 Found 100 hits with Last Name = 'brink' and Initial = 'm'
Found 100 hits with Last Name = 'brink' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50361983
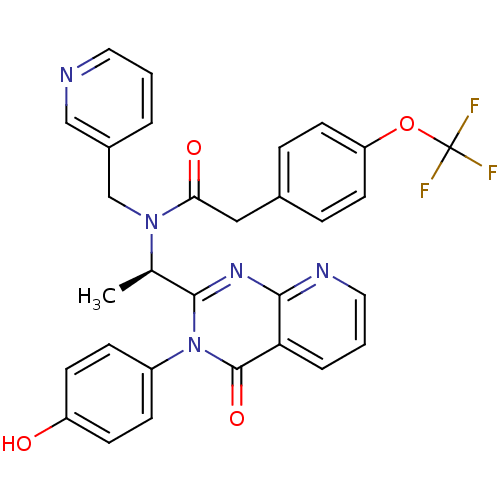
(CHEMBL1939697)Show SMILES C[C@@H](N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2ncccc2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24F3N5O4/c1-19(28-36-27-25(5-3-15-35-27)29(41)38(28)22-8-10-23(39)11-9-22)37(18-21-4-2-14-34-17-21)26(40)16-20-6-12-24(13-7-20)42-30(31,32)33/h2-15,17,19,39H,16,18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate assessed as unbound inhibitor concentration required for half maximal enz... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50361983

(CHEMBL1939697)Show SMILES C[C@@H](N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2ncccc2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24F3N5O4/c1-19(28-36-27-25(5-3-15-35-27)29(41)38(28)22-8-10-23(39)11-9-22)37(18-21-4-2-14-34-17-21)26(40)16-20-6-12-24(13-7-20)42-30(31,32)33/h2-15,17,19,39H,16,18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate assessed as unbound inhibitor concentration required for half maximal ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50361983

(CHEMBL1939697)Show SMILES C[C@@H](N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2ncccc2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24F3N5O4/c1-19(28-36-27-25(5-3-15-35-27)29(41)38(28)22-8-10-23(39)11-9-22)37(18-21-4-2-14-34-17-21)26(40)16-20-6-12-24(13-7-20)42-30(31,32)33/h2-15,17,19,39H,16,18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate assessed as residual enzyme activity after 2 to 10 mins by LC-MS/MS an... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50361983

(CHEMBL1939697)Show SMILES C[C@@H](N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2ncccc2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24F3N5O4/c1-19(28-36-27-25(5-3-15-35-27)29(41)38(28)22-8-10-23(39)11-9-22)37(18-21-4-2-14-34-17-21)26(40)16-20-6-12-24(13-7-20)42-30(31,32)33/h2-15,17,19,39H,16,18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate assessed as residual enzyme activity after 2 to 10 mins by LC-MS/MS analy... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50371232
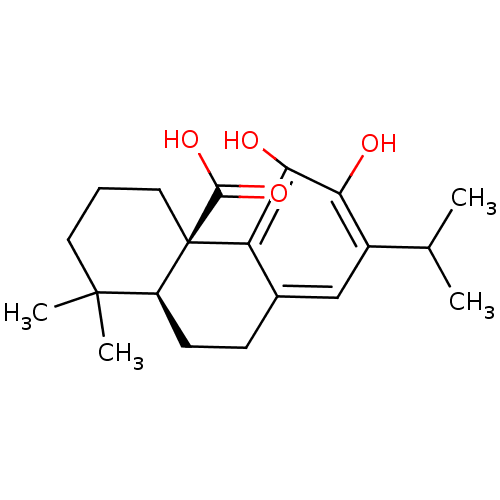
(CARNOSIC ACID)Show SMILES CC(C)c1cc2CC[C@H]3C(C)(C)CCC[C@]3(C(O)=O)c2c(O)c1O |r| Show InChI InChI=1S/C20H28O4/c1-11(2)13-10-12-6-7-14-19(3,4)8-5-9-20(14,18(23)24)15(12)17(22)16(13)21/h10-11,14,21-22H,5-9H2,1-4H3,(H,23,24)/t14-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human hepatocytes after 30 mins by liquid chromatography/tandem mass spectroscopy |
Drug Metab Dispos 40: 1263-7 (2012)
Article DOI: 10.1124/dmd.112.044909
BindingDB Entry DOI: 10.7270/Q24Q7WQX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50371232

(CARNOSIC ACID)Show SMILES CC(C)c1cc2CC[C@H]3C(C)(C)CCC[C@]3(C(O)=O)c2c(O)c1O |r| Show InChI InChI=1S/C20H28O4/c1-11(2)13-10-12-6-7-14-19(3,4)8-5-9-20(14,18(23)24)15(12)17(22)16(13)21/h10-11,14,21-22H,5-9H2,1-4H3,(H,23,24)/t14-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 activity in human hepatocytes after 30 mins by liquid chromatography/tandem mass spectroscopy |
Drug Metab Dispos 40: 1263-7 (2012)
Article DOI: 10.1124/dmd.112.044909
BindingDB Entry DOI: 10.7270/Q24Q7WQX |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008271
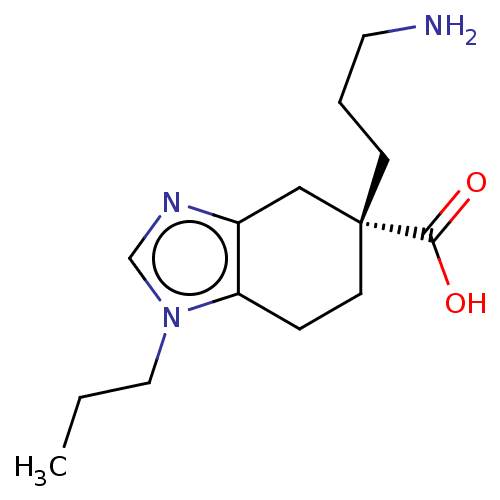
(CHEMBL3235132)Show InChI InChI=1S/C14H23N3O2/c1-2-8-17-10-16-11-9-14(13(18)19,5-3-7-15)6-4-12(11)17/h10H,2-9,15H2,1H3,(H,18,19)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a using hippuryl-L-arginine/hippuryl-L-lysine as substrate by liquid chromatographic analysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50361983

(CHEMBL1939697)Show SMILES C[C@@H](N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2ncccc2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24F3N5O4/c1-19(28-36-27-25(5-3-15-35-27)29(41)38(28)22-8-10-23(39)11-9-22)37(18-21-4-2-14-34-17-21)26(40)16-20-6-12-24(13-7-20)42-30(31,32)33/h2-15,17,19,39H,16,18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008279
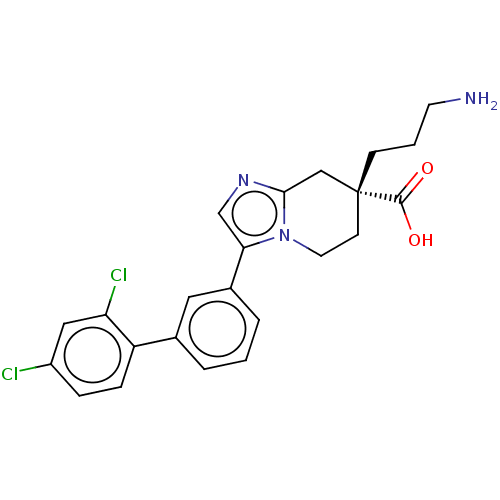
(CHEMBL3235140)Show SMILES Cl.NCCC[C@]1(CCn2c(C1)ncc2-c1cccc(c1)-c1ccc(Cl)cc1Cl)C(O)=O |r| Show InChI InChI=1S/C23H23Cl2N3O2.ClH/c24-17-5-6-18(19(25)12-17)15-3-1-4-16(11-15)20-14-27-21-13-23(22(29)30,7-2-9-26)8-10-28(20)21;/h1,3-6,11-12,14H,2,7-10,13,26H2,(H,29,30);1H/t23-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008272
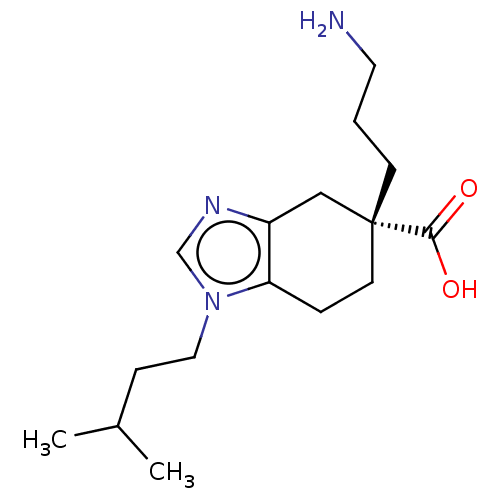
(CHEMBL3235133)Show SMILES Cl.CC(C)CCn1cnc2C[C@@](CCCN)(CCc12)C(O)=O |r| Show InChI InChI=1S/C16H27N3O2.ClH/c1-12(2)5-9-19-11-18-13-10-16(15(20)21,6-3-8-17)7-4-14(13)19;/h11-12H,3-10,17H2,1-2H3,(H,20,21);1H/t16-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008278
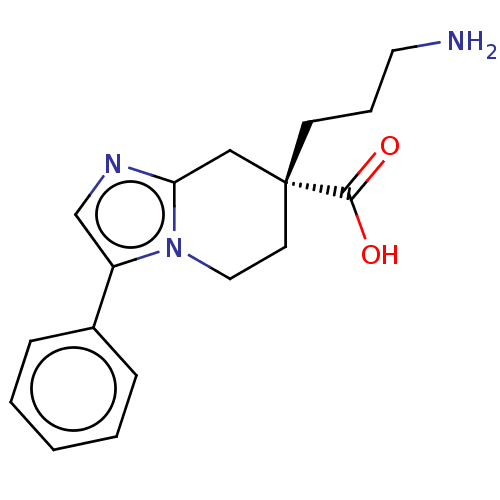
(CHEMBL3235139)Show SMILES NCCC[C@]1(CCn2c(C1)ncc2-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C17H21N3O2/c18-9-4-7-17(16(21)22)8-10-20-14(12-19-15(20)11-17)13-5-2-1-3-6-13/h1-3,5-6,12H,4,7-11,18H2,(H,21,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50361983

(CHEMBL1939697)Show SMILES C[C@@H](N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2ncccc2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24F3N5O4/c1-19(28-36-27-25(5-3-15-35-27)29(41)38(28)22-8-10-23(39)11-9-22)37(18-21-4-2-14-34-17-21)26(40)16-20-6-12-24(13-7-20)42-30(31,32)33/h2-15,17,19,39H,16,18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 30 mins followed by 10-fold dilution a... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008271

(CHEMBL3235132)Show InChI InChI=1S/C14H23N3O2/c1-2-8-17-10-16-11-9-14(13(18)19,5-3-7-15)6-4-12(11)17/h10H,2-9,15H2,1H3,(H,18,19)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008273
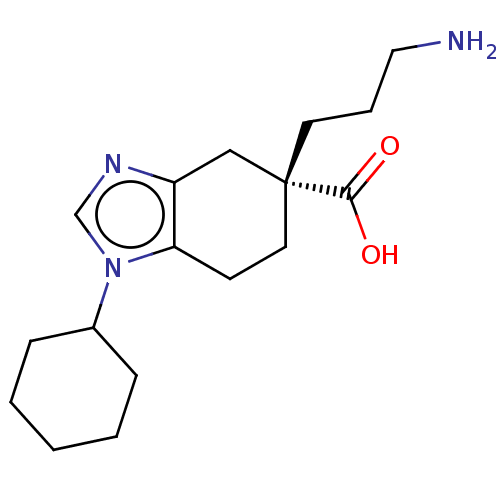
(CHEMBL3235134)Show InChI InChI=1S/C17H27N3O2/c18-10-4-8-17(16(21)22)9-7-15-14(11-17)19-12-20(15)13-5-2-1-3-6-13/h12-13H,1-11,18H2,(H,21,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008269

(CHEMBL3235130)Show InChI InChI=1S/C10H14N2O2S/c1-6(15)8(10(13)14)4-7-2-3-9(11)12-5-7/h2-3,5-6,8,15H,4H2,1H3,(H2,11,12)(H,13,14)/t6-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a using hippuryl-L-arginine/hippuryl-L-lysine as substrate by liquid chromatographic analysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by 10-fold dilution and ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 90 mins followed by 10-fold dilution a... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008272

(CHEMBL3235133)Show SMILES Cl.CC(C)CCn1cnc2C[C@@](CCCN)(CCc12)C(O)=O |r| Show InChI InChI=1S/C16H27N3O2.ClH/c1-12(2)5-9-19-11-18-13-10-16(15(20)21,6-3-8-17)7-4-14(13)19;/h11-12H,3-10,17H2,1-2H3,(H,20,21);1H/t16-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008274
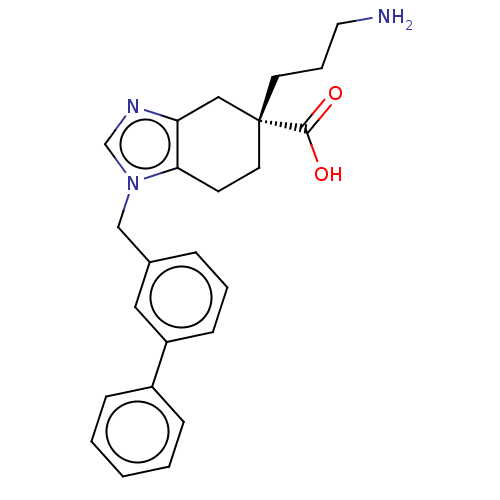
(CHEMBL3235135)Show SMILES NCCC[C@]1(CCc2c(C1)ncn2Cc1cccc(c1)-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H27N3O2/c25-13-5-11-24(23(28)29)12-10-22-21(15-24)26-17-27(22)16-18-6-4-9-20(14-18)19-7-2-1-3-8-19/h1-4,6-9,14,17H,5,10-13,15-16,25H2,(H,28,29)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008273

(CHEMBL3235134)Show InChI InChI=1S/C17H27N3O2/c18-10-4-8-17(16(21)22)9-7-15-14(11-17)19-12-20(15)13-5-2-1-3-6-13/h12-13H,1-11,18H2,(H,21,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008275
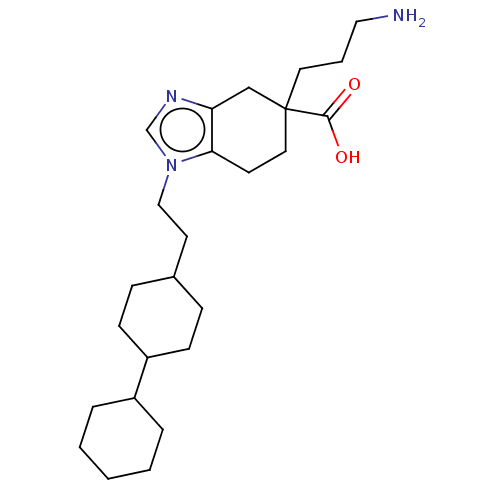
(CHEMBL3235136)Show SMILES NCCCC1(CCc2c(C1)ncn2CCC1CCC(CC1)C1CCCCC1)C(O)=O |(12.03,-18.01,;11.64,-19.5,;10.15,-19.91,;9.76,-21.4,;8.28,-21.8,;8.28,-20.26,;6.95,-19.48,;5.61,-20.25,;5.62,-21.8,;6.95,-22.56,;4.15,-22.29,;3.24,-21.04,;4.14,-19.78,;3.66,-18.32,;4.68,-17.17,;4.2,-15.71,;5.22,-14.56,;4.74,-13.1,;3.23,-12.78,;2.2,-13.93,;2.68,-15.4,;2.75,-11.32,;3.78,-10.18,;3.31,-8.72,;1.8,-8.39,;.77,-9.54,;1.25,-11.01,;9.04,-23.13,;8.26,-24.46,;10.58,-23.14,)| Show InChI InChI=1S/C25H41N3O2/c26-15-4-13-25(24(29)30)14-11-23-22(17-25)27-18-28(23)16-12-19-7-9-21(10-8-19)20-5-2-1-3-6-20/h18-21H,1-17,26H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008269

(CHEMBL3235130)Show InChI InChI=1S/C10H14N2O2S/c1-6(15)8(10(13)14)4-7-2-3-9(11)12-5-7/h2-3,5-6,8,15H,4H2,1H3,(H2,11,12)(H,13,14)/t6-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50226610
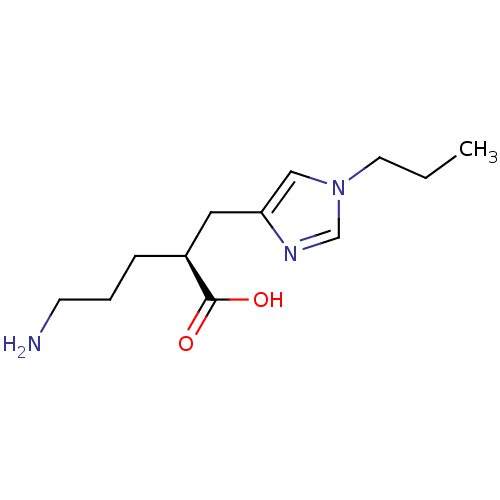
((2S)-5-AMINO-2-[(1-PROPYL-1H-IMIDAZOL-4-YL)METHYL]...)Show InChI InChI=1S/C12H21N3O2/c1-2-6-15-8-11(14-9-15)7-10(12(16)17)4-3-5-13/h8-10H,2-7,13H2,1H3,(H,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a using hippuryl-L-arginine/hippuryl-L-lysine as substrate by liquid chromatographic analysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008276
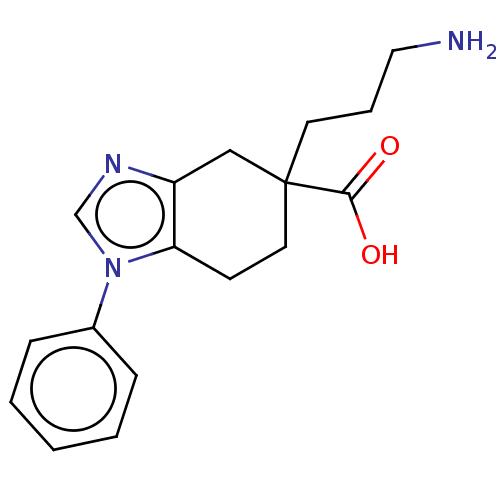
(CHEMBL3235137)Show InChI InChI=1S/C17H21N3O2/c18-10-4-8-17(16(21)22)9-7-15-14(11-17)19-12-20(15)13-5-2-1-3-6-13/h1-3,5-6,12H,4,7-11,18H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 585 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008270
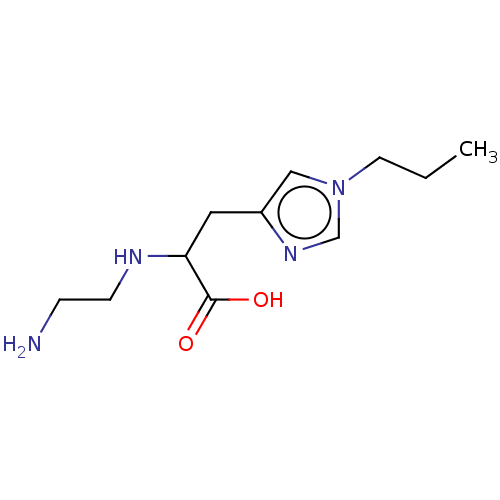
(CHEMBL3235131)Show InChI InChI=1S/C11H20N4O2/c1-2-5-15-7-9(14-8-15)6-10(11(16)17)13-4-3-12/h7-8,10,13H,2-6,12H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a using hippuryl-L-arginine/hippuryl-L-lysine as substrate by liquid chromatographic analysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50361983

(CHEMBL1939697)Show SMILES C[C@@H](N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2ncccc2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24F3N5O4/c1-19(28-36-27-25(5-3-15-35-27)29(41)38(28)22-8-10-23(39)11-9-22)37(18-21-4-2-14-34-17-21)26(40)16-20-6-12-24(13-7-20)42-30(31,32)33/h2-15,17,19,39H,16,18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008271

(CHEMBL3235132)Show InChI InChI=1S/C14H23N3O2/c1-2-8-17-10-16-11-9-14(13(18)19,5-3-7-15)6-4-12(11)17/h10H,2-9,15H2,1H3,(H,18,19)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008278

(CHEMBL3235139)Show SMILES NCCC[C@]1(CCn2c(C1)ncc2-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C17H21N3O2/c18-9-4-7-17(16(21)22)8-10-20-14(12-19-15(20)11-17)13-5-2-1-3-6-13/h1-3,5-6,12H,4,7-11,18H2,(H,21,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135933
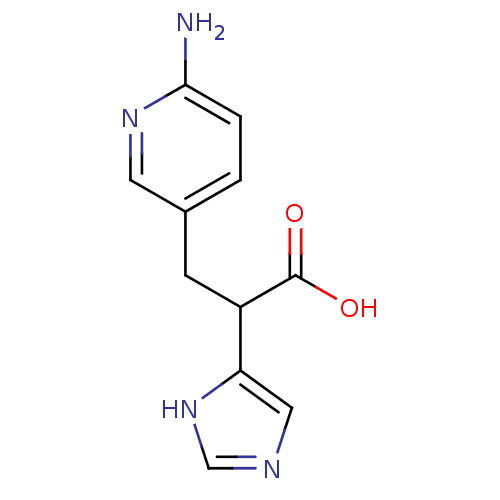
(3-(6-Amino-pyridin-3-yl)-2-(1H-imidazol-4-yl)-prop...)Show InChI InChI=1S/C11H12N4O2/c12-10-2-1-7(4-14-10)3-8(11(16)17)9-5-13-6-15-9/h1-2,4-6,8H,3H2,(H2,12,14)(H,13,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a using hippuryl-L-arginine/hippuryl-L-lysine as substrate by liquid chromatographic analysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50361983

(CHEMBL1939697)Show SMILES C[C@@H](N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2ncccc2c(=O)n1-c1ccc(O)cc1 |r| Show InChI InChI=1S/C30H24F3N5O4/c1-19(28-36-27-25(5-3-15-35-27)29(41)38(28)22-8-10-23(39)11-9-22)37(18-21-4-2-14-34-17-21)26(40)16-20-6-12-24(13-7-20)42-30(31,32)33/h2-15,17,19,39H,16,18H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 30 mins followed by 10-fold dilution a... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008277
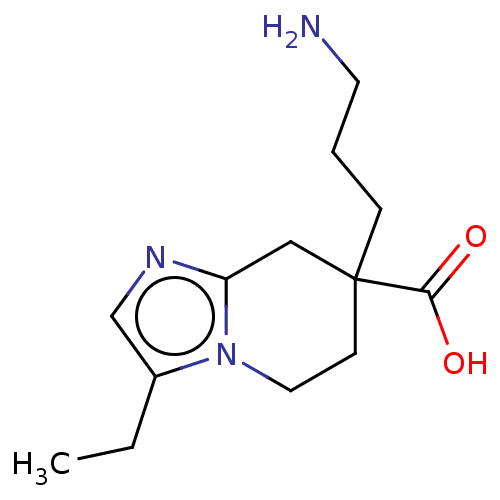
(CHEMBL3235138)Show InChI InChI=1S/C13H21N3O2/c1-2-10-9-15-11-8-13(12(17)18,4-3-6-14)5-7-16(10)11/h9H,2-8,14H2,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human TAF1a |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008274

(CHEMBL3235135)Show SMILES NCCC[C@]1(CCc2c(C1)ncn2Cc1cccc(c1)-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H27N3O2/c25-13-5-11-24(23(28)29)12-10-22-21(15-24)26-17-27(22)16-18-6-4-9-20(14-18)19-7-2-1-3-8-19/h1-4,6-9,14,17H,5,10-13,15-16,25H2,(H,28,29)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by 10-fold dilution and ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by substrate addition by... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008279

(CHEMBL3235140)Show SMILES Cl.NCCC[C@]1(CCn2c(C1)ncc2-c1cccc(c1)-c1ccc(Cl)cc1Cl)C(O)=O |r| Show InChI InChI=1S/C23H23Cl2N3O2.ClH/c24-17-5-6-18(19(25)12-17)15-3-1-4-16(11-15)20-14-27-21-13-23(22(29)30,7-2-9-26)8-10-28(20)21;/h1,3-6,11-12,14H,2,7-10,13,26H2,(H,29,30);1H/t23-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50226610

((2S)-5-AMINO-2-[(1-PROPYL-1H-IMIDAZOL-4-YL)METHYL]...)Show InChI InChI=1S/C12H21N3O2/c1-2-6-15-8-11(14-9-15)7-10(12(16)17)4-3-5-13/h8-10H,2-7,13H2,1H3,(H,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by substrate addition by... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008276

(CHEMBL3235137)Show InChI InChI=1S/C17H21N3O2/c18-10-4-8-17(16(21)22)9-7-15-14(11-17)19-12-20(15)13-5-2-1-3-6-13/h1-3,5-6,12H,4,7-11,18H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by 10-fold dilution and ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008270

(CHEMBL3235131)Show InChI InChI=1S/C11H20N4O2/c1-2-5-15-7-9(14-8-15)6-10(11(16)17)13-4-3-12/h7-8,10,13H,2-6,12H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 30 mins followed by 10-fold dilution a... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by substrate addition by... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by substrate addition by... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 90 mins followed by substrate addition... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by 10-fold dilution and ... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50008275

(CHEMBL3235136)Show SMILES NCCCC1(CCc2c(C1)ncn2CCC1CCC(CC1)C1CCCCC1)C(O)=O |(12.03,-18.01,;11.64,-19.5,;10.15,-19.91,;9.76,-21.4,;8.28,-21.8,;8.28,-20.26,;6.95,-19.48,;5.61,-20.25,;5.62,-21.8,;6.95,-22.56,;4.15,-22.29,;3.24,-21.04,;4.14,-19.78,;3.66,-18.32,;4.68,-17.17,;4.2,-15.71,;5.22,-14.56,;4.74,-13.1,;3.23,-12.78,;2.2,-13.93,;2.68,-15.4,;2.75,-11.32,;3.78,-10.18,;3.31,-8.72,;1.8,-8.39,;.77,-9.54,;1.25,-11.01,;9.04,-23.13,;8.26,-24.46,;10.58,-23.14,)| Show InChI InChI=1S/C25H41N3O2/c26-15-4-13-25(24(29)30)14-11-23-22(17-25)27-18-28(23)16-12-19-7-9-21(10-8-19)20-5-2-1-3-6-20/h18-21H,1-17,26H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of TAF1a in human plasma assessed as clot lysis |
Bioorg Med Chem 22: 2261-8 (2014)
Article DOI: 10.1016/j.bmc.2014.02.010
BindingDB Entry DOI: 10.7270/Q2DZ09TG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50004704

((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...)Show SMILES COc1ccc(cc1)[C@@H]1Sc2ccccc2N(CCN(C)C)C(=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 90 mins followed by substrate addition by... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 30 mins followed by substrate addition... |
Drug Metab Dispos 40: 1429-40 (2012)
Article DOI: 10.1124/dmd.112.045708
BindingDB Entry DOI: 10.7270/Q23N254Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data