 Found 56 hits with Last Name = 'bergqvist' and Initial = 's'
Found 56 hits with Last Name = 'bergqvist' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709
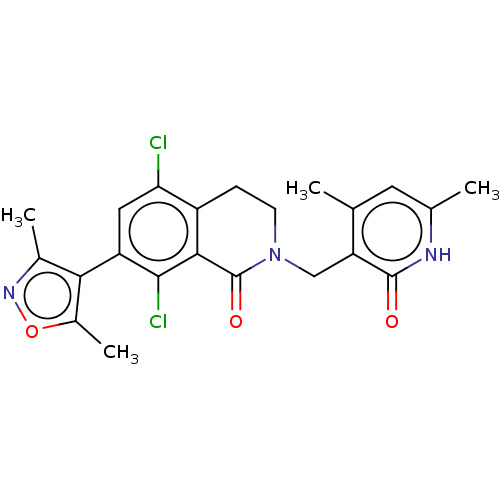
(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM92906
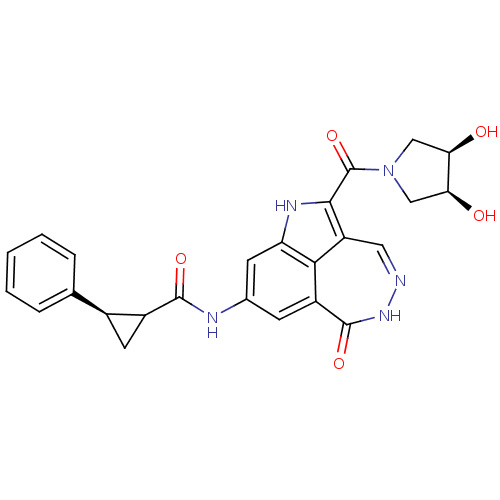
(CHK1 compound 1)Show SMILES O[C@H]1CN(C[C@H]1O)C(=O)c1[nH]c2cc(NC(=O)C3C[C@H]3c3ccccc3)cc3c2c1cn[nH]c3=O |r| Show InChI InChI=1S/C25H21N5O5/c31-19-10-30(11-20(19)32)25(35)22-17-9-26-29-24(34)16-6-13(7-18(28-22)21(16)17)27-23(33)15-8-14(15)12-4-2-1-3-5-12/h1-7,9,14-15,19-20,31-32H,8,10-11H2,(H,27,33)/t14-,15?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.75 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst... |
Biochemistry 48: 9823-30 (2009)
Article DOI: 10.1021/bi900258v
BindingDB Entry DOI: 10.7270/Q25M649B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193663
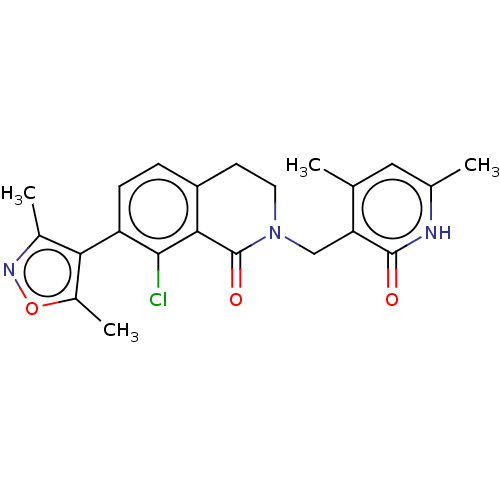
(CHEMBL3929944)Show SMILES Cc1noc(C)c1-c1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(62.09,-40.24,;61.77,-41.75,;62.8,-42.89,;62.03,-44.22,;60.52,-43.9,;59.38,-44.93,;60.36,-42.37,;59.02,-41.6,;57.69,-42.37,;56.37,-41.6,;56.38,-40.08,;55.05,-39.32,;55.05,-37.78,;56.38,-37,;56.38,-35.46,;55.04,-34.69,;55.05,-33.15,;56.38,-32.38,;53.72,-32.38,;52.38,-33.14,;51.05,-32.37,;52.38,-34.68,;53.71,-35.46,;53.71,-37,;57.7,-37.78,;59.04,-37.01,;57.7,-39.32,;59.02,-40.08,;60.35,-39.31,)| Show InChI InChI=1S/C22H22ClN3O3/c1-11-9-12(2)24-21(27)17(11)10-26-8-7-15-5-6-16(20(23)19(15)22(26)28)18-13(3)25-29-14(18)4/h5-6,9H,7-8,10H2,1-4H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM92906

(CHK1 compound 1)Show SMILES O[C@H]1CN(C[C@H]1O)C(=O)c1[nH]c2cc(NC(=O)C3C[C@H]3c3ccccc3)cc3c2c1cn[nH]c3=O |r| Show InChI InChI=1S/C25H21N5O5/c31-19-10-30(11-20(19)32)25(35)22-17-9-26-29-24(34)16-6-13(7-18(28-22)21(16)17)27-23(33)15-8-14(15)12-4-2-1-3-5-12/h1-7,9,14-15,19-20,31-32H,8,10-11H2,(H,27,33)/t14-,15?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.14 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 20 |
Pfizer
| Assay Description
Surface plasmon resonance (SPR) biosensor binding studies were conducted using a Biacore 3000 instrument (GE Healtchare). |
Biochemistry 48: 9823-30 (2009)
Article DOI: 10.1021/bi900258v
BindingDB Entry DOI: 10.7270/Q25M649B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM92908
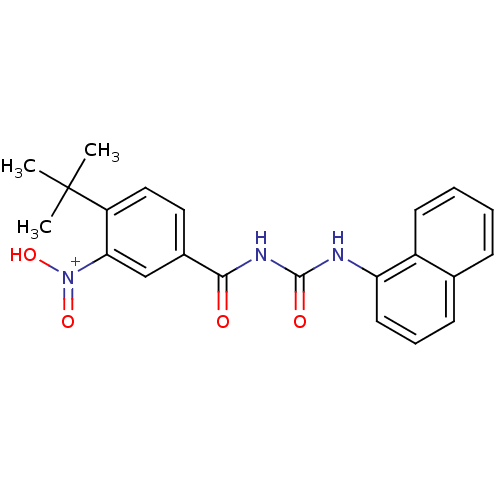
(CHK1 compound 3)Show SMILES CC(C)(C)c1ccc(cc1[N+](O)=O)C(=O)NC(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C22H21N3O4/c1-22(2,3)17-12-11-15(13-19(17)25(28)29)20(26)24-21(27)23-18-10-6-8-14-7-4-5-9-16(14)18/h4-13H,1-3H3,(H2-,23,24,26,27,28,29)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 146 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst... |
Biochemistry 48: 9823-30 (2009)
Article DOI: 10.1021/bi900258v
BindingDB Entry DOI: 10.7270/Q25M649B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM92908

(CHK1 compound 3)Show SMILES CC(C)(C)c1ccc(cc1[N+](O)=O)C(=O)NC(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C22H21N3O4/c1-22(2,3)17-12-11-15(13-19(17)25(28)29)20(26)24-21(27)23-18-10-6-8-14-7-4-5-9-16(14)18/h4-13H,1-3H3,(H2-,23,24,26,27,28,29)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | -36.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 20 |
Pfizer
| Assay Description
Surface plasmon resonance (SPR) biosensor binding studies were conducted using a Biacore 3000 instrument (GE Healtchare). |
Biochemistry 48: 9823-30 (2009)
Article DOI: 10.1021/bi900258v
BindingDB Entry DOI: 10.7270/Q25M649B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM92907
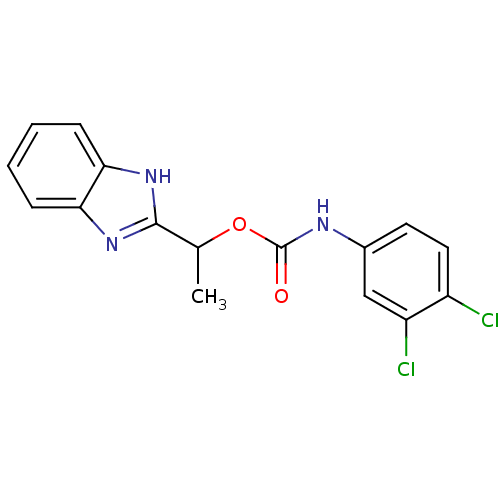
(CHK1 compound 2)Show SMILES CC(OC(=O)Nc1ccc(Cl)c(Cl)c1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C16H13Cl2N3O2/c1-9(15-20-13-4-2-3-5-14(13)21-15)23-16(22)19-10-6-7-11(17)12(18)8-10/h2-9H,1H3,(H,19,22)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.89E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst... |
Biochemistry 48: 9823-30 (2009)
Article DOI: 10.1021/bi900258v
BindingDB Entry DOI: 10.7270/Q25M649B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193663

(CHEMBL3929944)Show SMILES Cc1noc(C)c1-c1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(62.09,-40.24,;61.77,-41.75,;62.8,-42.89,;62.03,-44.22,;60.52,-43.9,;59.38,-44.93,;60.36,-42.37,;59.02,-41.6,;57.69,-42.37,;56.37,-41.6,;56.38,-40.08,;55.05,-39.32,;55.05,-37.78,;56.38,-37,;56.38,-35.46,;55.04,-34.69,;55.05,-33.15,;56.38,-32.38,;53.72,-32.38,;52.38,-33.14,;51.05,-32.37,;52.38,-34.68,;53.71,-35.46,;53.71,-37,;57.7,-37.78,;59.04,-37.01,;57.7,-39.32,;59.02,-40.08,;60.35,-39.31,)| Show InChI InChI=1S/C22H22ClN3O3/c1-11-9-12(2)24-21(27)17(11)10-26-8-7-15-5-6-16(20(23)19(15)22(26)28)18-13(3)25-29-14(18)4/h5-6,9H,7-8,10H2,1-4H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193708

(CHEMBL3981606)Show SMILES Cc1cc(C)c(CN2CCc3c(Cl)cc(O[C@@H]4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 |r| Show InChI InChI=1S/C21H22Cl2N2O4/c1-11-7-12(2)24-20(26)15(11)9-25-5-3-14-16(22)8-17(19(23)18(14)21(25)27)29-13-4-6-28-10-13/h7-8,13H,3-6,9-10H2,1-2H3,(H,24,26)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709

(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193712
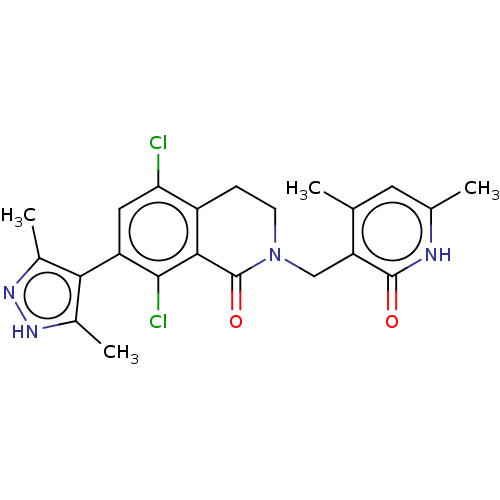
(CHEMBL3919969)Show SMILES Cc1n[nH]c(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(45.66,-39.4,;45.34,-40.91,;46.37,-42.05,;45.6,-43.38,;44.09,-43.06,;42.95,-44.09,;43.93,-41.53,;42.59,-40.76,;41.26,-41.53,;39.94,-40.76,;38.61,-41.53,;39.95,-39.24,;38.62,-38.48,;38.62,-36.94,;39.95,-36.16,;39.95,-34.62,;38.61,-33.85,;38.62,-32.31,;39.96,-31.54,;37.29,-31.54,;35.96,-32.3,;34.63,-31.53,;35.95,-33.84,;37.29,-34.62,;37.29,-36.16,;41.28,-36.94,;42.61,-36.17,;41.28,-38.48,;42.59,-39.24,;43.93,-38.47,)| Show InChI InChI=1S/C22H22Cl2N4O2/c1-10-7-11(2)25-21(29)16(10)9-28-6-5-14-17(23)8-15(20(24)19(14)22(28)30)18-12(3)26-27-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,29)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193724

(CHEMBL3975589)Show SMILES Cc1cc(C)c(CN2CCc3c(C)cc(O[C@@H]4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 |r| Show InChI InChI=1S/C22H25ClN2O4/c1-12-8-14(3)24-21(26)17(12)10-25-6-4-16-13(2)9-18(20(23)19(16)22(25)27)29-15-5-7-28-11-15/h8-9,15H,4-7,10-11H2,1-3H3,(H,24,26)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193656
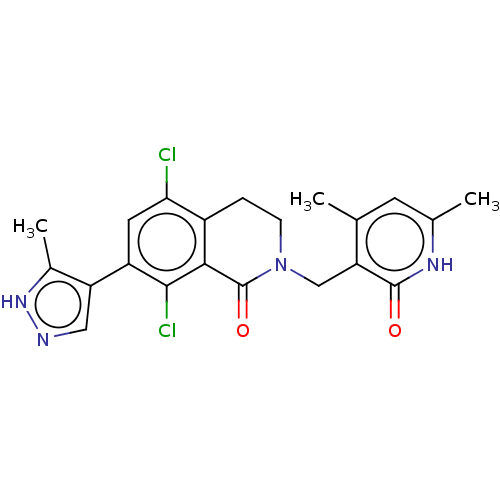
(CHEMBL3947760)Show SMILES Cc1[nH]ncc1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C21H20Cl2N4O2/c1-10-6-11(2)25-20(28)16(10)9-27-5-4-13-17(22)7-14(15-8-24-26-12(15)3)19(23)18(13)21(27)29/h6-8H,4-5,9H2,1-3H3,(H,24,26)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193706
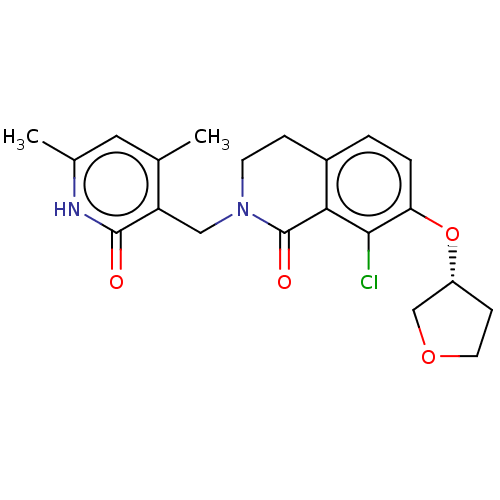
(CHEMBL3967105)Show SMILES Cc1cc(C)c(CN2CCc3ccc(O[C@@H]4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 |r| Show InChI InChI=1S/C21H23ClN2O4/c1-12-9-13(2)23-20(25)16(12)10-24-7-5-14-3-4-17(19(22)18(14)21(24)26)28-15-6-8-27-11-15/h3-4,9,15H,5-8,10-11H2,1-2H3,(H,23,25)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193660

(CHEMBL3928387)Show SMILES CC(C)Oc1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C20H23ClN2O3/c1-11(2)26-16-6-5-14-7-8-23(20(25)17(14)18(16)21)10-15-12(3)9-13(4)22-19(15)24/h5-6,9,11H,7-8,10H2,1-4H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193662

(CHEMBL3911607)Show SMILES Cc1cc(C)c(CN2CCc3c(Cl)cc(-c4cn[nH]c4)c(Cl)c3C2=O)c(=O)[nH]1 Show InChI InChI=1S/C20H18Cl2N4O2/c1-10-5-11(2)25-19(27)15(10)9-26-4-3-13-16(21)6-14(12-7-23-24-8-12)18(22)17(13)20(26)28/h5-8H,3-4,9H2,1-2H3,(H,23,24)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193658
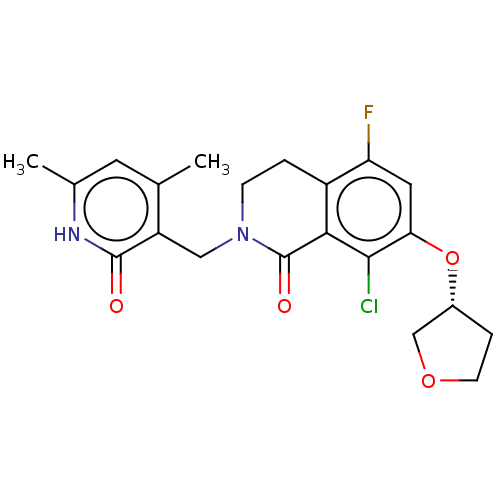
(CHEMBL3946272)Show SMILES Cc1cc(C)c(CN2CCc3c(F)cc(O[C@@H]4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 |r| Show InChI InChI=1S/C21H22ClFN2O4/c1-11-7-12(2)24-20(26)15(11)9-25-5-3-14-16(23)8-17(19(22)18(14)21(25)27)29-13-4-6-28-10-13/h7-8,13H,3-6,9-10H2,1-2H3,(H,24,26)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193663

(CHEMBL3929944)Show SMILES Cc1noc(C)c1-c1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(62.09,-40.24,;61.77,-41.75,;62.8,-42.89,;62.03,-44.22,;60.52,-43.9,;59.38,-44.93,;60.36,-42.37,;59.02,-41.6,;57.69,-42.37,;56.37,-41.6,;56.38,-40.08,;55.05,-39.32,;55.05,-37.78,;56.38,-37,;56.38,-35.46,;55.04,-34.69,;55.05,-33.15,;56.38,-32.38,;53.72,-32.38,;52.38,-33.14,;51.05,-32.37,;52.38,-34.68,;53.71,-35.46,;53.71,-37,;57.7,-37.78,;59.04,-37.01,;57.7,-39.32,;59.02,-40.08,;60.35,-39.31,)| Show InChI InChI=1S/C22H22ClN3O3/c1-11-9-12(2)24-21(27)17(11)10-26-8-7-15-5-6-16(20(23)19(15)22(26)28)18-13(3)25-29-14(18)4/h5-6,9H,7-8,10H2,1-4H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193705
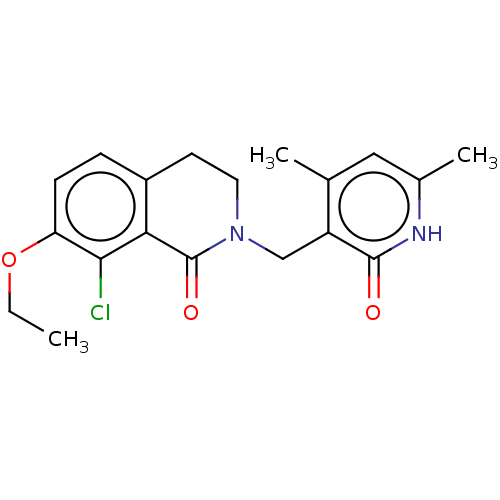
(CHEMBL3947273)Show SMILES CCOc1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C19H21ClN2O3/c1-4-25-15-6-5-13-7-8-22(19(24)16(13)17(15)20)10-14-11(2)9-12(3)21-18(14)23/h5-6,9H,4,7-8,10H2,1-3H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193714
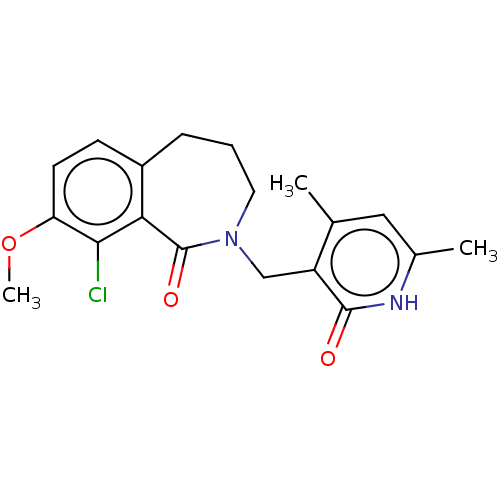
(CHEMBL3984943)Show SMILES COc1ccc2CCCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C19H21ClN2O3/c1-11-9-12(2)21-18(23)14(11)10-22-8-4-5-13-6-7-15(25-3)17(20)16(13)19(22)24/h6-7,9H,4-5,8,10H2,1-3H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193657
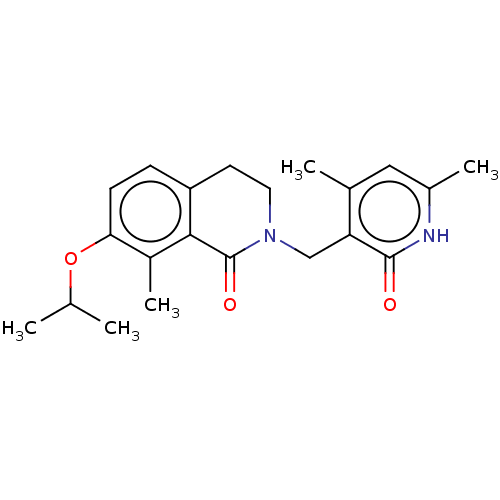
(CHEMBL3955994)Show SMILES CC(C)Oc1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1C Show InChI InChI=1S/C21H26N2O3/c1-12(2)26-18-7-6-16-8-9-23(21(25)19(16)15(18)5)11-17-13(3)10-14(4)22-20(17)24/h6-7,10,12H,8-9,11H2,1-5H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193708

(CHEMBL3981606)Show SMILES Cc1cc(C)c(CN2CCc3c(Cl)cc(O[C@@H]4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 |r| Show InChI InChI=1S/C21H22Cl2N2O4/c1-11-7-12(2)24-20(26)15(11)9-25-5-3-14-16(22)8-17(19(23)18(14)21(25)27)29-13-4-6-28-10-13/h7-8,13H,3-6,9-10H2,1-2H3,(H,24,26)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193724

(CHEMBL3975589)Show SMILES Cc1cc(C)c(CN2CCc3c(C)cc(O[C@@H]4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 |r| Show InChI InChI=1S/C22H25ClN2O4/c1-12-8-14(3)24-21(26)17(12)10-25-6-4-16-13(2)9-18(20(23)19(16)22(25)27)29-15-5-7-28-11-15/h8-9,15H,4-7,10-11H2,1-3H3,(H,24,26)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193704

(CHEMBL3973277)Show SMILES COc1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C18H19ClN2O3/c1-10-8-11(2)20-17(22)13(10)9-21-7-6-12-4-5-14(24-3)16(19)15(12)18(21)23/h4-5,8H,6-7,9H2,1-3H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50483918

(CHEMBL1779734)Show SMILES CCc1[nH]c2nc(C)nc(-c3cc(OC)c(Cl)cc3Cl)c2c1C#N Show InChI InChI=1S/C17H14Cl2N4O/c1-4-13-10(7-20)15-16(21-8(2)22-17(15)23-13)9-5-14(24-3)12(19)6-11(9)18/h5-6H,4H2,1-3H3,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193712

(CHEMBL3919969)Show SMILES Cc1n[nH]c(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(45.66,-39.4,;45.34,-40.91,;46.37,-42.05,;45.6,-43.38,;44.09,-43.06,;42.95,-44.09,;43.93,-41.53,;42.59,-40.76,;41.26,-41.53,;39.94,-40.76,;38.61,-41.53,;39.95,-39.24,;38.62,-38.48,;38.62,-36.94,;39.95,-36.16,;39.95,-34.62,;38.61,-33.85,;38.62,-32.31,;39.96,-31.54,;37.29,-31.54,;35.96,-32.3,;34.63,-31.53,;35.95,-33.84,;37.29,-34.62,;37.29,-36.16,;41.28,-36.94,;42.61,-36.17,;41.28,-38.48,;42.59,-39.24,;43.93,-38.47,)| Show InChI InChI=1S/C22H22Cl2N4O2/c1-10-7-11(2)25-21(29)16(10)9-28-6-5-14-17(23)8-15(20(24)19(14)22(28)30)18-12(3)26-27-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,29)(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 502 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193725
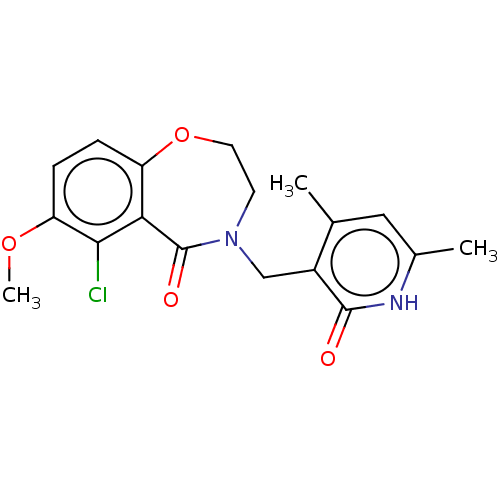
(CHEMBL3956280)Show SMILES COc1ccc2OCCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C18H19ClN2O4/c1-10-8-11(2)20-17(22)12(10)9-21-6-7-25-13-4-5-14(24-3)16(19)15(13)18(21)23/h4-5,8H,6-7,9H2,1-3H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50483917

(CHEMBL1738738)Show SMILES COc1cc(c(Cl)cc1Cl)-c1nc(C)nc2[nH]c(C)c(C#N)c12 Show InChI InChI=1S/C16H12Cl2N4O/c1-7-10(6-19)14-15(21-8(2)22-16(14)20-7)9-4-13(23-3)12(18)5-11(9)17/h4-5H,1-3H3,(H,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193706

(CHEMBL3967105)Show SMILES Cc1cc(C)c(CN2CCc3ccc(O[C@@H]4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 |r| Show InChI InChI=1S/C21H23ClN2O4/c1-12-9-13(2)23-20(25)16(12)10-24-7-5-14-3-4-17(19(22)18(14)21(24)26)28-15-6-8-27-11-15/h3-4,9,15H,5-8,10-11H2,1-2H3,(H,23,25)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 796 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193656

(CHEMBL3947760)Show SMILES Cc1[nH]ncc1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C21H20Cl2N4O2/c1-10-6-11(2)25-20(28)16(10)9-27-5-4-13-17(22)7-14(15-8-24-26-12(15)3)19(23)18(13)21(27)29/h6-8H,4-5,9H2,1-3H3,(H,24,26)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 873 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193658

(CHEMBL3946272)Show SMILES Cc1cc(C)c(CN2CCc3c(F)cc(O[C@@H]4CCOC4)c(Cl)c3C2=O)c(=O)[nH]1 |r| Show InChI InChI=1S/C21H22ClFN2O4/c1-11-7-12(2)24-20(26)15(11)9-25-5-3-14-16(23)8-17(19(22)18(14)21(25)27)29-13-4-6-28-10-13/h7-8,13H,3-6,9-10H2,1-2H3,(H,24,26)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 994 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50483920
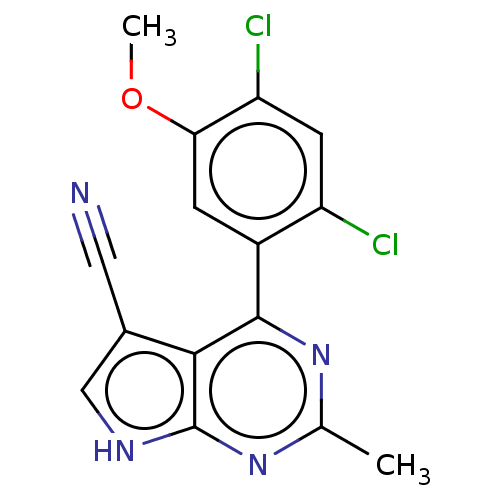
(CHEMBL1738737)Show SMILES COc1cc(c(Cl)cc1Cl)-c1nc(C)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C15H10Cl2N4O/c1-7-20-14(13-8(5-18)6-19-15(13)21-7)9-3-12(22-2)11(17)4-10(9)16/h3-4,6H,1-2H3,(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193660

(CHEMBL3928387)Show SMILES CC(C)Oc1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl Show InChI InChI=1S/C20H23ClN2O3/c1-11(2)26-16-6-5-14-7-8-23(20(25)17(14)18(16)21)10-15-12(3)9-13(4)22-19(15)24/h5-6,9,11H,7-8,10H2,1-4H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193671

(CHEMBL3983898)Show SMILES CC(C)Oc1cccc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1Cl Show InChI InChI=1S/C18H21ClN2O3/c1-10(2)24-15-7-5-6-13(16(15)19)17(22)20-9-14-11(3)8-12(4)21-18(14)23/h5-8,10H,9H2,1-4H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193717

(CHEMBL3932025)Show SMILES CC(C)Oc1cccc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C19H24N2O3/c1-11(2)24-17-8-6-7-15(14(17)5)18(22)20-10-16-12(3)9-13(4)21-19(16)23/h6-9,11H,10H2,1-5H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193707
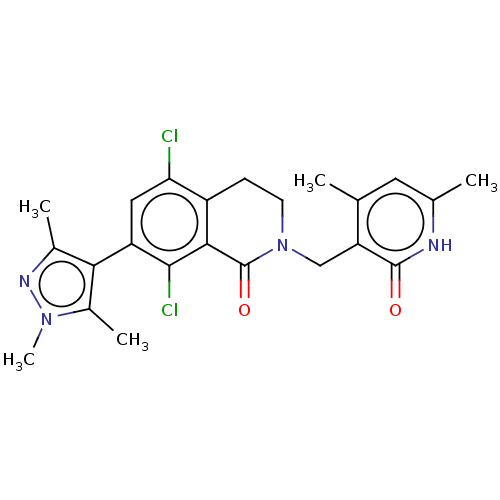
(CHEMBL3938963)Show SMILES Cc1nn(C)c(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(56.67,-10.92,;56.34,-12.43,;57.37,-13.57,;56.6,-14.91,;57.23,-16.31,;55.1,-14.59,;53.95,-15.62,;54.94,-13.06,;53.6,-12.29,;52.27,-13.05,;50.95,-12.28,;49.61,-13.05,;50.95,-10.76,;49.62,-10,;49.62,-8.46,;50.95,-7.68,;50.95,-6.14,;49.62,-5.37,;49.63,-3.83,;50.96,-3.07,;48.3,-3.06,;46.96,-3.83,;45.63,-3.05,;46.96,-5.37,;48.29,-6.15,;48.29,-7.69,;52.28,-8.46,;53.62,-7.7,;52.28,-10,;53.6,-10.77,;54.93,-10,)| Show InChI InChI=1S/C23H24Cl2N4O2/c1-11-8-12(2)26-22(30)17(11)10-29-7-6-15-18(24)9-16(21(25)20(15)23(29)31)19-13(3)27-28(5)14(19)4/h8-9H,6-7,10H2,1-5H3,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193662

(CHEMBL3911607)Show SMILES Cc1cc(C)c(CN2CCc3c(Cl)cc(-c4cn[nH]c4)c(Cl)c3C2=O)c(=O)[nH]1 Show InChI InChI=1S/C20H18Cl2N4O2/c1-10-5-11(2)25-19(27)15(10)9-26-4-3-13-16(21)6-14(12-7-23-24-8-12)18(22)17(13)20(26)28/h5-8H,3-4,9H2,1-2H3,(H,23,24)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50483916

(CHEMBL1779589)Show InChI InChI=1S/C14H10Cl3N3O/c1-6-19-13(12-10(17)5-18-14(12)20-6)7-3-11(21-2)9(16)4-8(7)15/h3-5H,1-2H3,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193666
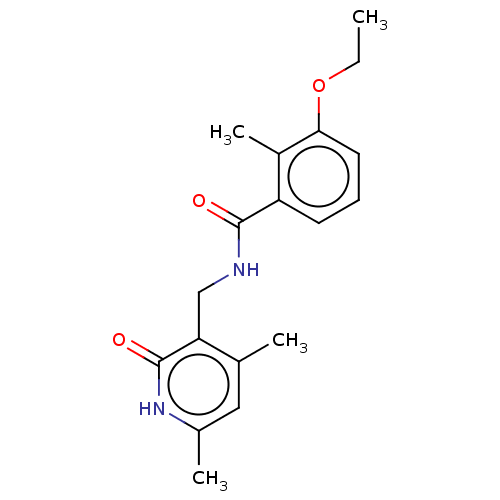
(CHEMBL3895097)Show InChI InChI=1S/C18H22N2O3/c1-5-23-16-8-6-7-14(13(16)4)17(21)19-10-15-11(2)9-12(3)20-18(15)22/h6-9H,5,10H2,1-4H3,(H,19,21)(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193711

(CHEMBL3955846 | US10478426, Example 36)Show InChI InChI=1S/C17H20N2O3/c1-10-8-11(2)19-17(21)14(10)9-18-16(20)13-6-5-7-15(22-4)12(13)3/h5-8H,9H2,1-4H3,(H,18,20)(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193726

(CHEMBL3914083)Show InChI InChI=1S/C16H17ClN2O3/c1-9-7-10(2)19-16(21)12(9)8-18-15(20)11-5-4-6-13(22-3)14(11)17/h4-7H,8H2,1-3H3,(H,18,20)(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193661
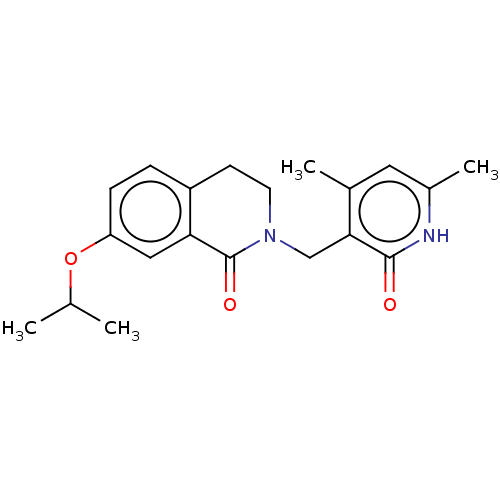
(CHEMBL3986389)Show SMILES CC(C)Oc1ccc2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1 Show InChI InChI=1S/C20H24N2O3/c1-12(2)25-16-6-5-15-7-8-22(20(24)17(15)10-16)11-18-13(3)9-14(4)21-19(18)23/h5-6,9-10,12H,7-8,11H2,1-4H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50483919

(CHEMBL1779582)Show InChI InChI=1S/C11H11N3/c1-8-7-10(14-11(12)13-8)9-5-3-2-4-6-9/h2-7H,1H3,(H2,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50483915
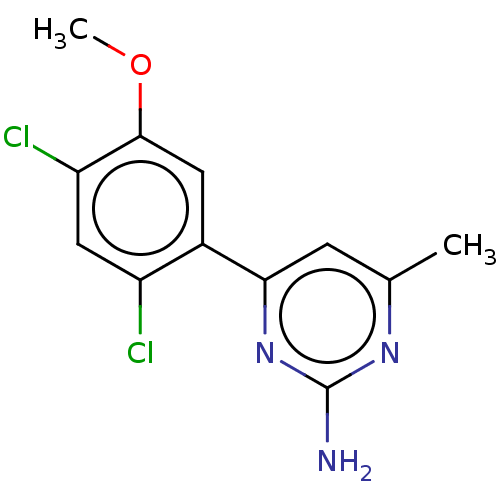
(CHEMBL1738736)Show InChI InChI=1S/C12H11Cl2N3O/c1-6-3-10(17-12(15)16-6)7-4-11(18-2)9(14)5-8(7)13/h3-5H,1-2H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50343361
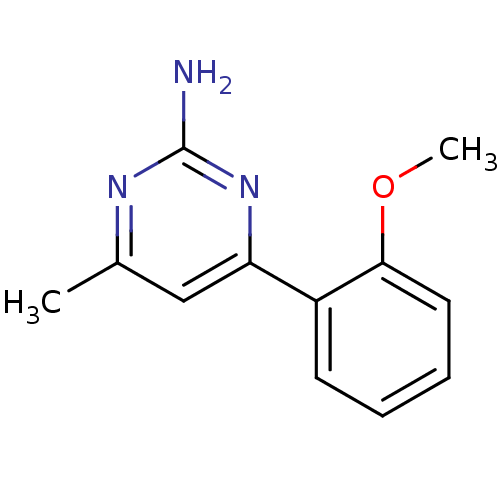
(4-(2-methoxyphenyl)-6-methylpyrimidin-2-amine | CH...)Show InChI InChI=1S/C12H13N3O/c1-8-7-10(15-12(13)14-8)9-5-3-4-6-11(9)16-2/h3-7H,1-2H3,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM81730
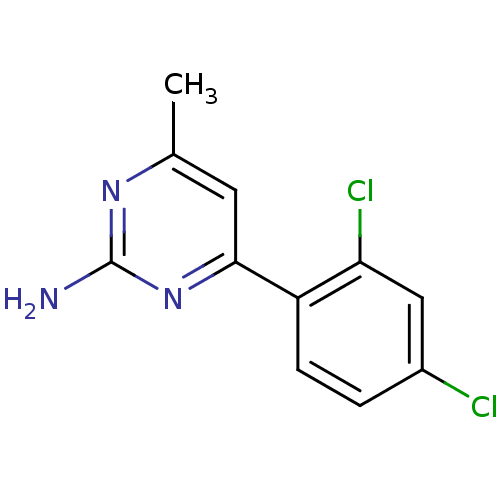
(HSP90 Inhibitor, 4)Show InChI InChI=1S/C11H9Cl2N3/c1-6-4-10(16-11(14)15-6)8-3-2-7(12)5-9(8)13/h2-5H,1H3,(H2,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193707

(CHEMBL3938963)Show SMILES Cc1nn(C)c(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(56.67,-10.92,;56.34,-12.43,;57.37,-13.57,;56.6,-14.91,;57.23,-16.31,;55.1,-14.59,;53.95,-15.62,;54.94,-13.06,;53.6,-12.29,;52.27,-13.05,;50.95,-12.28,;49.61,-13.05,;50.95,-10.76,;49.62,-10,;49.62,-8.46,;50.95,-7.68,;50.95,-6.14,;49.62,-5.37,;49.63,-3.83,;50.96,-3.07,;48.3,-3.06,;46.96,-3.83,;45.63,-3.05,;46.96,-5.37,;48.29,-6.15,;48.29,-7.69,;52.28,-8.46,;53.62,-7.7,;52.28,-10,;53.6,-10.77,;54.93,-10,)| Show InChI InChI=1S/C23H24Cl2N4O2/c1-11-8-12(2)26-22(30)17(11)10-29-7-6-15-18(24)9-16(21(25)20(15)23(29)31)19-13(3)27-28(5)14(19)4/h8-9H,6-7,10H2,1-5H3,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as reduction in H3K27Me3 levels after 72 hrs by ELISA |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50483921
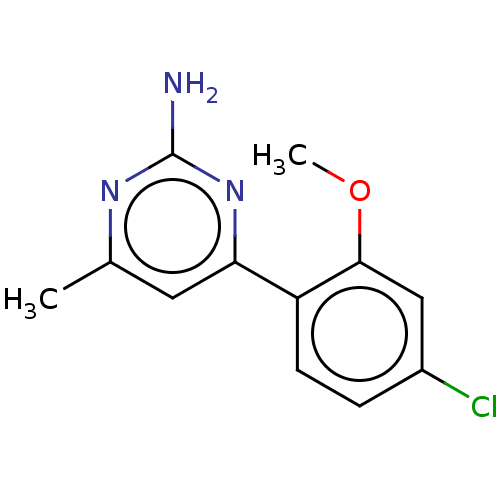
(CHEMBL1779583)Show InChI InChI=1S/C12H12ClN3O/c1-7-5-10(16-12(14)15-7)9-4-3-8(13)6-11(9)17-2/h3-6H,1-2H3,(H2,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193710
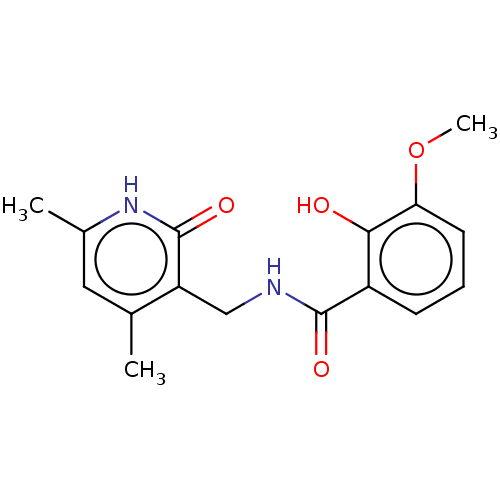
(CHEMBL3975884)Show InChI InChI=1S/C16H18N2O4/c1-9-7-10(2)18-16(21)12(9)8-17-15(20)11-5-4-6-13(22-3)14(11)19/h4-7,19H,8H2,1-3H3,(H,17,20)(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EZH2 (unknown origin) expressed in baculovirus infected SF9 cells co-expressing SUZ12/EED/RbAp48 complex using HeLa cells der... |
J Med Chem 59: 8306-25 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00515
BindingDB Entry DOI: 10.7270/Q2J1053B |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50483914

(CHEMBL1779580)Show InChI InChI=1S/C13H15ClN4O2/c1-13(2,3)20-12(19)18-6-4-5-9(18)8-7-10(14)17-11(15)16-8/h4-7H,1-3H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 in human H1299 cells assessed as degradation of Akt protein after 24 hrs by luminex assay |
Bioorg Med Chem Lett 21: 3557-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.130
BindingDB Entry DOI: 10.7270/Q2H99820 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data