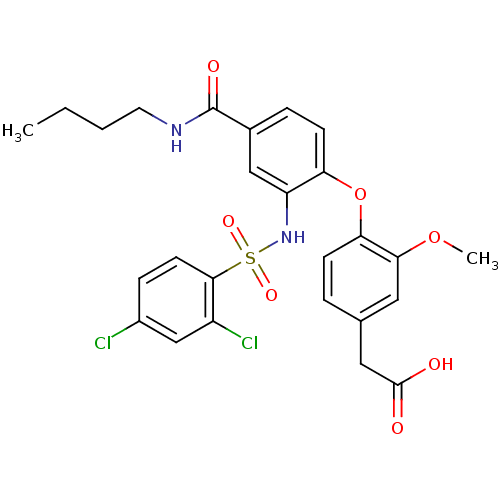 (2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PGD2-induced CRTH2 receptor internalization of CD16 negative granulocytes in human whole blood by flow cytometry | Bioorg Med Chem Lett 19: 6419-23 (2009) Article DOI: 10.1016/j.bmcl.2009.09.052 BindingDB Entry DOI: 10.7270/Q25Q4X20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50326659 (3,10-dimethoxy-17-methyl-(10S)-17-azatetracyclo[7....) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]-U-69,593 from kappa receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50229378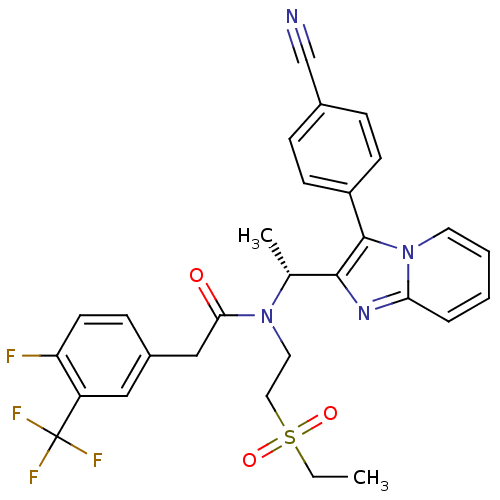 ((R)-N-(1-(3-(4-cyanophenyl)H-imidazo[1,2-a]pyridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA | Bioorg Med Chem Lett 18: 688-93 (2008) Article DOI: 10.1016/j.bmcl.2007.11.060 BindingDB Entry DOI: 10.7270/Q2WQ03HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]DAMGO from mu receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM50316302 (((2R,3S,4R,5R)-5-(2-amino-7-methyl-6-oxo-1H-purin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Competitive inhibition of [3H]m7-GTP binding to human FLAG-His6 tagged eIF4E expressed in Escherichia coli by scintillation proximity assay | J Med Chem 55: 3837-51 (2012) Article DOI: 10.1021/jm300037x BindingDB Entry DOI: 10.7270/Q27082GX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50326659 (3,10-dimethoxy-17-methyl-(10S)-17-azatetracyclo[7....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]DPDPE from delta receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50285003 (3,10-dimethoxy-17-phenethyl-17-azatetracyclo[7.5.3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]DPDPE from delta receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]-U-69,593 from kappa receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]DPDPE from delta receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50302828 (2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Antagonist activity at DP receptor in human platelets assessed as inhibition of PGD2-induced cAMP production by competitive ELISA | Bioorg Med Chem Lett 19: 6419-23 (2009) Article DOI: 10.1016/j.bmcl.2009.09.052 BindingDB Entry DOI: 10.7270/Q25Q4X20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50285003 (3,10-dimethoxy-17-phenethyl-17-azatetracyclo[7.5.3...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity by its ability to displace [3H]-U-69,593 from kappa receptor in homogenates of guinea pig brain | Bioorg Med Chem Lett 5: 1923-1926 (1995) Article DOI: 10.1016/0960-894X(95)00325-N BindingDB Entry DOI: 10.7270/Q2FX79XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50130929 (CHEMBL3633189) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of [3H]-U-69,593 binding to Opioid receptor kappa 1 | Bioorg Med Chem Lett 25: 5449-53 (2015) Article DOI: 10.1016/j.bmcl.2015.06.095 BindingDB Entry DOI: 10.7270/Q2R2136F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50302828 (2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of EP2 expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP production | Bioorg Med Chem Lett 19: 6419-23 (2009) Article DOI: 10.1016/j.bmcl.2009.09.052 BindingDB Entry DOI: 10.7270/Q25Q4X20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50302828 (2-(4-(4-(butylcarbamoyl)-2-(2,4-dichlorophenylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of EP4 expressed in HEK293 cells assessed as inhibition of PGE2-induced cAMP production | Bioorg Med Chem Lett 19: 6419-23 (2009) Article DOI: 10.1016/j.bmcl.2009.09.052 BindingDB Entry DOI: 10.7270/Q25Q4X20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215340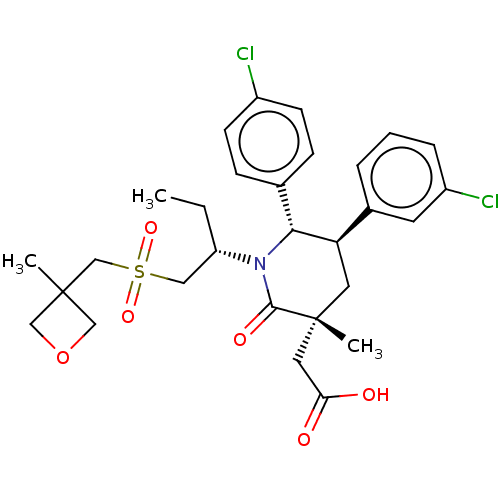 (US9296736, 308 | US9593129, Example 308) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215379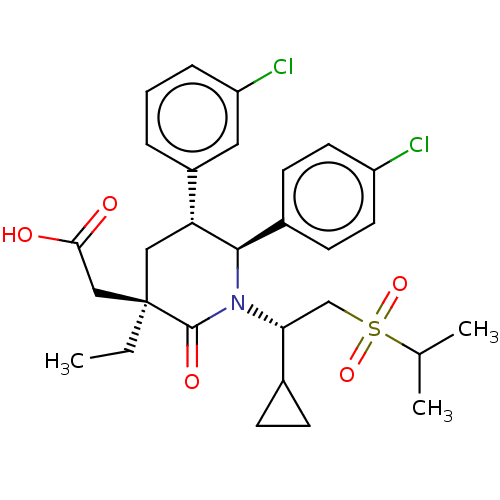 (US9296736, 358 | US9593129, Example 358) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215366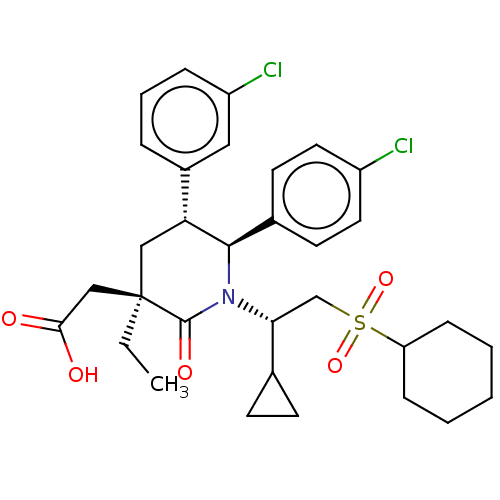 (US9296736, 334 | US9593129, Example 334) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50448936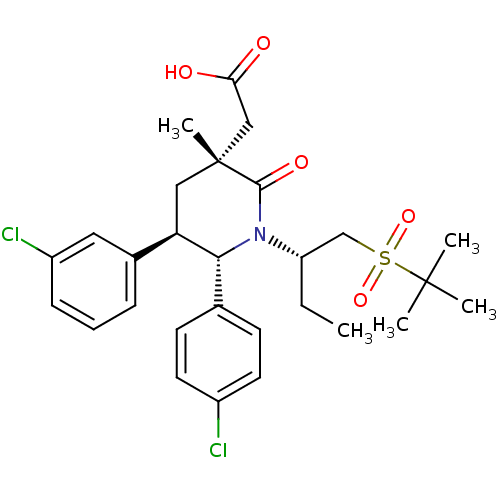 (CHEMBL3125527 | US9296736, 342 | US9593129, Exampl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215373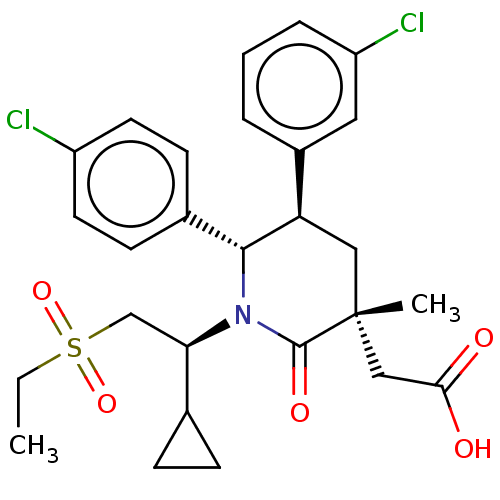 (US9296736, 349 | US9593129, Example 349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50448963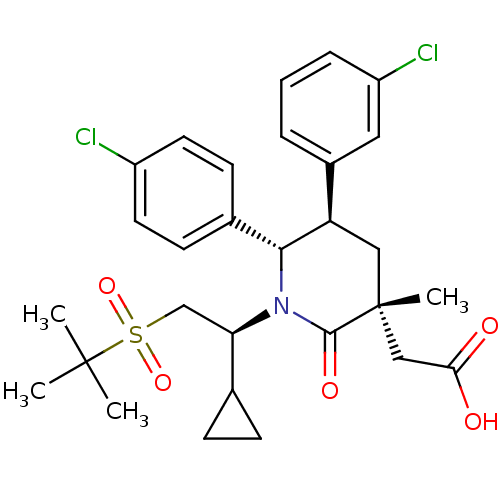 (CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215375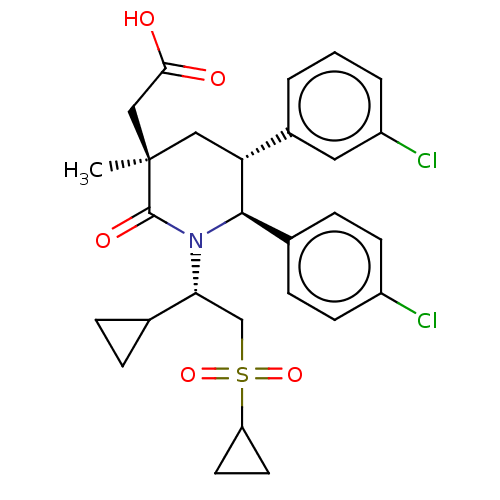 (US9296736, 353 | US9593129, Example 353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215385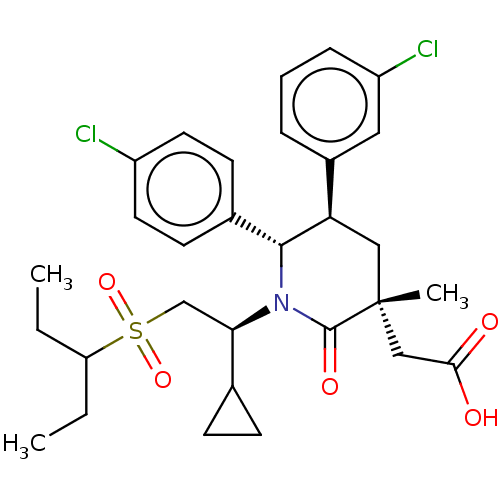 (US9296736, 366 | US9593129, Example 366) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215366 (US9296736, 334 | US9593129, Example 334) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50448936 (CHEMBL3125527 | US9296736, 342 | US9593129, Exampl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215307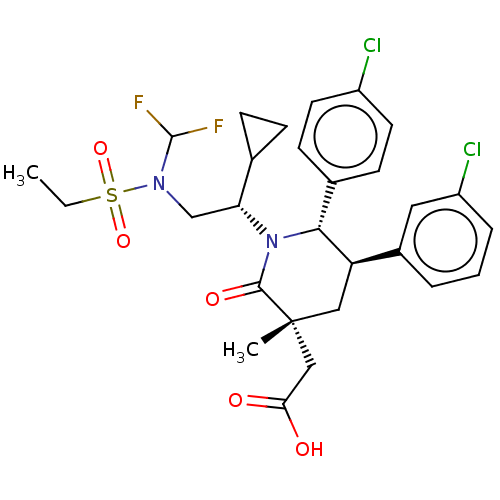 (US9296736, 268 | US9593129, Example 268) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215308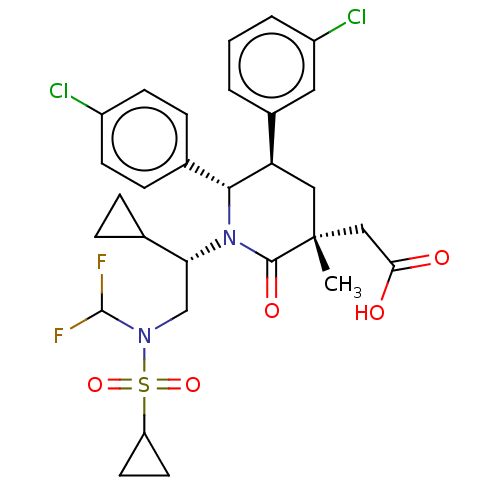 (US9296736, 269 | US9593129, Example 269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM299594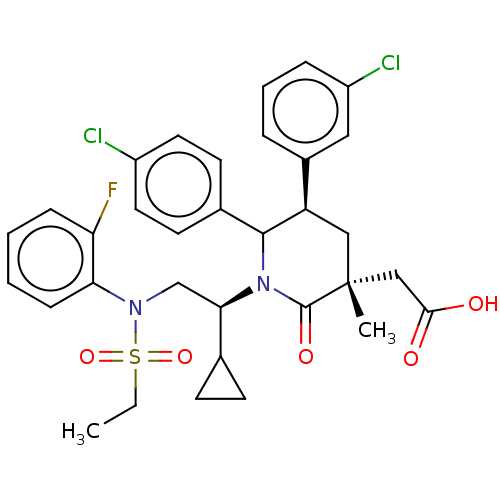 (2-((3R,5R,6S)-5-(3-Chlorophenyl)-6-(4-chlorophenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215394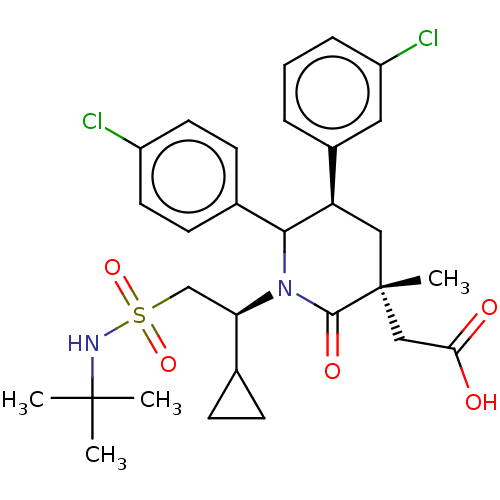 (US9296736, 375 | US9593129, Example 375) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50053040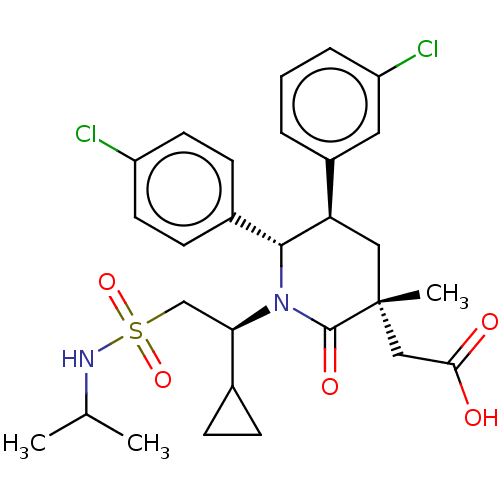 (CHEMBL3318760 | US9296736, 378 | US9593129, Exampl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50053052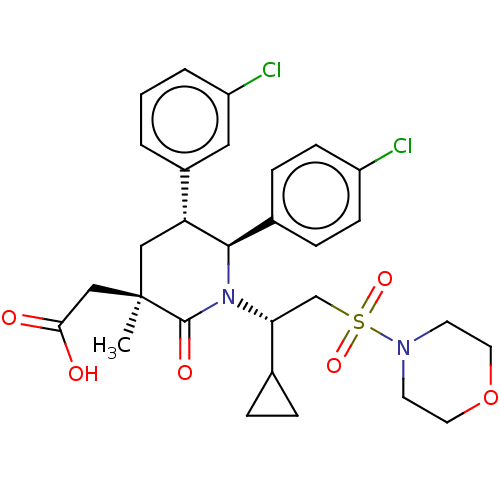 (CHEMBL3318767 | US9296736, 379 | US9593129, Exampl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50448945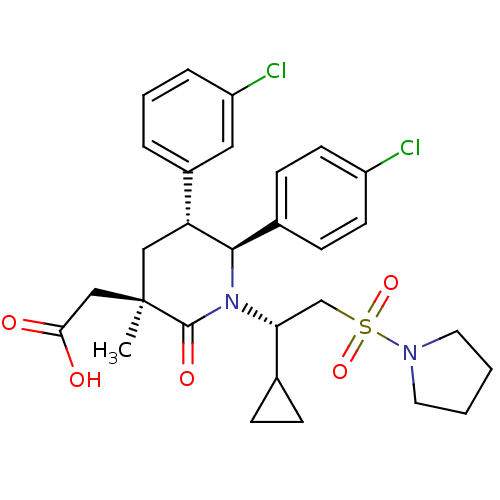 (CHEMBL3125521 | US9296736, 381 | US9593129, Exampl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215410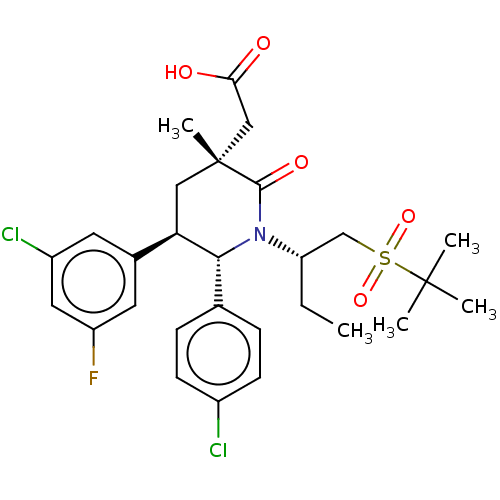 (US9296736, 398 | US9593129, Example 398) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215307 (US9296736, 268 | US9593129, Example 268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215310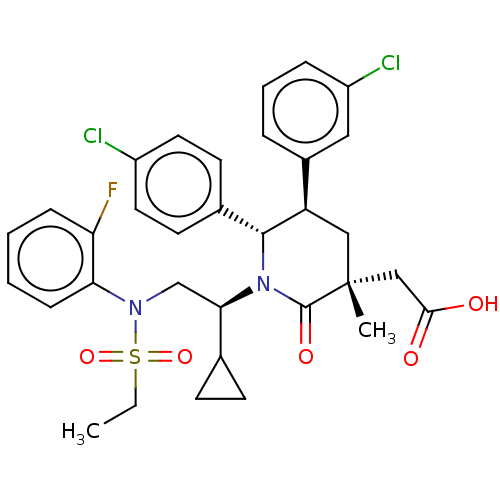 (US9296736, 272) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215385 (US9296736, 366 | US9593129, Example 366) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215390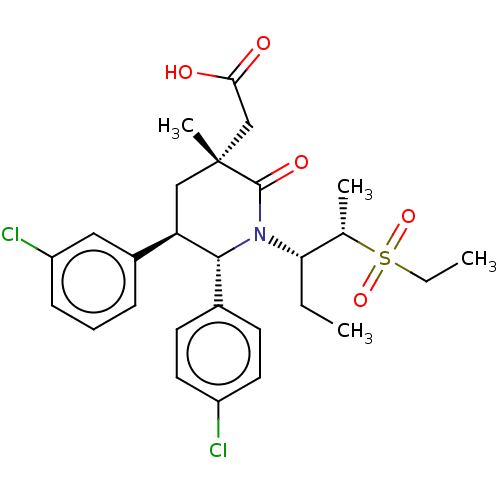 (US9296736, 371 | US9593129, Example 371) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215394 (US9296736, 375 | US9593129, Example 375) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50053040 (CHEMBL3318760 | US9296736, 378 | US9593129, Exampl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50053052 (CHEMBL3318767 | US9296736, 379 | US9593129, Exampl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM50448945 (CHEMBL3125521 | US9296736, 381 | US9593129, Exampl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215410 (US9296736, 398 | US9593129, Example 398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215412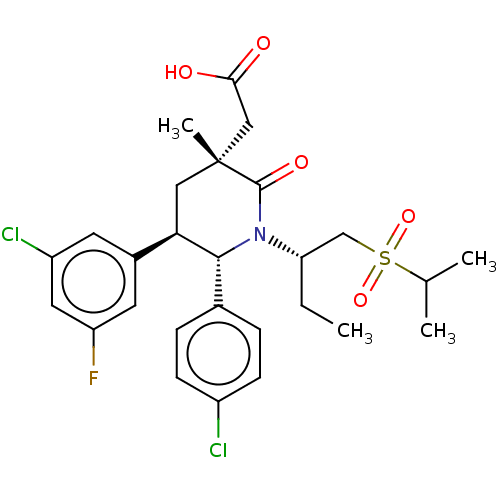 (US9296736, 399 | US9593129, Example 399) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215340 (US9296736, 308 | US9593129, Example 308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215343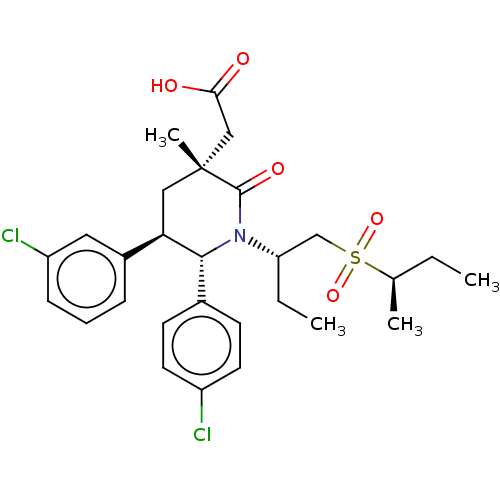 (US9296736, 311 | US9296736, 312 | US9593129, Examp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215346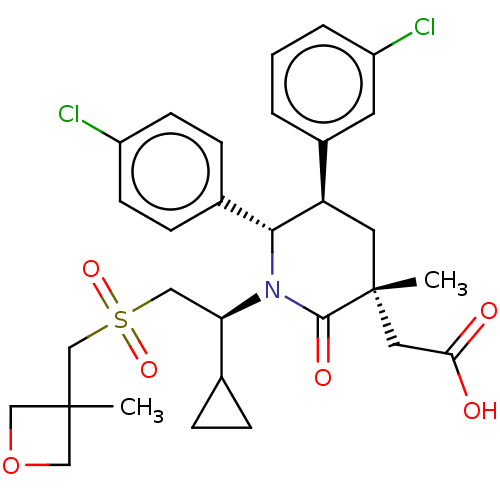 (US9296736, 314 | US9593129, Example 314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215354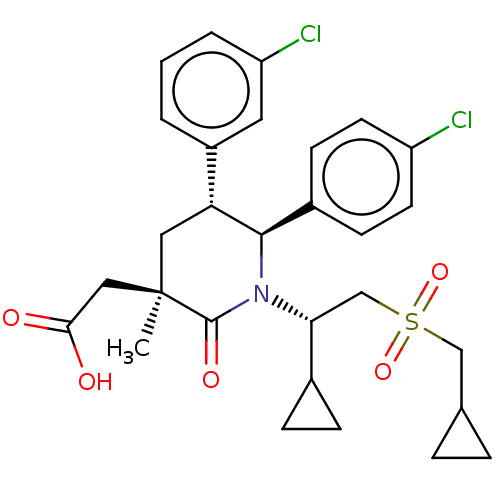 (US9296736, 322 | US9593129, Example 322) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9593129 (2017) BindingDB Entry DOI: 10.7270/Q20P1232 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215354 (US9296736, 322 | US9593129, Example 322) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188] (Homo sapiens (Human)) | BDBM215370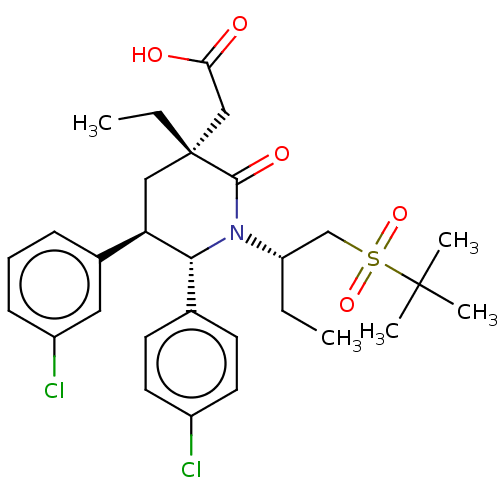 (US9296736, 346 | US9593129, Example 346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US9296736 (2016) BindingDB Entry DOI: 10.7270/Q29022M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6202 total ) | Next | Last >> |