

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474150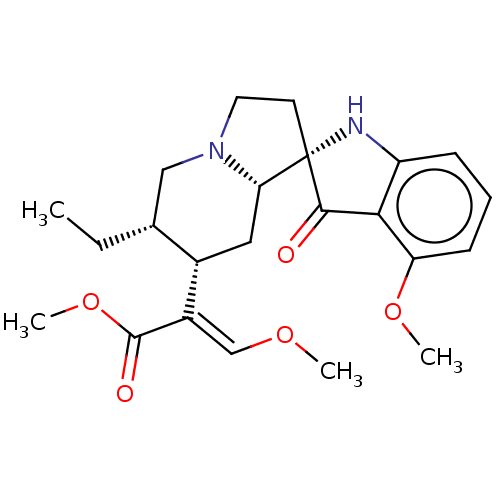 (CHEMBL58362) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University School of Pharmacy Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor (unknown origin) | J Med Chem 56: 4840-8 (2013) Article DOI: 10.1021/jm400143z BindingDB Entry DOI: 10.7270/Q2FT8PZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50080514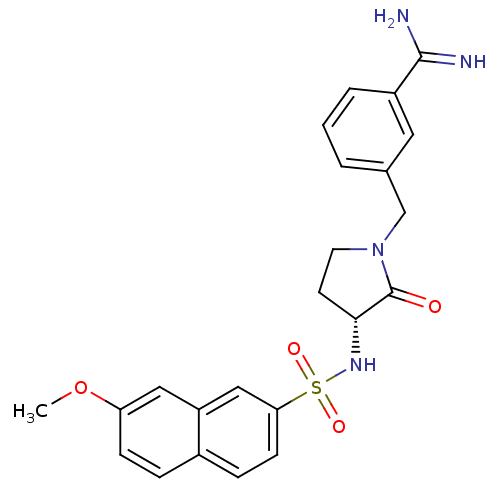 (3-[(R)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human Coagulation factor Xa | J Med Chem 42: 3557-71 (1999) Article DOI: 10.1021/jm990040h BindingDB Entry DOI: 10.7270/Q2M04640 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203905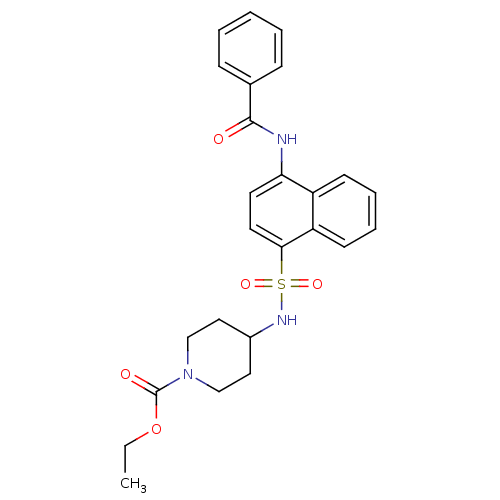 (4-(4-benzoylamino-naphthalene-1-sulfonylamino)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50272695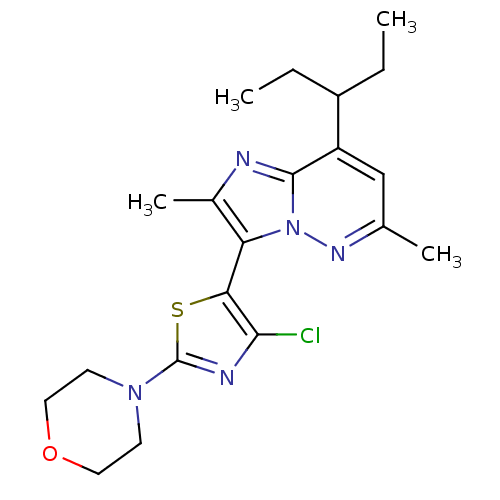 (3-(4-chloro-2-morpholinothiazol-5-yl)-2,6-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from human CRF1 receptor expressed in IMR32 cells | Bioorg Med Chem Lett 18: 4486-90 (2008) Article DOI: 10.1016/j.bmcl.2008.07.063 BindingDB Entry DOI: 10.7270/Q2NG4RJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50272695 (3-(4-chloro-2-morpholinothiazol-5-yl)-2,6-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CRF-1 receptor | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260806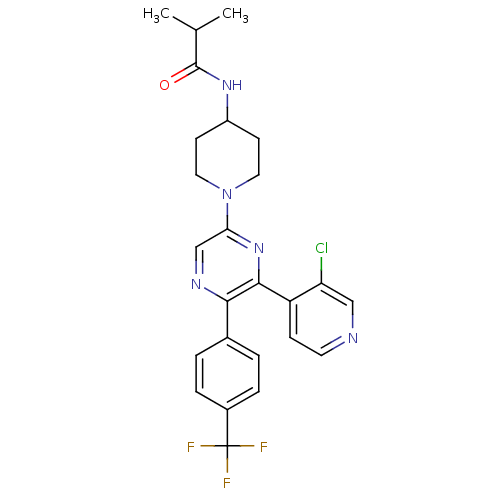 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203831 (CHEMBL374939 | N-(4-{[(1-butyrylpiperidin-4-yl)ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by PDSP Ki Database | J Pharmacol Exp Ther 282: 1011-9 (1997) BindingDB Entry DOI: 10.7270/Q2CC0Z63 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307292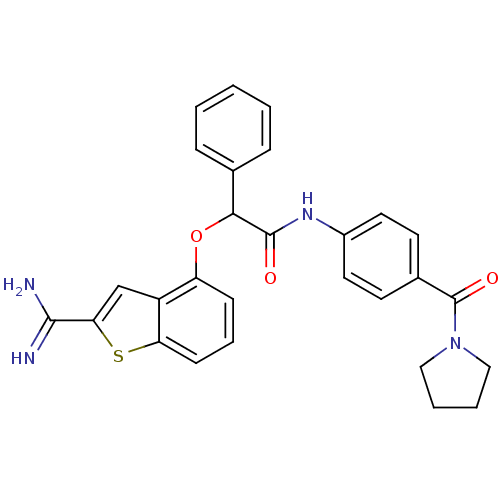 (2-(2-Carbamimidoyl-benzo[b]thiophen-4-yloxy)-2-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd. Curated by ChEMBL | Assay Description Inhibition of factor 10a by amidolytic assay | J Med Chem 53: 1473-82 (2010) Article DOI: 10.1021/jm901476x BindingDB Entry DOI: 10.7270/Q2P84C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307291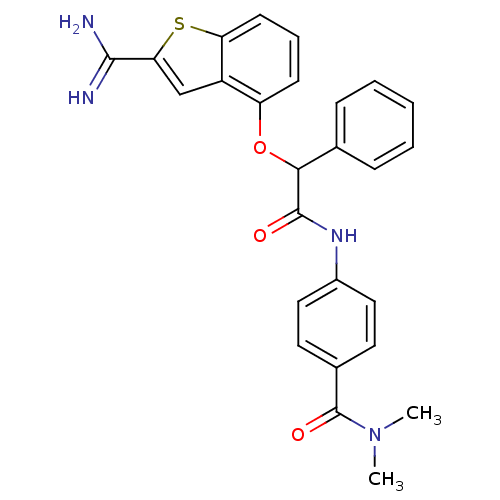 (4-[2-(2-Carbamimidoyl-benzo[b]thiophen-4-yloxy)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd. Curated by ChEMBL | Assay Description Inhibition of factor 10a by amidolytic assay | J Med Chem 53: 1473-82 (2010) Article DOI: 10.1021/jm901476x BindingDB Entry DOI: 10.7270/Q2P84C0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by PDSP Ki Database | J Pharmacol Exp Ther 282: 1011-9 (1997) BindingDB Entry DOI: 10.7270/Q2CC0Z63 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260767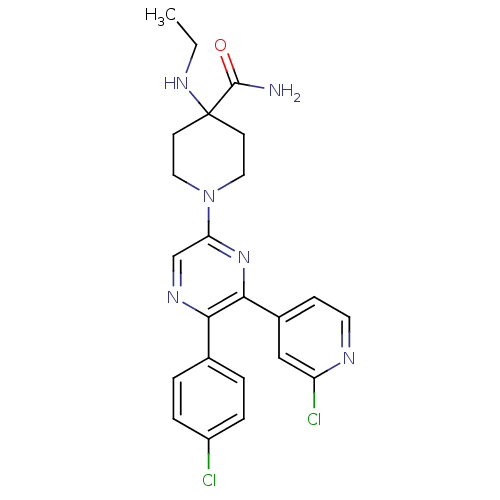 (1-(5-(4-chlorophenyl)-6-(2-chloropyridin-4-yl)pyra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14059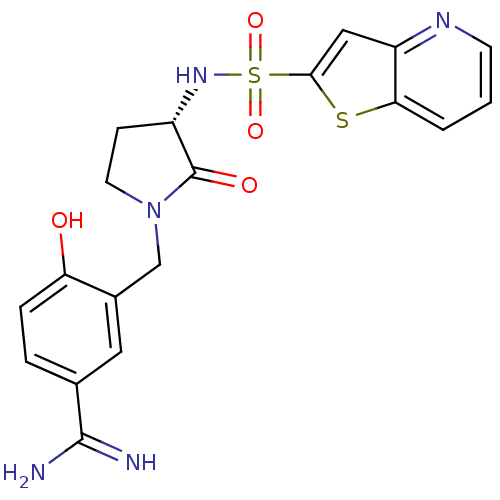 (4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | -51.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 43: 3226-32 (2000) Article DOI: 10.1021/jm000940u BindingDB Entry DOI: 10.7270/Q25H7DHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14059 (4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203834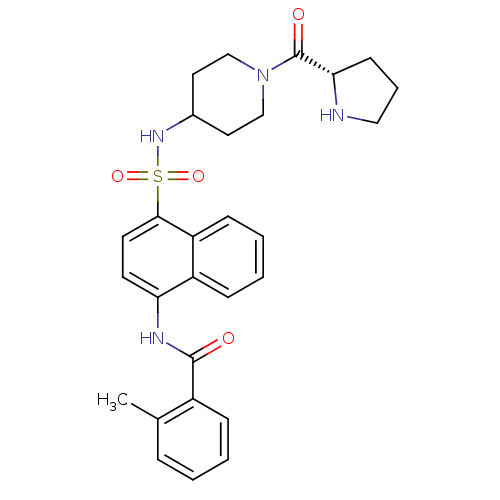 (2-methyl-N-{4-[({1-[(2S)-pyrrolidin-2-ylcarbonyl]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203832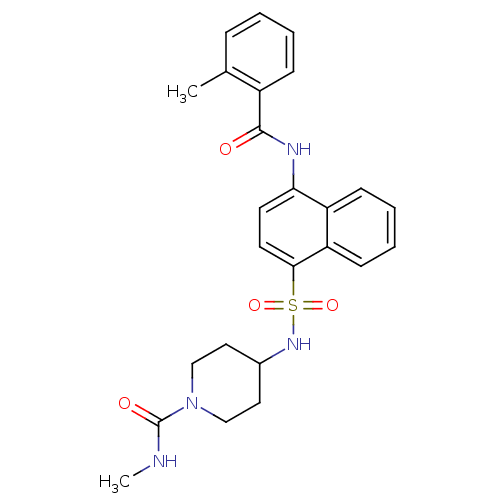 (4-[4-(2-methyl-benzoylamino)-naphthalene-1-sulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203834 (2-methyl-N-{4-[({1-[(2S)-pyrrolidin-2-ylcarbonyl]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260805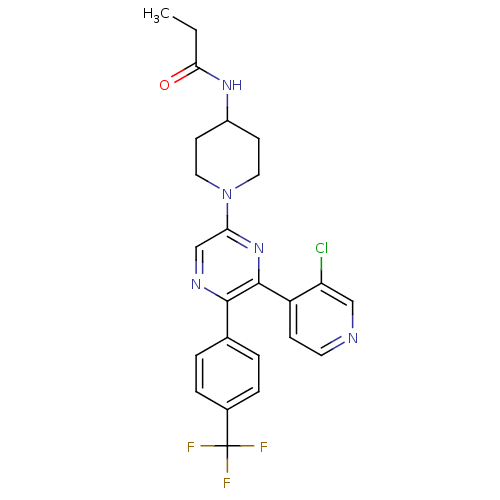 (CHEMBL524804 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260681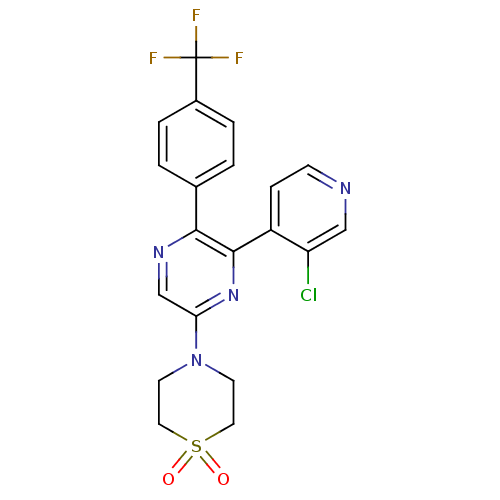 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260768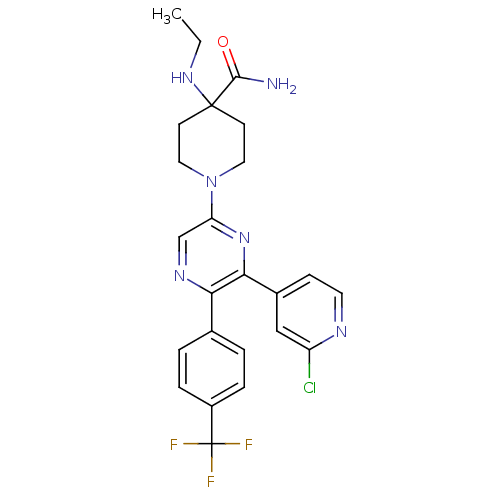 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50132951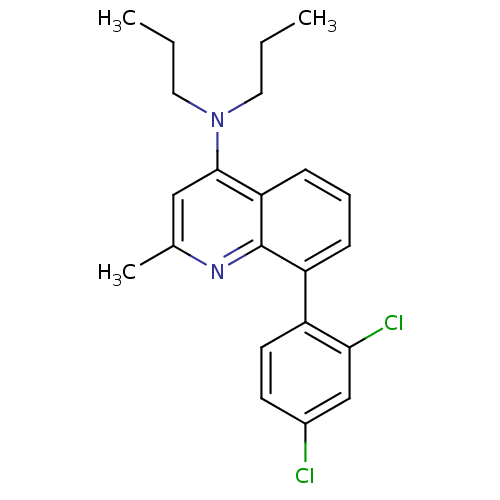 (8-(2,4-dichlorophenyl)-2-methyl-N,N-dipropylquinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]sauvagine from CRF1 receptor expressed in human IMR32 cells | Bioorg Med Chem Lett 18: 891-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.050 BindingDB Entry DOI: 10.7270/Q28S4PPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50071958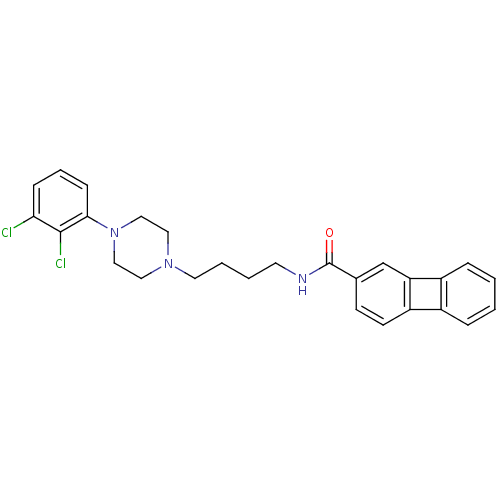 (Biphenylene-2-carboxylic acid {4-[4-(2,3-dichloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against human Dopamine receptor D3 transfected in CHO cell membranes to stimulate [3H]-thymidine uptake | Bioorg Med Chem Lett 8: 2715-8 (1999) BindingDB Entry DOI: 10.7270/Q200018M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50132951 (8-(2,4-dichlorophenyl)-2-methyl-N,N-dipropylquinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CRF-1 receptor | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12597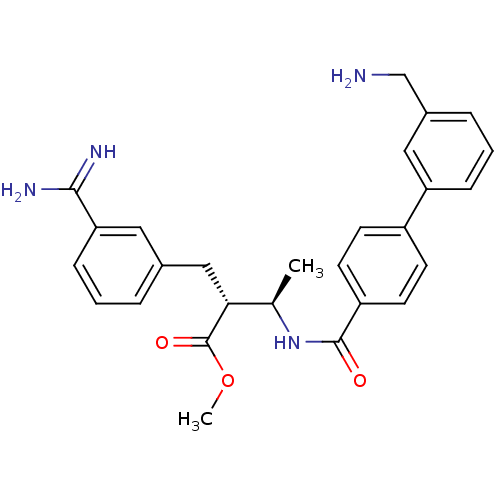 (CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 43: 3226-32 (2000) Article DOI: 10.1021/jm000940u BindingDB Entry DOI: 10.7270/Q25H7DHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50071958 (Biphenylene-2-carboxylic acid {4-[4-(2,3-dichloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity at dopamine receptor D3 | J Med Chem 44: 3175-86 (2001) BindingDB Entry DOI: 10.7270/Q2WD419S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12597 (CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 46: 685-90 (2003) Article DOI: 10.1021/jm0203837 BindingDB Entry DOI: 10.7270/Q2VH5M2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260682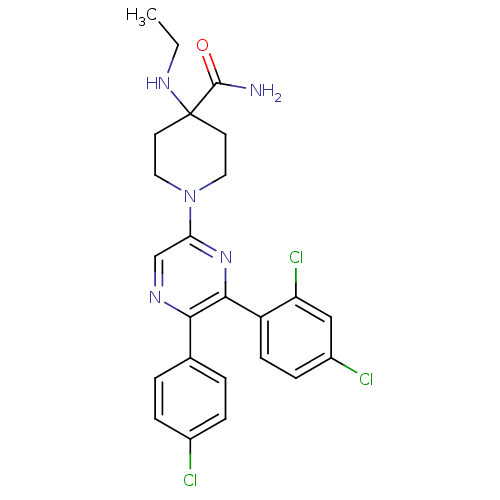 (1-(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12594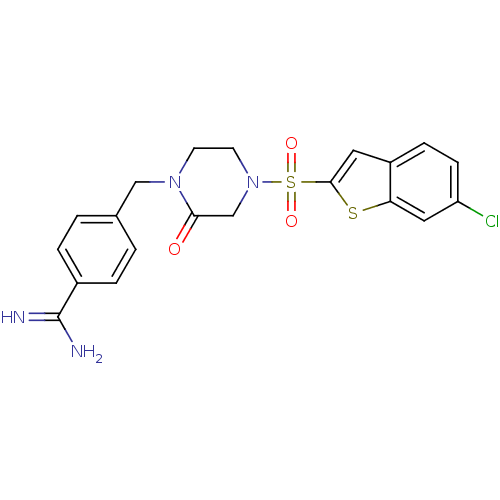 (4-({4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-2-OXO...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Coagulation factor Xa | Bioorg Med Chem Lett 12: 919-22 (2002) BindingDB Entry DOI: 10.7270/Q28C9VJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50111966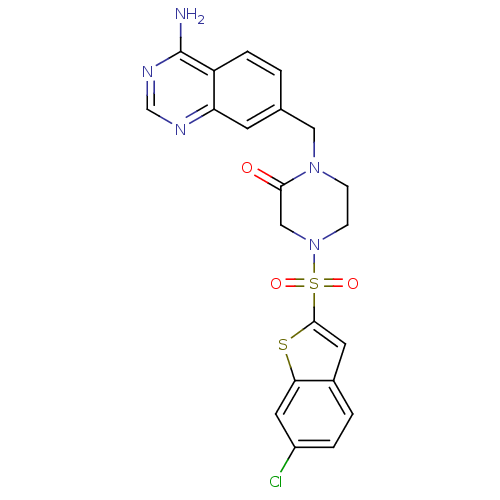 (1-(4-Amino-quinazolin-7-ylmethyl)-4-(6-chloro-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Coagulation factor Xa | Bioorg Med Chem Lett 12: 919-22 (2002) BindingDB Entry DOI: 10.7270/Q28C9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348782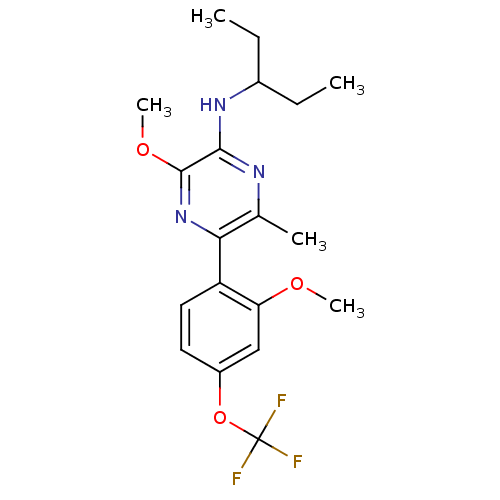 (CHEMBL1807053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Binding affinity towards Dopamine type 2 receptor was determined by displacement assays using [3H]-YM 09151 as the competitive ligand | Bioorg Med Chem Lett 12: 3111-5 (2002) BindingDB Entry DOI: 10.7270/Q2DZ07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50116112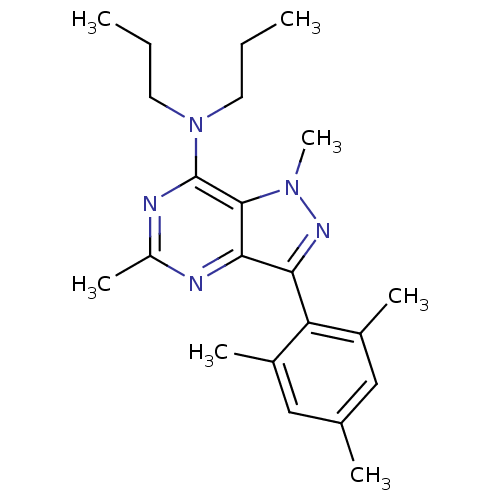 (CHEMBL67484 | [1,5-Dimethyl-3-(2,4,6-trimethyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from Corticotropin releasing factor receptor 1 endogenously expressed in IMR-32 human neuroblastoma cells | Bioorg Med Chem Lett 12: 2133-6 (2002) BindingDB Entry DOI: 10.7270/Q2SQ8ZPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203919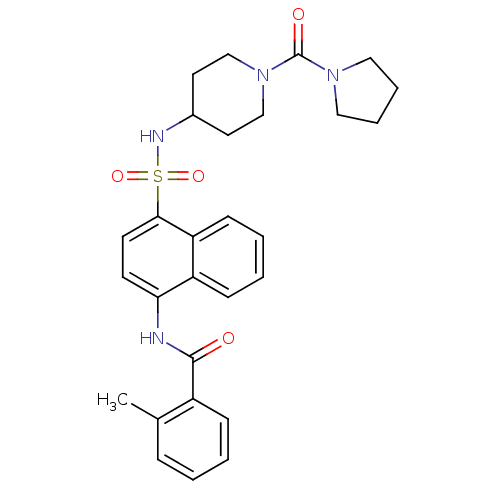 (2-methyl-N-[4-({[1-(pyrrolidin-1-ylcarbonyl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348778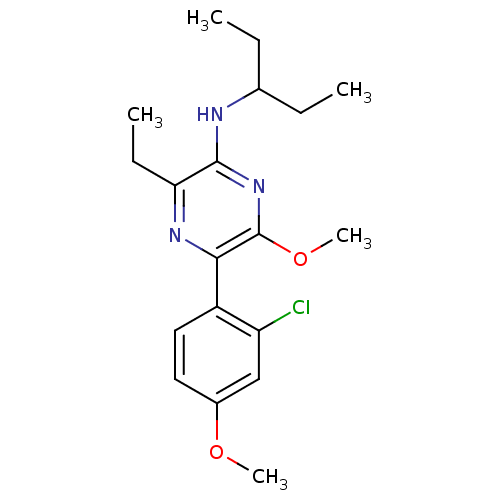 (CHEMBL1807058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12596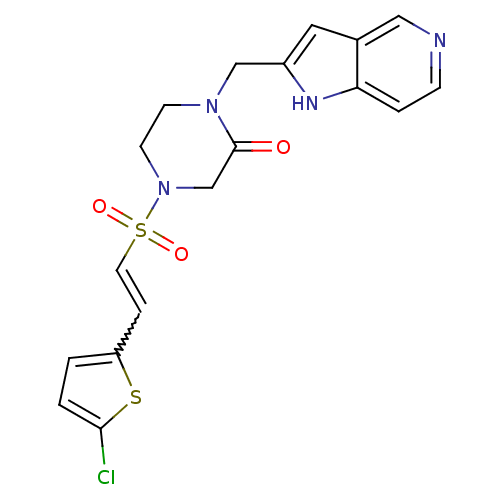 (4-{[(E)-2-(5-CHLOROTHIEN-2-YL)VINYL]SULFONYL}-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 46: 685-90 (2003) Article DOI: 10.1021/jm0203837 BindingDB Entry DOI: 10.7270/Q2VH5M2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260803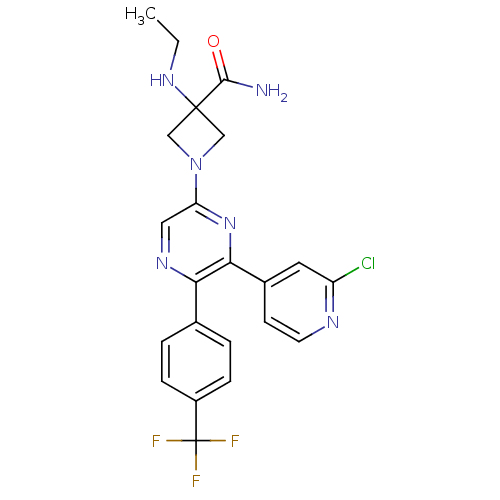 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12594 (4-({4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-2-OXO...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 46: 685-90 (2003) Article DOI: 10.1021/jm0203837 BindingDB Entry DOI: 10.7270/Q2VH5M2S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348829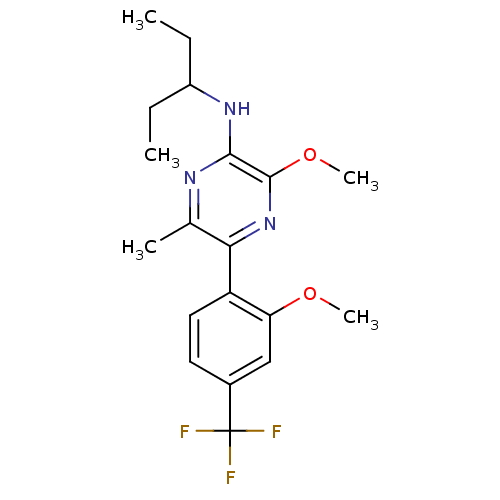 (CHEMBL1807054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50071959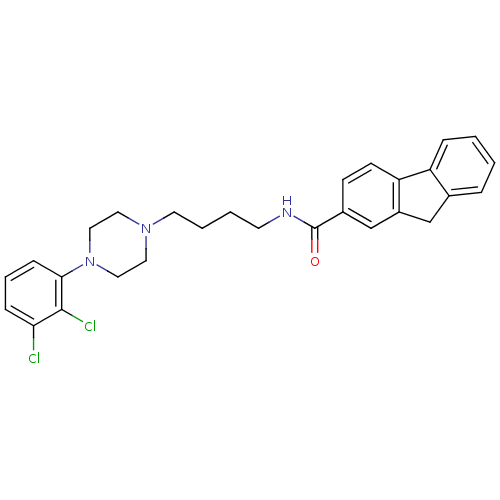 (9H-Fluorene-2-carboxylic acid {4-[4-(2,3-dichloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against human Dopamine receptor D3 transfected in CHO cell membranes to stimulate [3H]-thymidine uptake | Bioorg Med Chem Lett 8: 2715-8 (1999) BindingDB Entry DOI: 10.7270/Q200018M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50104122 (9-Oxo-9H-fluorene-4-carboxylic acid {4-[4-(2,3-dic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity at dopamine receptor D3 | J Med Chem 44: 3175-86 (2001) BindingDB Entry DOI: 10.7270/Q2WD419S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50104138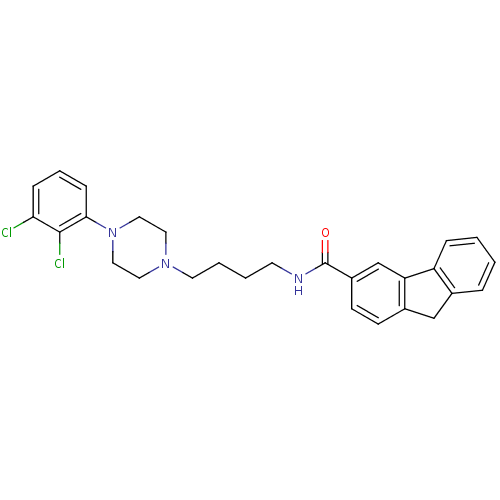 (9H-Fluorene-3-carboxylic acid {4-[4-(2,3-dichloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity at dopamine receptor D3 | J Med Chem 44: 3175-86 (2001) BindingDB Entry DOI: 10.7270/Q2WD419S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50348783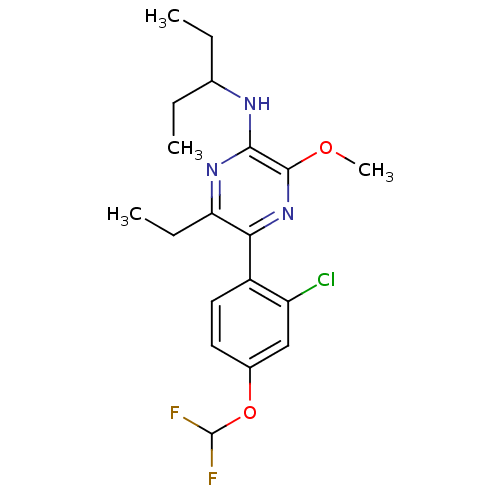 (CHEMBL1807052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [125I]-sauvagine from human CRF-1 receptor expressed in human IMR-32 cells after 2 hrs by scintillation counting | J Med Chem 54: 4187-206 (2011) Article DOI: 10.1021/jm200365y BindingDB Entry DOI: 10.7270/Q2XS5VR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50081505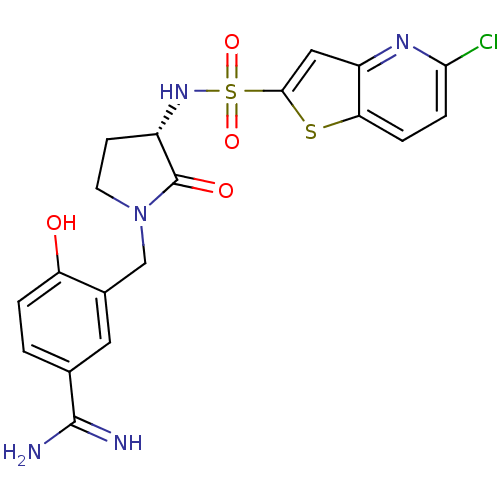 (3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity against serine protease factor Xa (fXa) | Bioorg Med Chem Lett 9: 2753-8 (1999) BindingDB Entry DOI: 10.7270/Q2W66K02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203914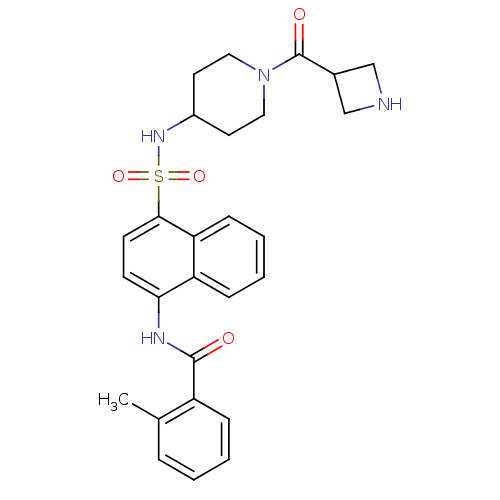 (CHEMBL375854 | N-[4-({[1-(azetidin-3-ylcarbonyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50092053 (2-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-(2,3-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Binding affinity towards human Dopamine Receptor D4 was determined via standard competitive displacement assays using [3H]-YM 09151 as radioligand | Bioorg Med Chem Lett 12: 3105-9 (2002) BindingDB Entry DOI: 10.7270/Q2JM2908 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50092053 (2-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-(2,3-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Displacement of [3H]-YM 09151 from D4 receptor | Bioorg Med Chem Lett 13: 701-4 (2003) BindingDB Entry DOI: 10.7270/Q2B56J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3460 total ) | Next | Last >> |