

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16385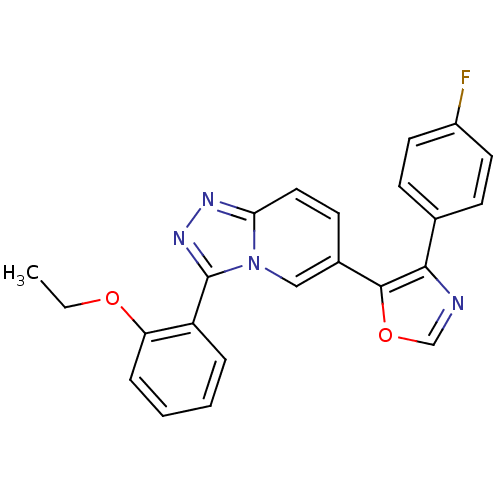 (5-[3-(2-ethoxyphenyl)-[1,2,4]triazolo[3,4-a]pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16384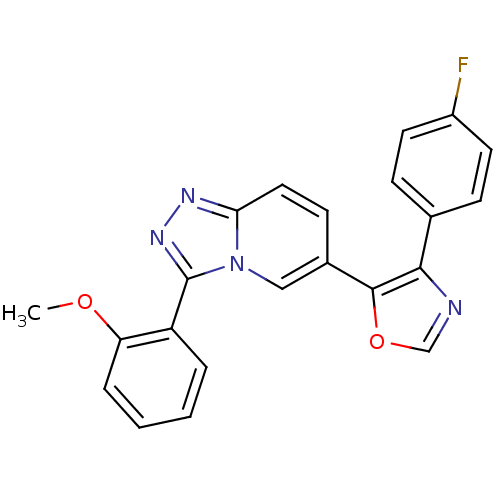 (4-(4-fluorophenyl)-5-[3-(2-methoxyphenyl)-[1,2,4]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16390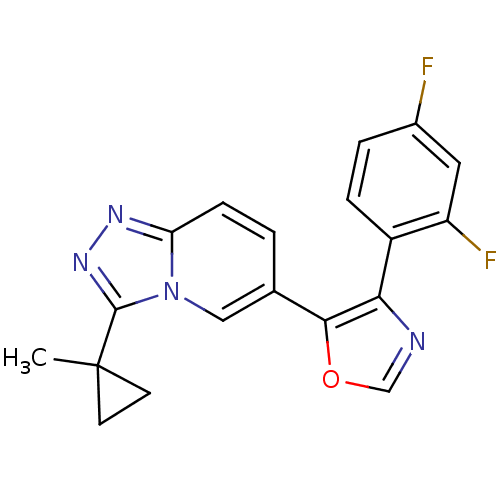 (4-(2,4-difluorophenyl)-5-[3-(1-methylcyclopropyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50457826 (CHEMBL4216500) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkA in human U2OS cells assessed as inhibition of beta-NGF-induced receptor phosphorylation by measuring reduc... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50457839 (CHEMBL4211921) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkC in human U2OS cells assessed as inhibition of NT3-induced receptor phosphorylation by measuring reduction ... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16392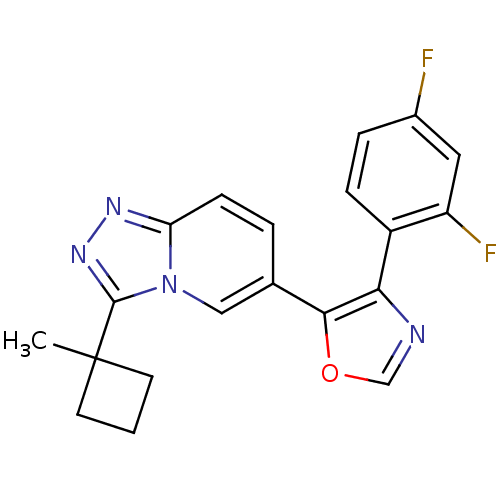 (4-(2,4-difluorophenyl)-5-[3-(1-methylcyclobutyl)-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50457833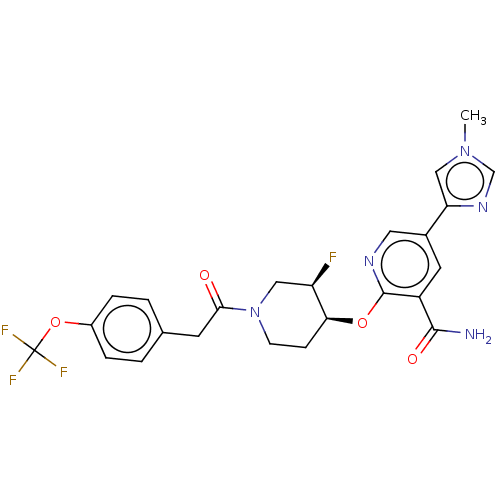 (CHEMBL4210892) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkC in human U2OS cells assessed as inhibition of NT3-induced receptor phosphorylation by measuring reduction ... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16393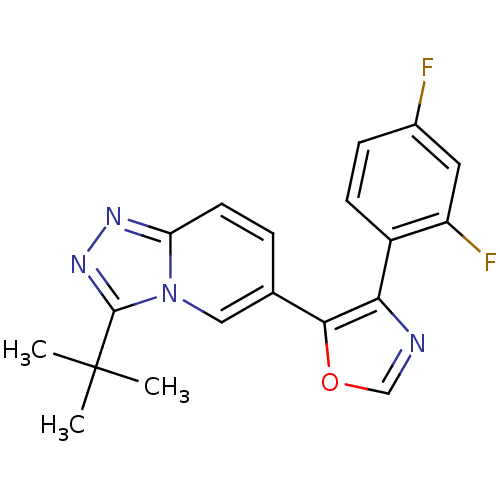 (5-{3-tert-butyl-[1,2,4]triazolo[3,4-a]pyridin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16398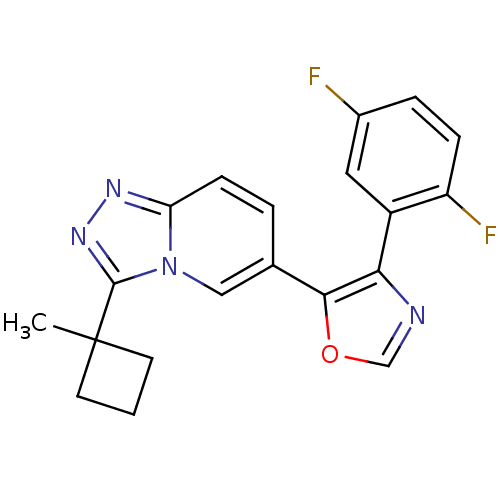 (4-(2,5-difluorophenyl)-5-[3-(1-methylcyclobutyl)-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16404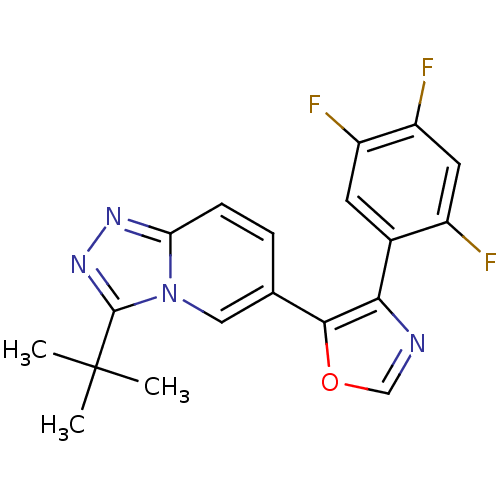 (5-{3-tert-butyl-[1,2,4]triazolo[3,4-a]pyridin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16346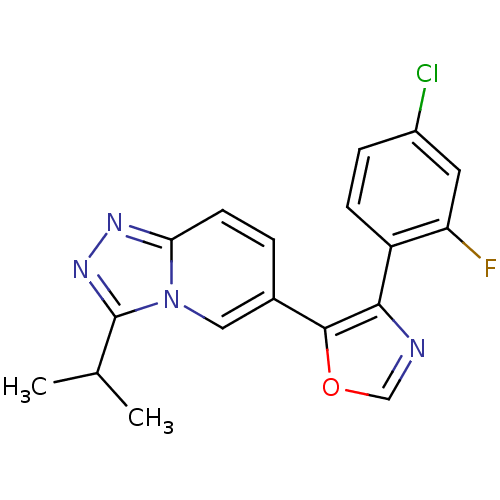 (4-(4-chloro-2-fluorophenyl)-5-[3-(propan-2-yl)-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50457825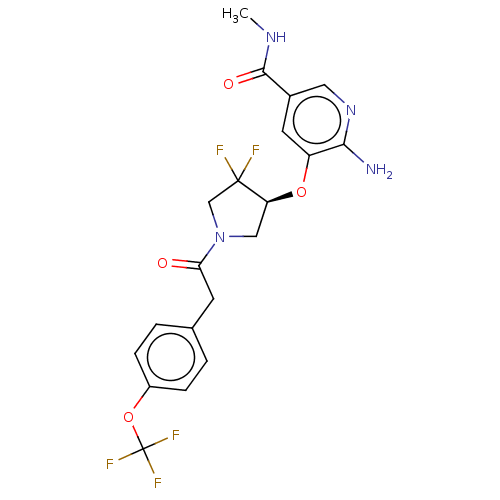 (CHEMBL4207042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkC in human U2OS cells assessed as inhibition of NT3-induced receptor phosphorylation by measuring reduction ... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50457837 (CHEMBL4215846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkC in human U2OS cells assessed as inhibition of NT3-induced receptor phosphorylation by measuring reduction ... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16401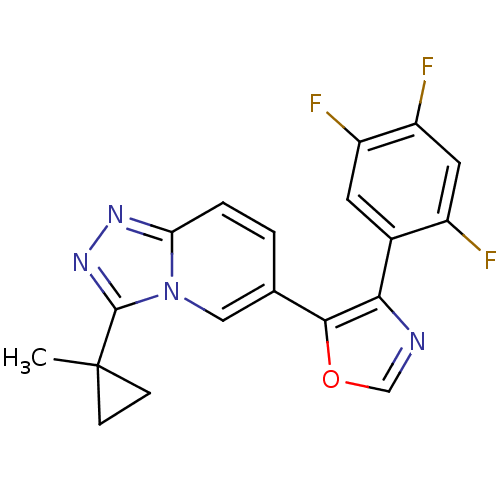 (5-[3-(1-methylcyclopropyl)-[1,2,4]triazolo[3,4-a]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16403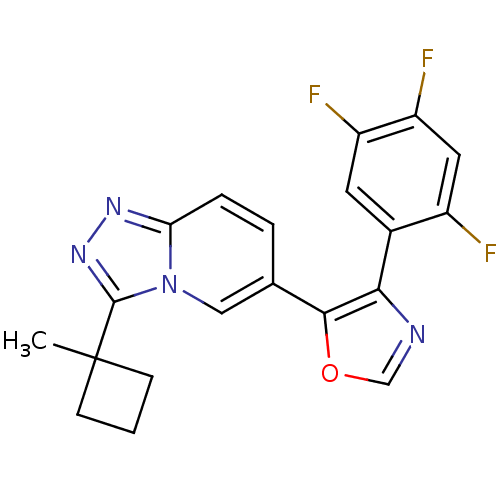 (5-[3-(1-methylcyclobutyl)-[1,2,4]triazolo[3,4-a]py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16348 (4-(2-chloro-4-fluorophenyl)-5-[3-(propan-2-yl)-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50457833 (CHEMBL4210892) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkB in human U2OS cells assessed as inhibition of BDNF-induced receptor phosphorylation by measuring reduction... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50457839 (CHEMBL4211921) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkB in human U2OS cells assessed as inhibition of BDNF-induced receptor phosphorylation by measuring reduction... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16405 (3-(2,5-difluorophenyl)-4-[3-(propan-2-yl)-[1,2,4]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16396 (4-(2,5-difluorophenyl)-5-[3-(1-methylcyclopropyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16340 (4-(2-chlorophenyl)-5-[3-(propan-2-yl)-[1,2,4]triaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16351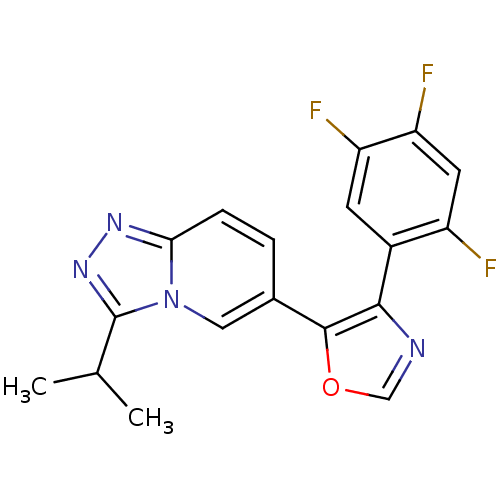 (5-[3-(propan-2-yl)-[1,2,4]triazolo[3,4-a]pyridin-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16389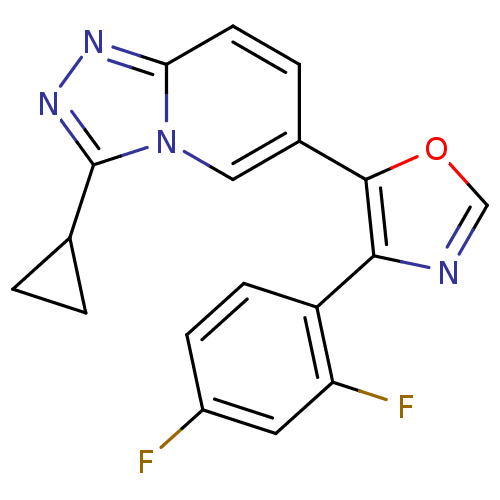 (5-{3-cyclopropyl-[1,2,4]triazolo[3,4-a]pyridin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16386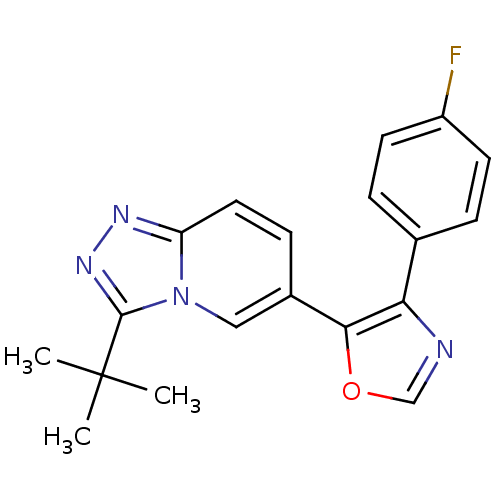 (5-{3-tert-butyl-[1,2,4]triazolo[3,4-a]pyridin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16391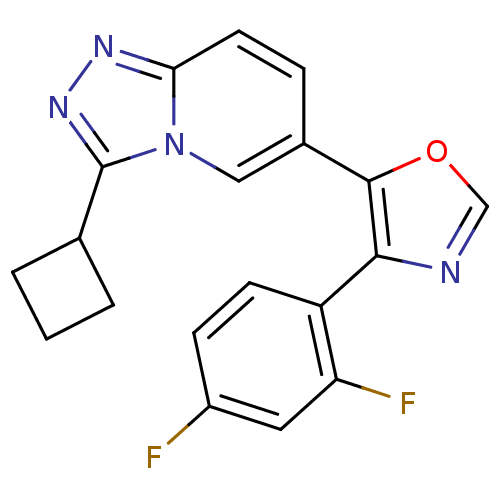 (5-{3-cyclobutyl-[1,2,4]triazolo[3,4-a]pyridin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50457827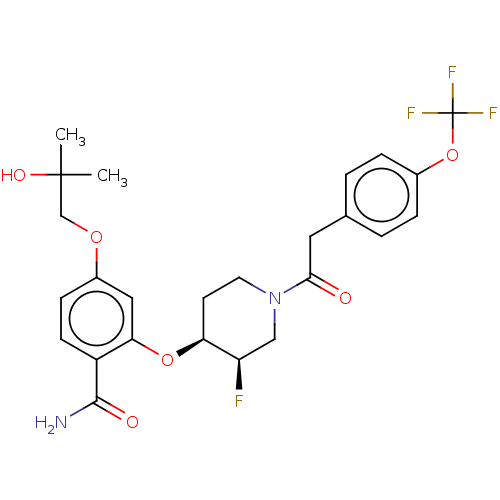 (CHEMBL4203759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkC in human U2OS cells assessed as inhibition of NT3-induced receptor phosphorylation by measuring reduction ... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16350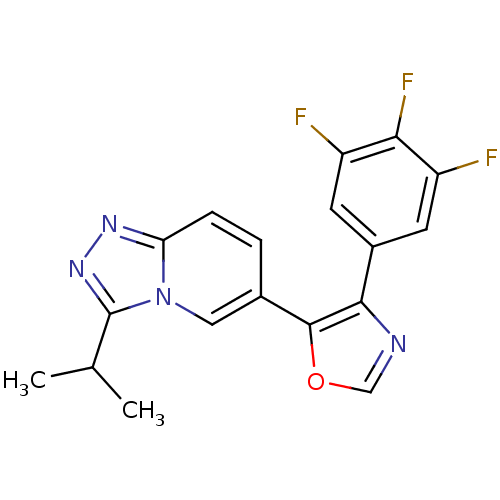 (5-[3-(propan-2-yl)-[1,2,4]triazolo[3,4-a]pyridin-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50457824 (CHEMBL4204174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkB in human U2OS cells assessed as inhibition of BDNF-induced receptor phosphorylation by measuring reduction... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16337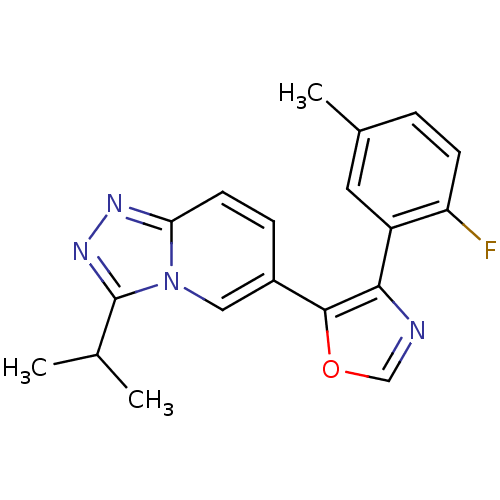 (4-(2-fluoro-5-methylphenyl)-5-[3-(propan-2-yl)-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16341 (4-(2,4-difluorophenyl)-5-[3-(propan-2-yl)-[1,2,4]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16379 (N-ethyl-6-[4-(4-fluorophenyl)-1,3-oxazol-5-yl]-N-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50305692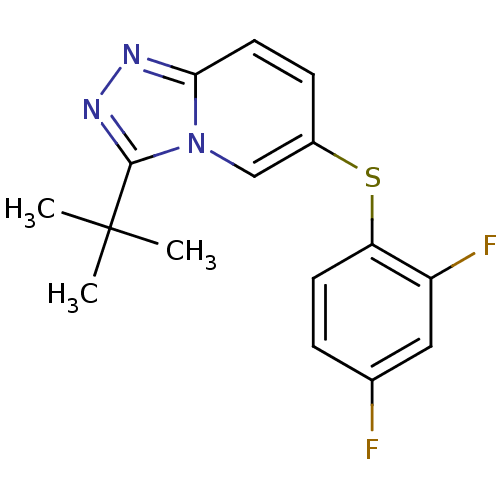 (3-tert-butyl-6-(2,4-difluorophenylthio)-[1,2,4]tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of p38 alpha | Bioorg Med Chem Lett 20: 469-73 (2010) Article DOI: 10.1016/j.bmcl.2009.11.114 BindingDB Entry DOI: 10.7270/Q2G160XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM15414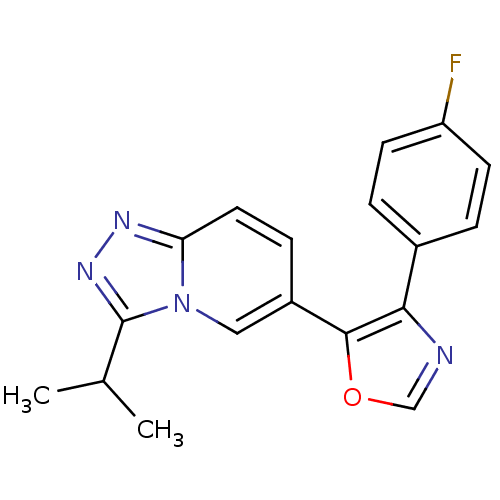 (4-(4-fluorophenyl)-5-[3-(propan-2-yl)-[1,2,4]triaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50457828 (CHEMBL4218103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkC in human U2OS cells assessed as inhibition of NT3-induced receptor phosphorylation by measuring reduction ... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16347 (4-(5-chloro-2-fluorophenyl)-5-[3-(propan-2-yl)-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16364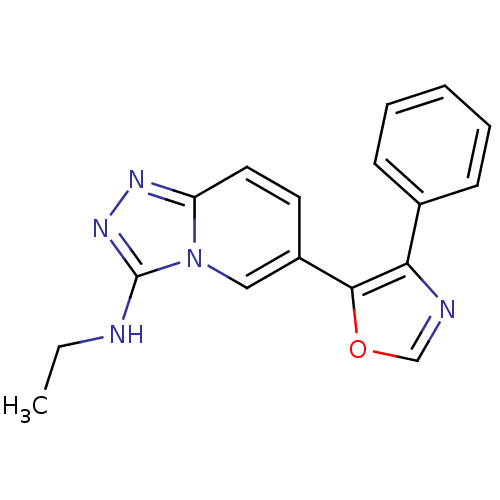 (N-ethyl-6-(4-phenyl-1,3-oxazol-5-yl)-[1,2,4]triazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16377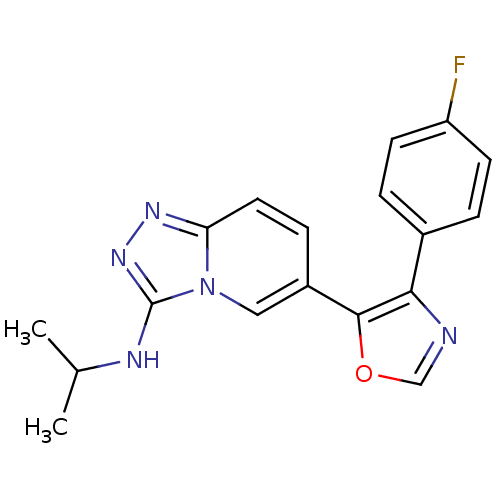 (6-[4-(4-fluorophenyl)-1,3-oxazol-5-yl]-N-(propan-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16359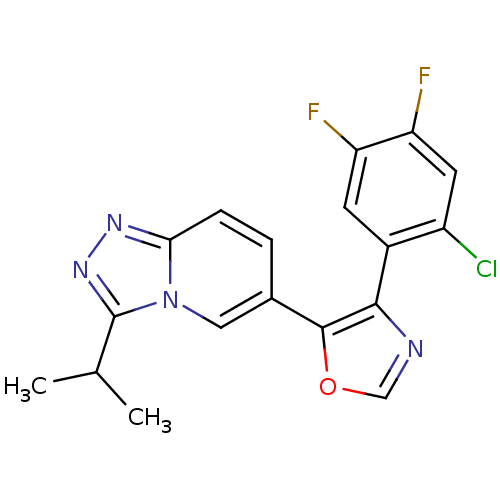 (4-(2-chloro-4,5-difluorophenyl)-5-[3-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16342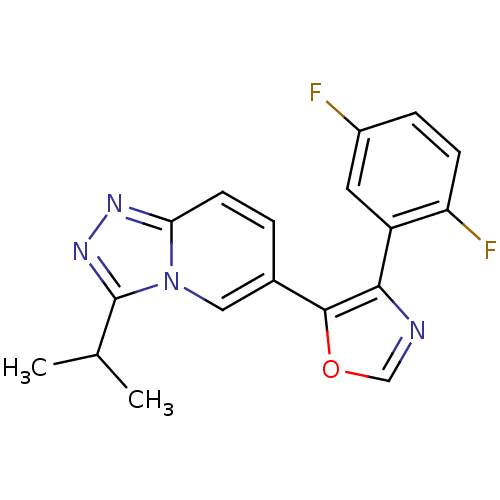 (4-(2,5-difluorophenyl)-5-[3-(propan-2-yl)-[1,2,4]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50457837 (CHEMBL4215846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkB in human U2OS cells assessed as inhibition of BDNF-induced receptor phosphorylation by measuring reduction... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50457825 (CHEMBL4207042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkB in human U2OS cells assessed as inhibition of BDNF-induced receptor phosphorylation by measuring reduction... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16407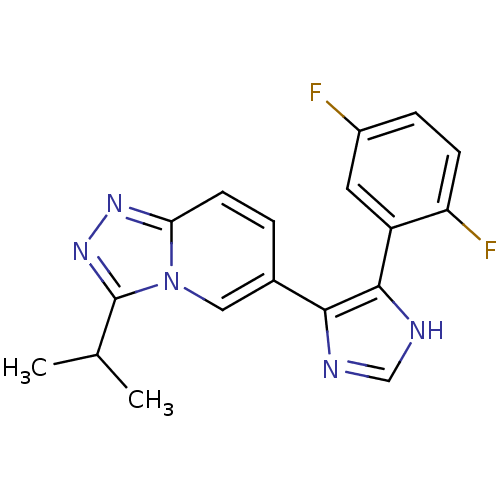 (4-(2,5-difluorophenyl)-5-[3-(propan-2-yl)-[1,2,4]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16336 (4-(4-fluoro-3-methylphenyl)-5-[3-(propan-2-yl)-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50457829 (CHEMBL4208510) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkB in human U2OS cells assessed as inhibition of BDNF-induced receptor phosphorylation by measuring reduction... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50457837 (CHEMBL4215846) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkA in human U2OS cells assessed as inhibition of beta-NGF-induced receptor phosphorylation by measuring reduc... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50305696 (3-(2,4-difluorophenyl)-6-(2,4-difluorophenylthio)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of p38 alpha | Bioorg Med Chem Lett 20: 469-73 (2010) Article DOI: 10.1016/j.bmcl.2009.11.114 BindingDB Entry DOI: 10.7270/Q2G160XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16352 (5-[3-(propan-2-yl)-[1,2,4]triazolo[3,4-a]pyridin-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16372 (N-ethyl-6-[4-(4-fluorophenyl)-1,3-oxazol-5-yl]-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description The IC50s were determined by incubating each test compound with activated p38-alpha in a 96-well plate that had been pre-treated with ATF-2-GST to al... | Bioorg Med Chem Lett 16: 4339-44 (2006) Article DOI: 10.1016/j.bmcl.2006.05.056 BindingDB Entry DOI: 10.7270/Q2251GFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50457839 (CHEMBL4211921) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at prolink-tagged TrkA in human U2OS cells assessed as inhibition of beta-NGF-induced receptor phosphorylation by measuring reduc... | J Med Chem 61: 6779-6800 (2018) Article DOI: 10.1021/acs.jmedchem.8b00633 BindingDB Entry DOI: 10.7270/Q2K64MPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50305704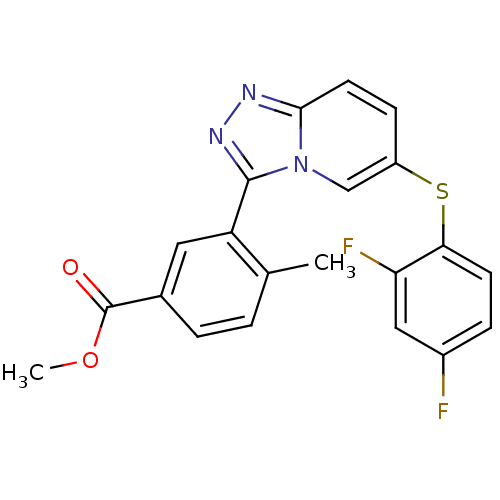 (CHEMBL595338 | methyl 3-(6-(2,4-difluorophenylthio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of p38 alpha | Bioorg Med Chem Lett 20: 469-73 (2010) Article DOI: 10.1016/j.bmcl.2009.11.114 BindingDB Entry DOI: 10.7270/Q2G160XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |