

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307542 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50509860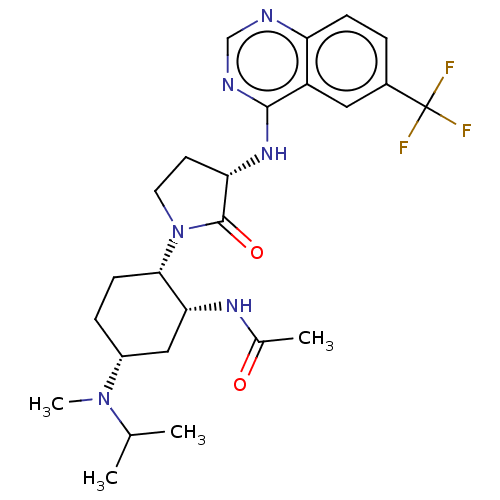 (CHEMBL4442783) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxis | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50557872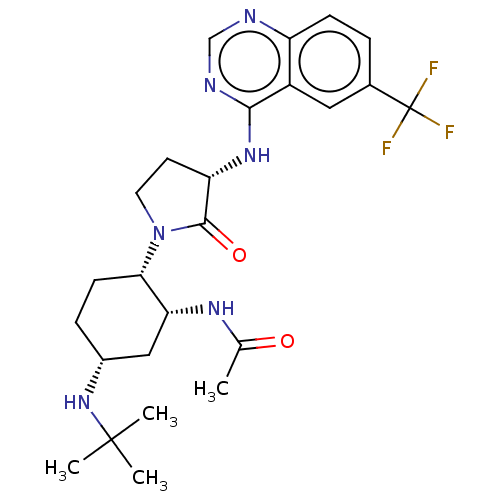 (CHEMBL4781426) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxis | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50578294 (Bms 813160 | Bms-813160) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxis | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50578294 (Bms 813160 | Bms-813160) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 in human peripheral T cells assessed as reduction in MIP-1beta-induced chemotaxis | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50557872 (CHEMBL4781426) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 in human peripheral T cells assessed as reduction in MIP-1beta-induced chemotaxis | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307547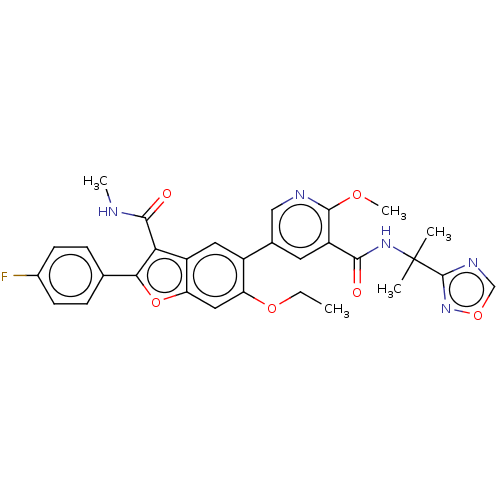 (BDBM307549 | N-(2-(1,2,4-oxadiazol-3-yl)propan-2-y...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307550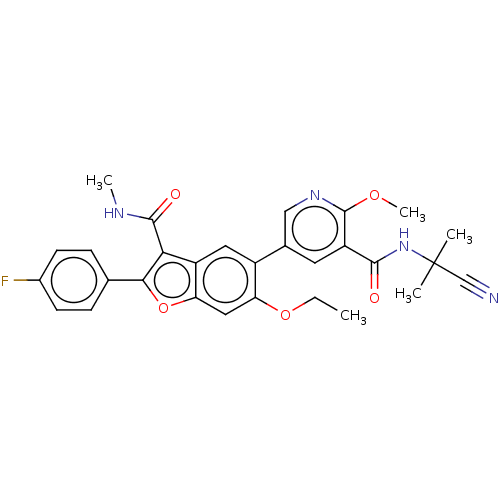 (N-(2-cyanopropan-2-yl)-5-(6-ethoxy-2-(4-fluorophen...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307545 (6-Ethoxy-2-(4-fluorophenyl)-5-(4-methoxy-3-((1-(py...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307566 (5-(3-((2-(1,2,4-thiadiazol-3-yl)propan-2-yl)carbam...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307561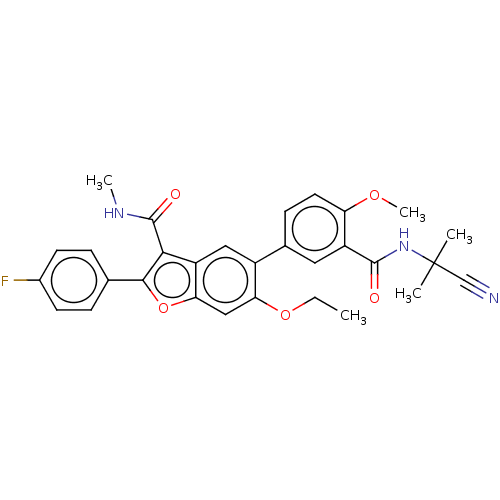 (5-(3-((2-cyanopropan-2-yl)carbamoyl)-4-(methoxy-d3...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307542 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307558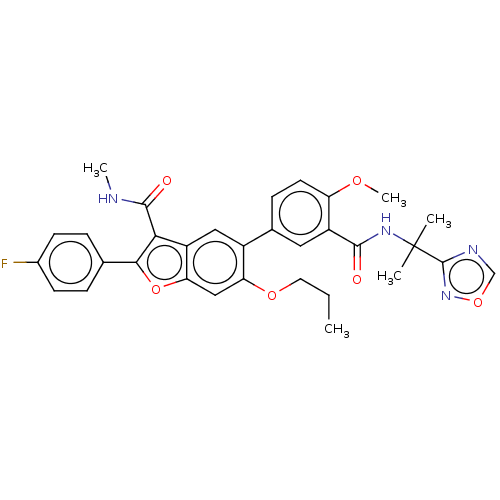 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307548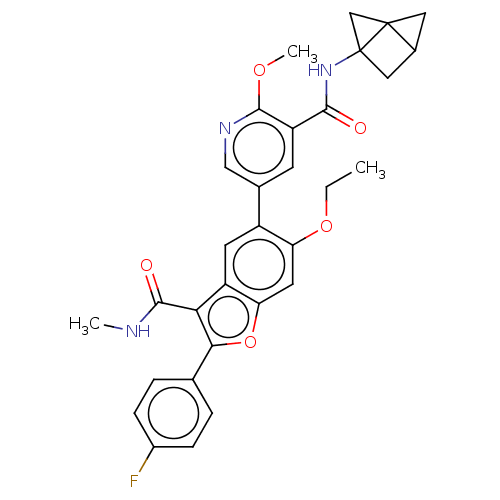 (N-(bicyclo[1.1.1]pentan-1-yl)-5-(6-ethoxy-2-(4-flu...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50557872 (CHEMBL4781426) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human whole blood assessed as inhibition of CCL2-induced CD11b upregulation | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307546 (5-(6-Ethoxy-2-(4-fluorophenyl)-3-(methylcarbamoyl)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307547 (BDBM307549 | N-(2-(1,2,4-oxadiazol-3-yl)propan-2-y...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307551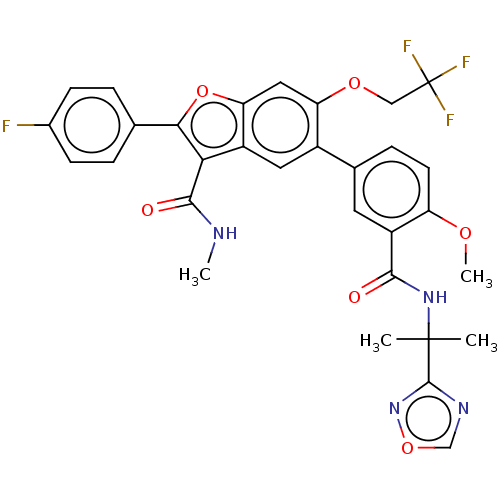 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307562 (5-(2-(4-Fluorophenyl)-3-(methylcarbamoyl)-6-(2,2,2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307543 (5-(3-((2-Cyanopropan-2-yl)carbamoyl)phenyl)-2-(4-f...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307539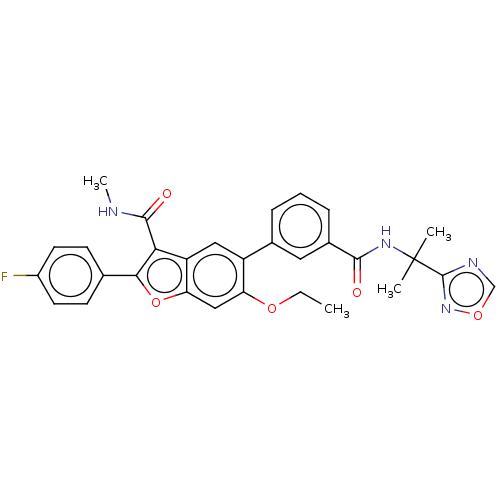 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50578294 (Bms 813160 | Bms-813160) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]MIP-1beta from CCR5 in human peripheral T cells | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307537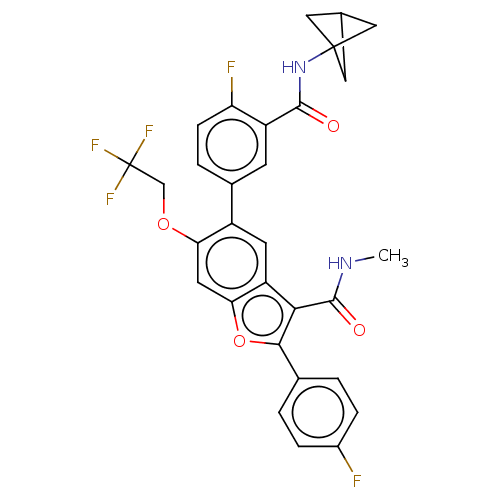 (5-(3-(Bicyclo[1.1.1]pentan-1-ylcarbamoyl)-4-fluoro...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307567 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307535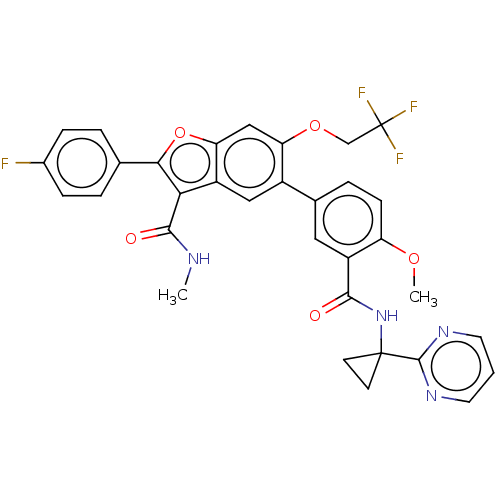 (2-(4-Fluorophenyl)-5-(4-methoxy-3-((1-(pyrimidin-2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307552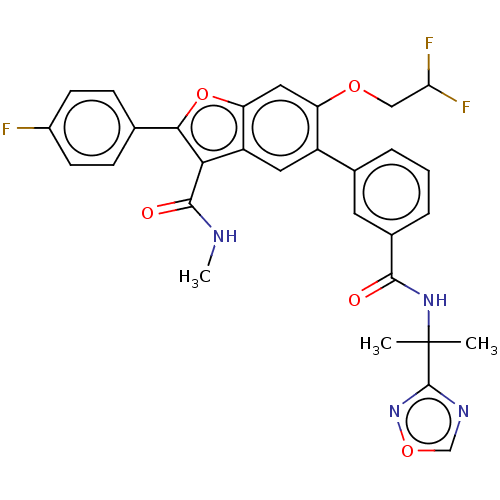 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307538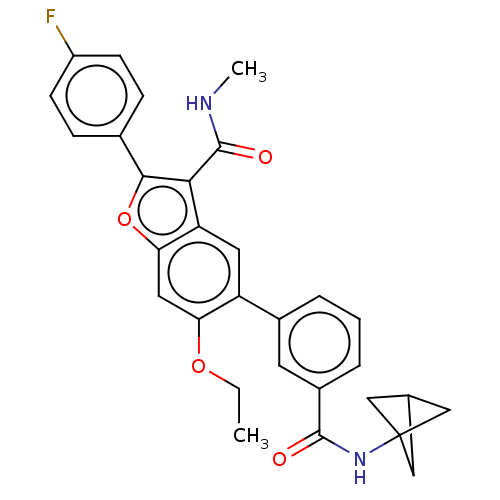 (5-(3-(Bicyclo[1.1.1]pentan-1-ylcarbamoyl)phenyl)-6...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307536 (5-(4-Fluoro-3-((1-(pyrimidin-2-yl)cyclopropyl)carb...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307554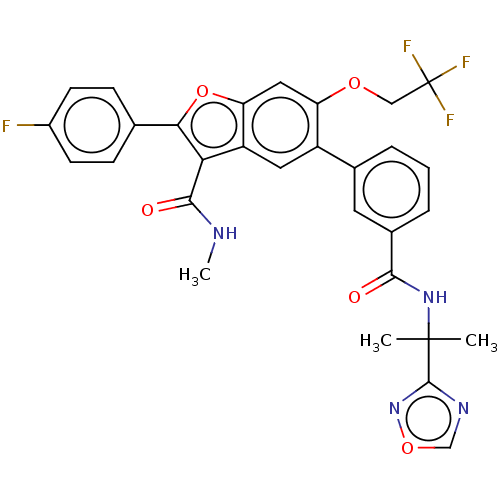 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307553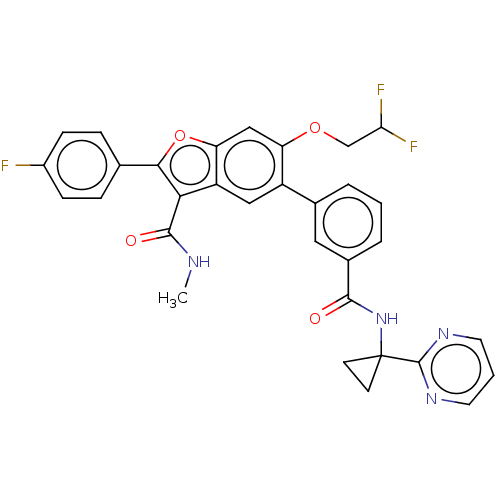 (6-(2,2-difluoroethoxy)-2-(4-fluorophenyl)-N-methyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50578294 (Bms 813160 | Bms-813160) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human whole blood assessed as inhibition of CCL2-induced CD11b upregulation | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307541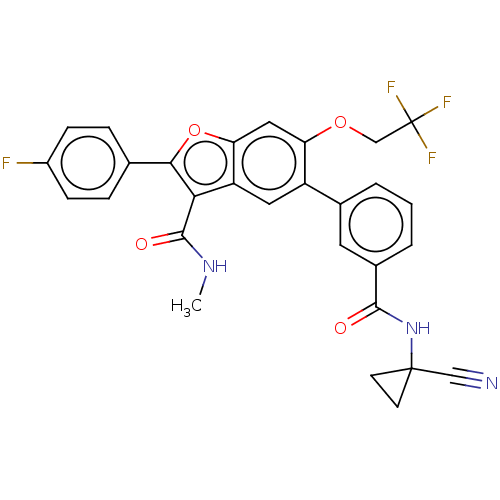 (5-(3-((1-Cyanocyclopropyl)carbamoyl)phenyl)-2-(4-f...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307539 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50578294 (Bms 813160 | Bms-813160) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 in human whole blood assessed as inhibition of MIP-1beta-induced CD11b upregulation | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307540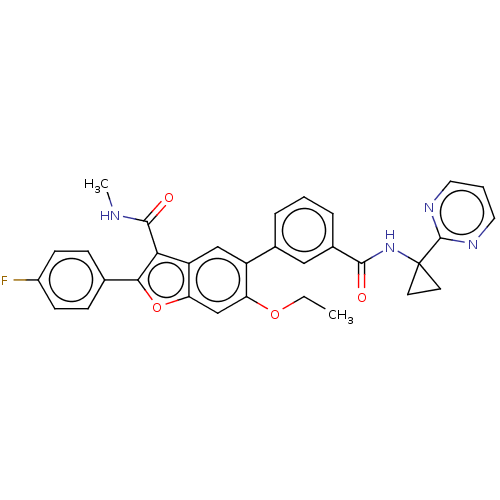 (6-Ethoxy-2-(4-fluorophenyl)-N-methyl-5-(3-((1-(pyr...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307565 (5-(3-((2-(3H-imidazo[4,5-c]pyridin-2-yl)propan-2-y...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50578294 (Bms 813160 | Bms-813160) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-CCL2 from CCR2 in human PBMC cells | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307556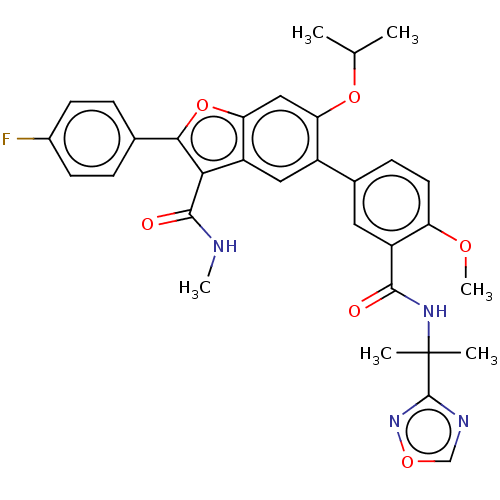 (5-(3-((2-(1,2,4-Oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307534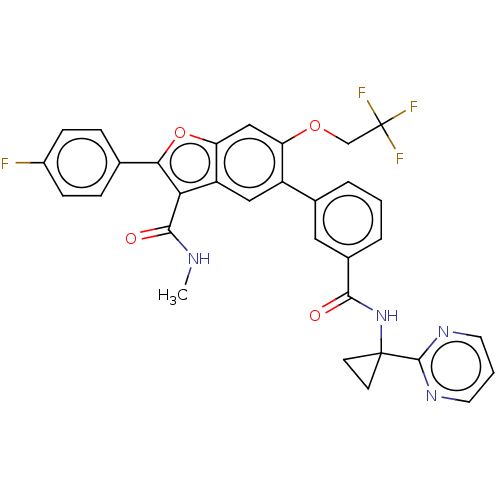 (2-(4-Fluorophenyl)-N-methyl-5-(3-((1-(pyrimidin-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50239250 (CHEMBL4086080) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of microsomal CYP2C8 (unknown origin) | J Med Chem 60: 4369-4385 (2017) Article DOI: 10.1021/acs.jmedchem.7b00328 BindingDB Entry DOI: 10.7270/Q20R9RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307559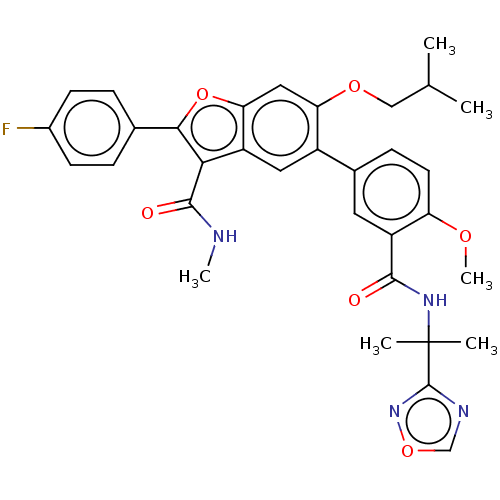 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50557872 (CHEMBL4781426) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 in human whole blood assessed as inhibition of MIP-1beta-induced CD11b upregulation | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Mus musculus) | BDBM50578294 (Bms 813160 | Bms-813160) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-CCL2 from mouse CCR2 | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50239251 (CHEMBL4096241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of microsomal CYP3A4 (unknown origin) | J Med Chem 60: 4369-4385 (2017) Article DOI: 10.1021/acs.jmedchem.7b00328 BindingDB Entry DOI: 10.7270/Q20R9RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50239250 (CHEMBL4086080) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of microsomal CYP3A4 (unknown origin) | J Med Chem 60: 4369-4385 (2017) Article DOI: 10.1021/acs.jmedchem.7b00328 BindingDB Entry DOI: 10.7270/Q20R9RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50239251 (CHEMBL4096241) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of microsomal CYP2C8 (unknown origin) | J Med Chem 60: 4369-4385 (2017) Article DOI: 10.1021/acs.jmedchem.7b00328 BindingDB Entry DOI: 10.7270/Q20R9RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50239252 (CHEMBL4078188) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of microsomal CYP2C8 (unknown origin) | J Med Chem 60: 4369-4385 (2017) Article DOI: 10.1021/acs.jmedchem.7b00328 BindingDB Entry DOI: 10.7270/Q20R9RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50239253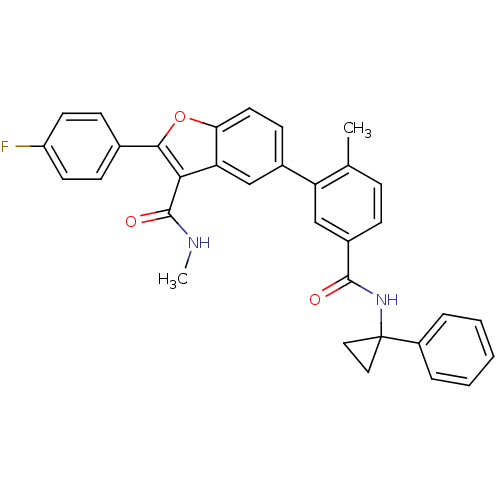 (CHEMBL4088517) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of microsomal CYP2C8 (unknown origin) | J Med Chem 60: 4369-4385 (2017) Article DOI: 10.1021/acs.jmedchem.7b00328 BindingDB Entry DOI: 10.7270/Q20R9RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50239253 (CHEMBL4088517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of microsomal CYP3A4 (unknown origin) | J Med Chem 60: 4369-4385 (2017) Article DOI: 10.1021/acs.jmedchem.7b00328 BindingDB Entry DOI: 10.7270/Q20R9RKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307557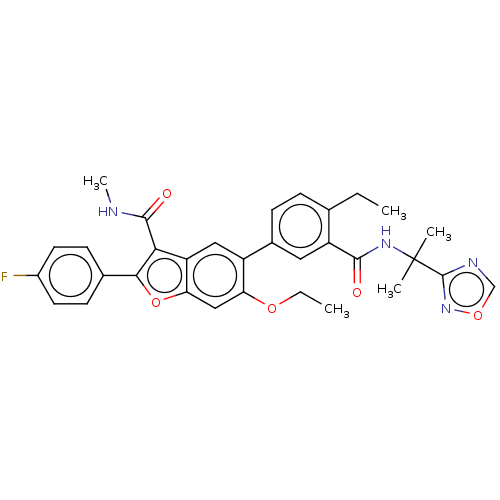 (5-(3-((2-(1,2,4-oxadiazol-3-yl)propan-2-yl)carbamo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To evaluate compound efficacy, titrated compounds were transferred to sterile 384-well tissue culture treated plates, and the plates were seeded with... | US Patent US10150747 (2018) BindingDB Entry DOI: 10.7270/Q2H70HWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |