 Found 632 hits with Last Name = 'brown' and Initial = 'ds'
Found 632 hits with Last Name = 'brown' and Initial = 'ds' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000293
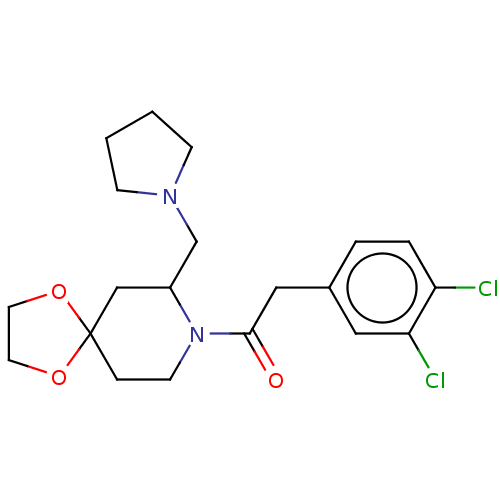
(2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...)Show SMILES Clc1ccc(CC(=O)N2CCC3(CC2CN2CCCC2)OCCO3)cc1Cl Show InChI InChI=1S/C20H26Cl2N2O3/c21-17-4-3-15(11-18(17)22)12-19(25)24-8-5-20(26-9-10-27-20)13-16(24)14-23-6-1-2-7-23/h3-4,11,16H,1-2,5-10,12-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000288
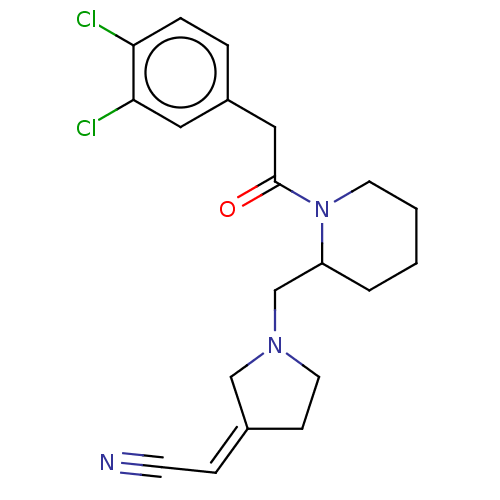
((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...)Show SMILES Clc1ccc(CC(=O)N2CCCCC2CN2CC\C(C2)=C\C#N)cc1Cl Show InChI InChI=1S/C20H23Cl2N3O/c21-18-5-4-16(11-19(18)22)12-20(26)25-9-2-1-3-17(25)14-24-10-7-15(13-24)6-8-23/h4-6,11,17H,1-3,7,9-10,12-14H2/b15-6- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000271
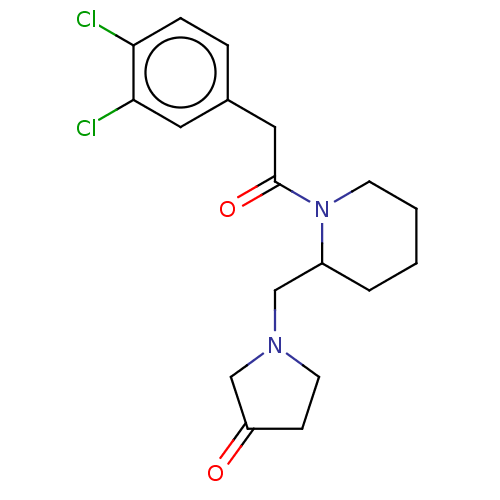
(1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2-...)Show InChI InChI=1S/C18H22Cl2N2O2/c19-16-5-4-13(9-17(16)20)10-18(24)22-7-2-1-3-14(22)11-21-8-6-15(23)12-21/h4-5,9,14H,1-3,6-8,10-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000260
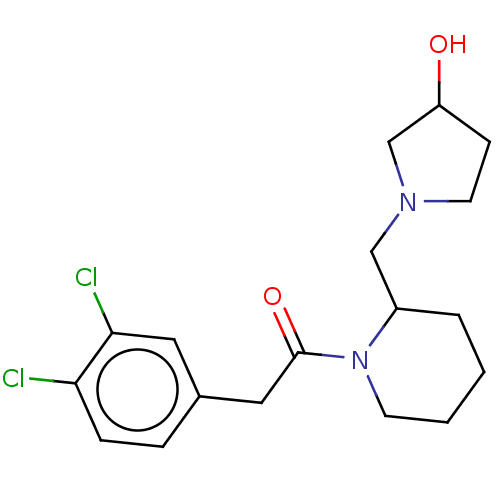
(2-(3,4-Dichloro-phenyl)-1-[2-(3-hydroxy-pyrrolidin...)Show InChI InChI=1S/C18H24Cl2N2O2/c19-16-5-4-13(9-17(16)20)10-18(24)22-7-2-1-3-14(22)11-21-8-6-15(23)12-21/h4-5,9,14-15,23H,1-3,6-8,10-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402421
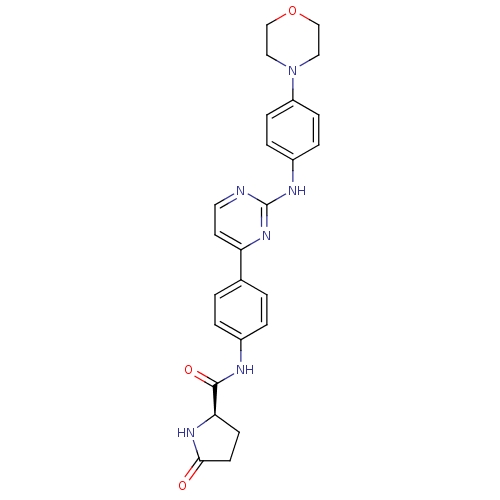
(CHEMBL2208035)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C25H26N6O3/c32-23-10-9-22(29-23)24(33)27-18-3-1-17(2-4-18)21-11-12-26-25(30-21)28-19-5-7-20(8-6-19)31-13-15-34-16-14-31/h1-8,11-12,22H,9-10,13-16H2,(H,27,33)(H,29,32)(H,26,28,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50272192
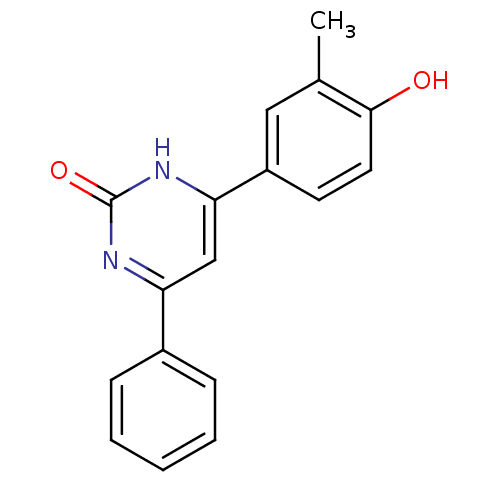
(4-(4-hydroxy-3-methylphenyl)-6-phenylpyrimidin-2(1...)Show InChI InChI=1S/C17H14N2O2/c1-11-9-13(7-8-16(11)20)15-10-14(18-17(21)19-15)12-5-3-2-4-6-12/h2-10,20H,1H3,(H,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402413

(CHEMBL2208032)Show SMILES C[C@@H](N)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O2/c1-16(24)22(30)26-18-4-2-17(3-5-18)21-10-11-25-23(28-21)27-19-6-8-20(9-7-19)29-12-14-31-15-13-29/h2-11,16H,12-15,24H2,1H3,(H,26,30)(H,25,27,28)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000284

(2-(3,4-Dichloro-phenyl)-1-(2-methyl-6-pyrrolidin-1...)Show SMILES C[C@H]1CCC[C@@H](CN2CCCC2)N1C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H26Cl2N2O/c1-14-5-4-6-16(13-22-9-2-3-10-22)23(14)19(24)12-15-7-8-17(20)18(21)11-15/h7-8,11,14,16H,2-6,9-10,12-13H2,1H3/t14-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402409
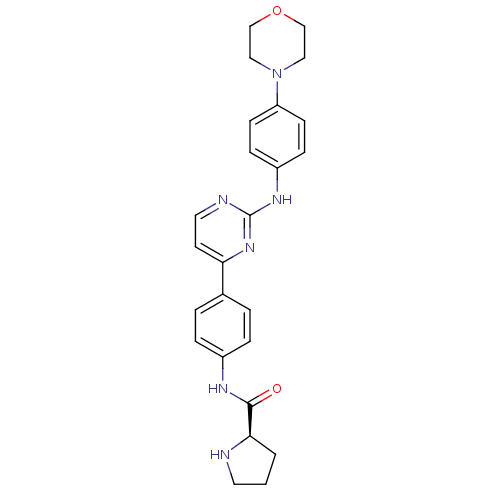
(CHEMBL2208034)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCCN1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(23-2-1-12-26-23)28-19-5-3-18(4-6-19)22-11-13-27-25(30-22)29-20-7-9-21(10-8-20)31-14-16-33-17-15-31/h3-11,13,23,26H,1-2,12,14-17H2,(H,28,32)(H,27,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402412
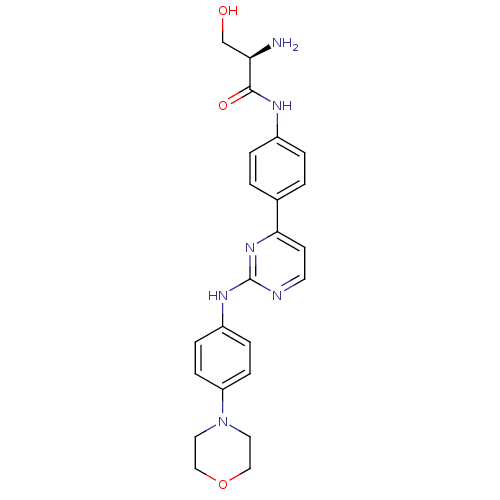
(CHEMBL2208033)Show SMILES N[C@H](CO)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O3/c24-20(15-30)22(31)26-17-3-1-16(2-4-17)21-9-10-25-23(28-21)27-18-5-7-19(8-6-18)29-11-13-32-14-12-29/h1-10,20,30H,11-15,24H2,(H,26,31)(H,25,27,28)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385075

(CHEMBL2035626)Show SMILES NCc1ccc(c(Cl)c1)-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C17H11BrClN3O2/c18-9-2-4-13-11(6-9)14-15(24-13)17(23)22-16(21-14)10-3-1-8(7-20)5-12(10)19/h1-6H,7,20H2,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385078
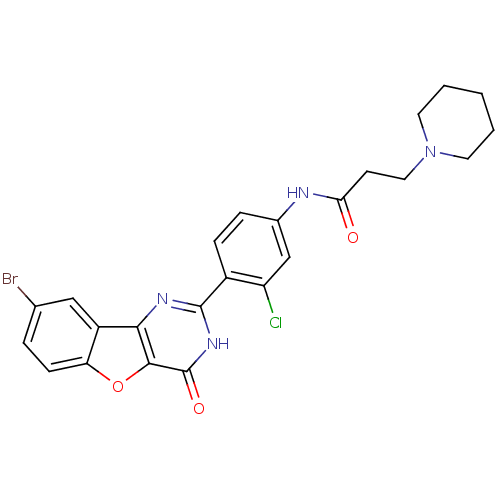
(CHEMBL2035629)Show SMILES Clc1cc(NC(=O)CCN2CCCCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C24H22BrClN4O3/c25-14-4-7-19-17(12-14)21-22(33-19)24(32)29-23(28-21)16-6-5-15(13-18(16)26)27-20(31)8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385084

(CHEMBL2035636)Show SMILES CN1C[C@@H]2CC[C@H]1CN2Cc1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C18H19BrN4O2/c1-22-7-12-4-3-11(22)8-23(12)9-15-20-16-13-6-10(19)2-5-14(13)25-17(16)18(24)21-15/h2,5-6,11-12H,3-4,7-9H2,1H3,(H,20,21,24)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383714
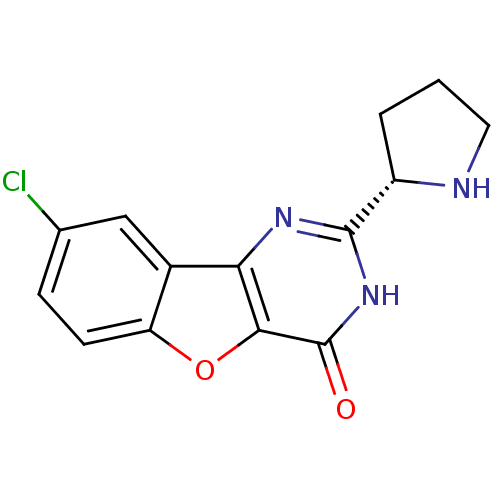
(CHEMBL2030402)Show SMILES Clc1ccc2oc3c(nc([nH]c3=O)[C@@H]3CCCN3)c2c1 |r| Show InChI InChI=1S/C14H12ClN3O2/c15-7-3-4-10-8(6-7)11-12(20-10)14(19)18-13(17-11)9-2-1-5-16-9/h3-4,6,9,16H,1-2,5H2,(H,17,18,19)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000292

(2-(3,4-Dichloro-phenyl)-1-[2-(2,5-dihydro-pyrrol-1...)Show SMILES Clc1ccc(CC(=O)N2CCCCC2CN2CC=CC2)cc1Cl |c:18| Show InChI InChI=1S/C18H22Cl2N2O/c19-16-7-6-14(11-17(16)20)12-18(23)22-10-2-1-5-15(22)13-21-8-3-4-9-21/h3-4,6-7,11,15H,1-2,5,8-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50310998

(CHEMBL1077458 | N-(4-(2-(4-morpholinophenylamino)p...)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-18-4-2-17(3-5-18)21-10-11-23-22(26-21)25-19-6-8-20(9-7-19)27-12-14-29-15-13-27/h2-11H,12-15H2,1H3,(H,24,28)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383727
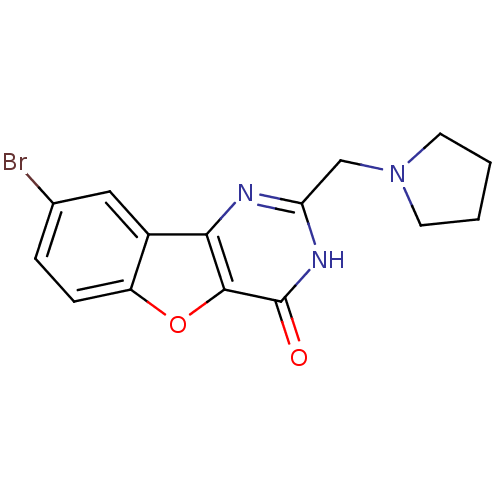
(CHEMBL2030389)Show InChI InChI=1S/C15H14BrN3O2/c16-9-3-4-11-10(7-9)13-14(21-11)15(20)18-12(17-13)8-19-5-1-2-6-19/h3-4,7H,1-2,5-6,8H2,(H,17,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383736
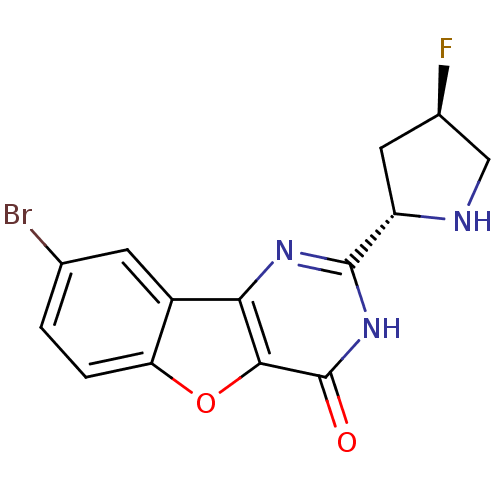
(CHEMBL2030400)Show SMILES F[C@H]1CN[C@@H](C1)c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C14H11BrFN3O2/c15-6-1-2-10-8(3-6)11-12(21-10)14(20)19-13(18-11)9-4-7(16)5-17-9/h1-3,7,9,17H,4-5H2,(H,18,19,20)/t7-,9+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383711
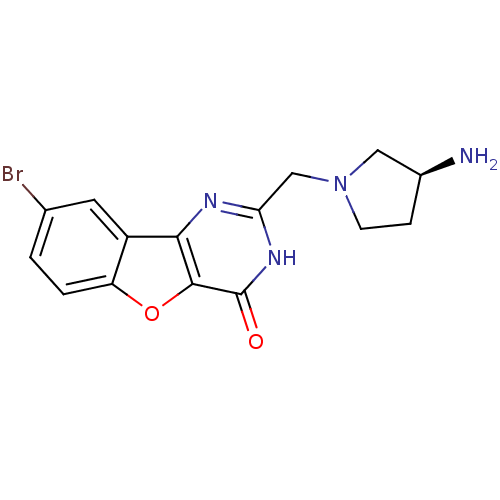
(CHEMBL2030387)Show SMILES N[C@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by chemiluminescence assay |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383740
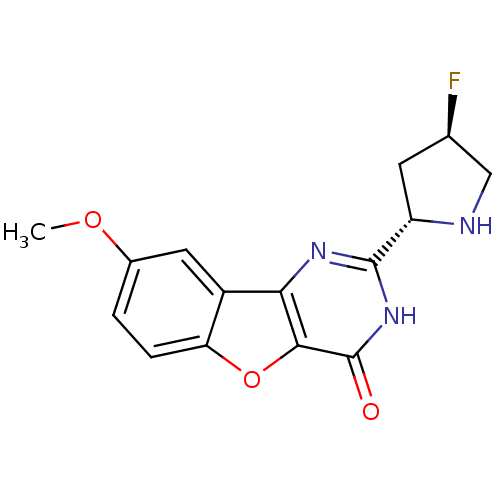
(CHEMBL2030405)Show SMILES COc1ccc2oc3c(nc([nH]c3=O)[C@@H]3C[C@@H](F)CN3)c2c1 |r| Show InChI InChI=1S/C15H14FN3O3/c1-21-8-2-3-11-9(5-8)12-13(22-11)15(20)19-14(18-12)10-4-7(16)6-17-10/h2-3,5,7,10,17H,4,6H2,1H3,(H,18,19,20)/t7-,10+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383711

(CHEMBL2030387)Show SMILES N[C@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383739
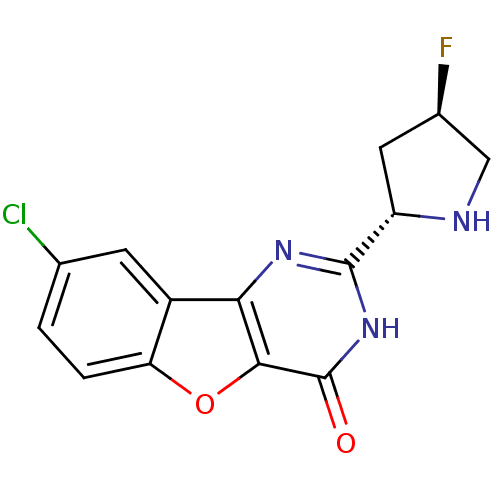
(CHEMBL2030404)Show SMILES F[C@H]1CN[C@@H](C1)c1nc2c3cc(Cl)ccc3oc2c(=O)[nH]1 |r| Show InChI InChI=1S/C14H11ClFN3O2/c15-6-1-2-10-8(3-6)11-12(21-10)14(20)19-13(18-11)9-4-7(16)5-17-9/h1-3,7,9,17H,4-5H2,(H,18,19,20)/t7-,9+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50383725
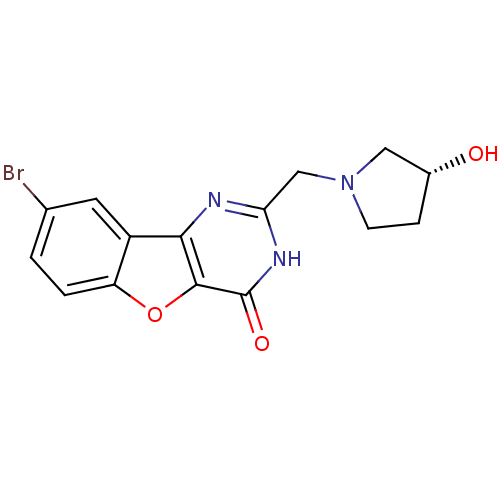
(CHEMBL2030386)Show SMILES O[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H14BrN3O3/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)18-12(17-13)7-19-4-3-9(20)6-19/h1-2,5,9,20H,3-4,6-7H2,(H,17,18,21)/t9-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM3 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385077

(CHEMBL2035628)Show SMILES Clc1cc(CNC2CCNCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C22H20BrClN4O2/c23-13-2-4-18-16(10-13)19-20(30-18)22(29)28-21(27-19)15-3-1-12(9-17(15)24)11-26-14-5-7-25-8-6-14/h1-4,9-10,14,25-26H,5-8,11H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385076

(CHEMBL2035627)Show SMILES Clc1cc(ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C24H22BrClN4O3/c25-15-5-7-19-17(13-15)20-21(33-19)24(32)29-22(28-20)16-6-4-14(12-18(16)26)23(31)27-8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402416

(CHEMBL2208025)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(c2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-18-7-5-17(6-8-18)21-9-10-23-22(26-21)25-19-3-2-4-20(15-19)27-11-13-29-14-12-27/h2-10,15H,11-14H2,1H3,(H,24,28)(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402424

(CHEMBL2208027)Show SMILES CC(=O)Nc1ccc(cc1)-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1F Show InChI InChI=1S/C22H22FN5O2/c1-15(29)25-17-4-2-16(3-5-17)21-20(23)14-24-22(27-21)26-18-6-8-19(9-7-18)28-10-12-30-13-11-28/h2-9,14H,10-13H2,1H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50384389
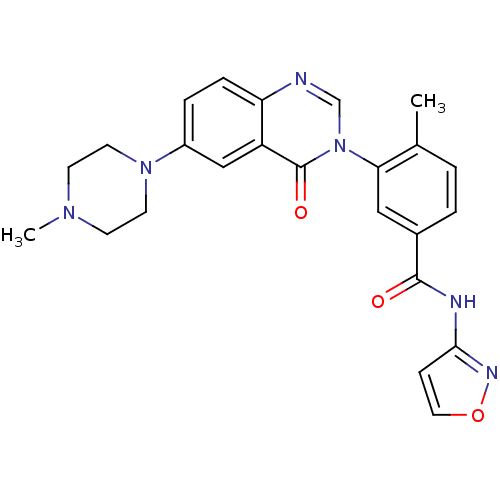
(CHEMBL2031461)Show SMILES CN1CCN(CC1)c1ccc2ncn(-c3cc(ccc3C)C(=O)Nc3ccon3)c(=O)c2c1 Show InChI InChI=1S/C24H24N6O3/c1-16-3-4-17(23(31)26-22-7-12-33-27-22)13-21(16)30-15-25-20-6-5-18(14-19(20)24(30)32)29-10-8-28(2)9-11-29/h3-7,12-15H,8-11H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.68 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha-mediated TNFalpha secretion in LPS-stimulated human whole blood assessed as IC50 equals to free drug concentration at human wh... |
Bioorg Med Chem Lett 22: 3879-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.116
BindingDB Entry DOI: 10.7270/Q2VH5PWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000274

((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...)Show SMILES COC(=O)\C=C1\CCN(CC2CCCCN2C(=O)Cc2ccc(Cl)c(Cl)c2)C1 Show InChI InChI=1S/C21H26Cl2N2O3/c1-28-21(27)12-16-7-9-24(13-16)14-17-4-2-3-8-25(17)20(26)11-15-5-6-18(22)19(23)10-15/h5-6,10,12,17H,2-4,7-9,11,13-14H2,1H3/b16-12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383730
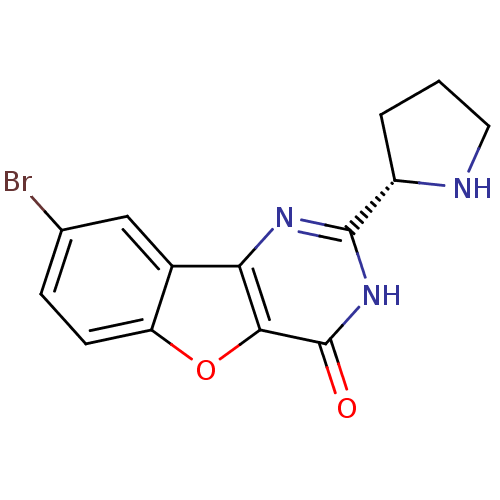
(CHEMBL2030393)Show SMILES Brc1ccc2oc3c(nc([nH]c3=O)[C@@H]3CCCN3)c2c1 |r| Show InChI InChI=1S/C14H12BrN3O2/c15-7-3-4-10-8(6-7)11-12(20-10)14(19)18-13(17-11)9-2-1-5-16-9/h3-4,6,9,16H,1-2,5H2,(H,17,18,19)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383725

(CHEMBL2030386)Show SMILES O[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H14BrN3O3/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)18-12(17-13)7-19-4-3-9(20)6-19/h1-2,5,9,20H,3-4,6-7H2,(H,17,18,21)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50385078

(CHEMBL2035629)Show SMILES Clc1cc(NC(=O)CCN2CCCCC2)ccc1-c1nc2c3cc(Br)ccc3oc2c(=O)[nH]1 Show InChI InChI=1S/C24H22BrClN4O3/c25-14-4-7-19-17(12-14)21-22(33-19)24(32)29-23(28-21)16-6-5-15(13-18(16)26)27-20(31)8-11-30-9-2-1-3-10-30/h4-7,12-13H,1-3,8-11H2,(H,27,31)(H,28,29,32) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM3 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000286
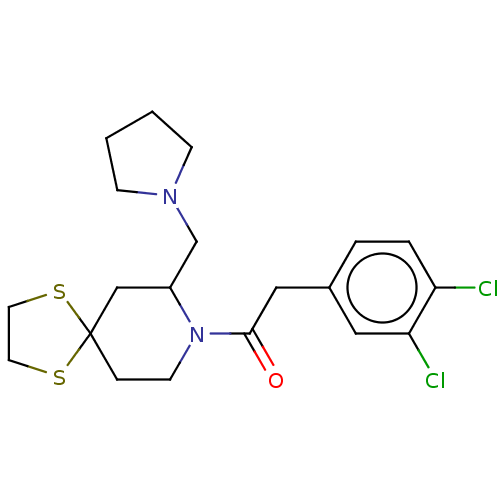
(2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...)Show SMILES Clc1ccc(CC(=O)N2CCC3(CC2CN2CCCC2)SCCS3)cc1Cl Show InChI InChI=1S/C20H26Cl2N2OS2/c21-17-4-3-15(11-18(17)22)12-19(25)24-8-5-20(26-9-10-27-20)13-16(24)14-23-6-1-2-7-23/h3-4,11,16H,1-2,5-10,12-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402415
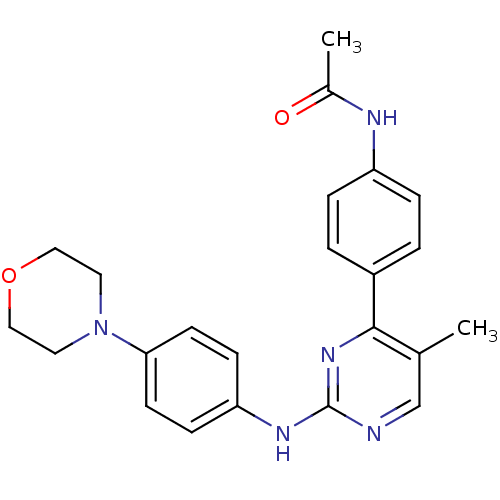
(CHEMBL2208028)Show SMILES CC(=O)Nc1ccc(cc1)-c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1C Show InChI InChI=1S/C23H25N5O2/c1-16-15-24-23(27-22(16)18-3-5-19(6-4-18)25-17(2)29)26-20-7-9-21(10-8-20)28-11-13-30-14-12-28/h3-10,15H,11-14H2,1-2H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402411

(CHEMBL2207759)Show SMILES CN1CCC[C@@H]1C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C26H30N6O2/c1-31-14-2-3-24(31)25(33)28-20-6-4-19(5-7-20)23-12-13-27-26(30-23)29-21-8-10-22(11-9-21)32-15-17-34-18-16-32/h4-13,24H,2-3,14-18H2,1H3,(H,28,33)(H,27,29,30)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383725

(CHEMBL2030386)Show SMILES O[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H14BrN3O3/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)18-12(17-13)7-19-4-3-9(20)6-19/h1-2,5,9,20H,3-4,6-7H2,(H,17,18,21)/t9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402420

(CHEMBL2207758)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCNC1 |r| Show InChI InChI=1S/C25H28N6O2/c32-24(19-9-11-26-17-19)28-20-3-1-18(2-4-20)23-10-12-27-25(30-23)29-21-5-7-22(8-6-21)31-13-15-33-16-14-31/h1-8,10,12,19,26H,9,11,13-17H2,(H,28,32)(H,27,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Delta-type/Kappa-type/Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000091
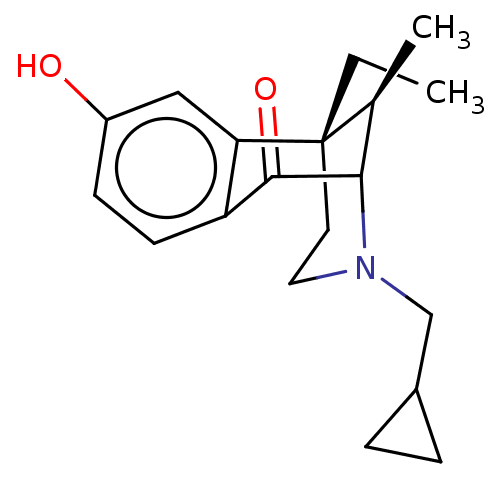
((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...)Show SMILES CC[C@]12CCN(CC3CC3)C([C@@H]1C)C(=O)c1ccc(O)cc21 |TLB:6:5:13.21.15:11,14:13:11:5.3.4,THB:20:21:11:5.3.4| Show InChI InChI=1S/C19H25NO2/c1-3-19-8-9-20(11-13-4-5-13)17(12(19)2)18(22)15-7-6-14(21)10-16(15)19/h6-7,10,12-13,17,21H,3-5,8-9,11H2,1-2H3/t12-,17?,19-/m0/s1 | PDB
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against opioid receptor in Guinea pig ileum |
J Med Chem 35: 48-56 (1992)
BindingDB Entry DOI: 10.7270/Q2J105DC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383726
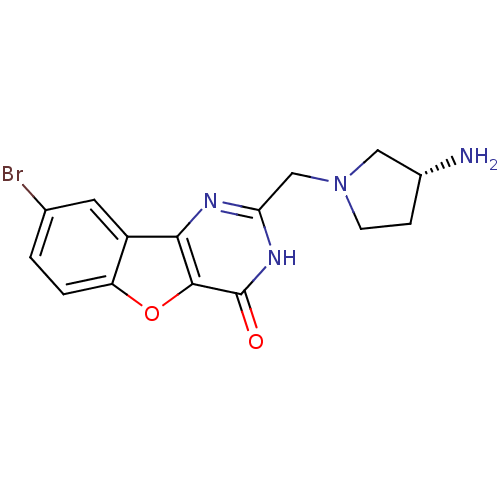
(CHEMBL2030388)Show SMILES N[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by chemiluminescence assay |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383726

(CHEMBL2030388)Show SMILES N[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50383726

(CHEMBL2030388)Show SMILES N[C@@H]1CCN(Cc2nc3c4cc(Br)ccc4oc3c(=O)[nH]2)C1 |r| Show InChI InChI=1S/C15H15BrN4O2/c16-8-1-2-11-10(5-8)13-14(22-11)15(21)19-12(18-13)7-20-4-3-9(17)6-20/h1-2,5,9H,3-4,6-7,17H2,(H,18,19,21)/t9-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM3 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383738
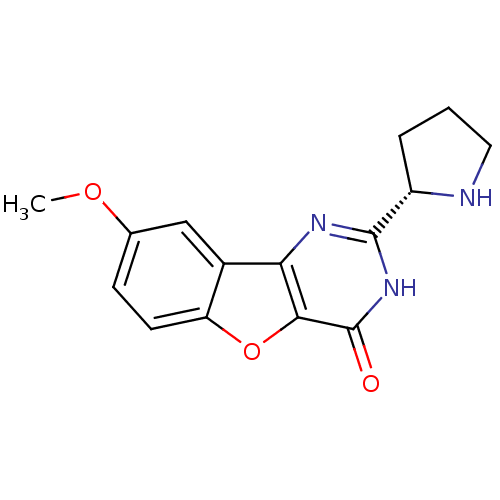
(CHEMBL2030403)Show SMILES COc1ccc2oc3c(nc([nH]c3=O)[C@@H]3CCCN3)c2c1 |r| Show InChI InChI=1S/C15H15N3O3/c1-20-8-4-5-11-9(7-8)12-13(21-11)15(19)18-14(17-12)10-3-2-6-16-10/h4-5,7,10,16H,2-3,6H2,1H3,(H,17,18,19)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50383733
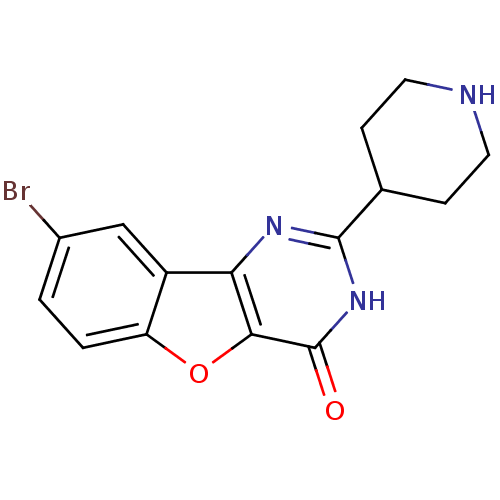
(CHEMBL2030396)Show InChI InChI=1S/C15H14BrN3O2/c16-9-1-2-11-10(7-9)12-13(21-11)15(20)19-14(18-12)8-3-5-17-6-4-8/h1-2,7-8,17H,3-6H2,(H,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000297
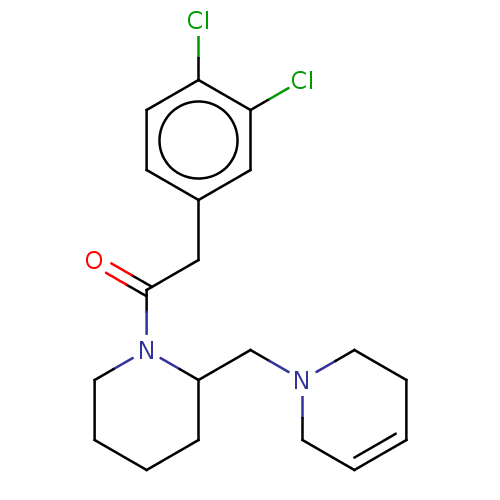
(2-(3,4-Dichloro-phenyl)-1-[2-(3,6-dihydro-2H-pyrid...)Show SMILES Clc1ccc(CC(=O)N2CCCCC2CN2CCC=CC2)cc1Cl |c:19| Show InChI InChI=1S/C19H24Cl2N2O/c20-17-8-7-15(12-18(17)21)13-19(24)23-11-5-2-6-16(23)14-22-9-3-1-4-10-22/h1,3,7-8,12,16H,2,4-6,9-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation |
J Med Chem 35: 490-501 (1992)
BindingDB Entry DOI: 10.7270/Q2TQ60GV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50290631

((6,7-Dimethoxy-quinazolin-4-yl)-isoquinolin-4-yl-a...)Show InChI InChI=1S/C19H16N4O2/c1-24-17-7-14-15(8-18(17)25-2)21-11-22-19(14)23-16-10-20-9-12-5-3-4-6-13(12)16/h3-11H,1-2H3,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of Epidermal growth factor receptor from A431 vulval squamous carcinoma cells |
Bioorg Med Chem Lett 7: 2723-2728 (1997)
Article DOI: 10.1016/S0960-894X(97)10059-2
BindingDB Entry DOI: 10.7270/Q2TD9XCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402417
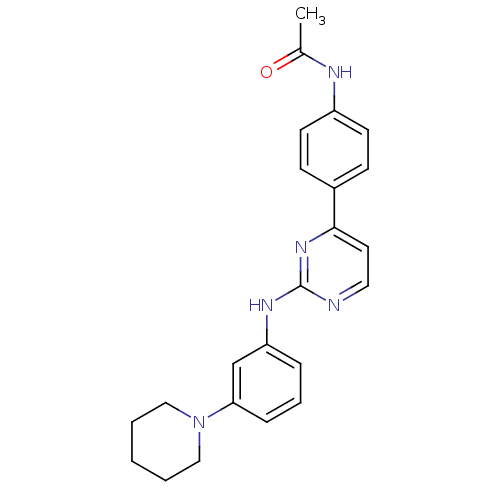
(CHEMBL2208024)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(c2)N2CCCCC2)n1 Show InChI InChI=1S/C23H25N5O/c1-17(29)25-19-10-8-18(9-11-19)22-12-13-24-23(27-22)26-20-6-5-7-21(16-20)28-14-3-2-4-15-28/h5-13,16H,2-4,14-15H2,1H3,(H,25,29)(H,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50384389

(CHEMBL2031461)Show SMILES CN1CCN(CC1)c1ccc2ncn(-c3cc(ccc3C)C(=O)Nc3ccon3)c(=O)c2c1 Show InChI InChI=1S/C24H24N6O3/c1-16-3-4-17(23(31)26-22-7-12-33-27-22)13-21(16)30-15-25-20-6-5-18(14-19(20)24(30)32)29-10-8-28(2)9-11-29/h3-7,12-15H,8-11H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.61 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha kinase |
Bioorg Med Chem Lett 22: 3879-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.116
BindingDB Entry DOI: 10.7270/Q2VH5PWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50384389

(CHEMBL2031461)Show SMILES CN1CCN(CC1)c1ccc2ncn(-c3cc(ccc3C)C(=O)Nc3ccon3)c(=O)c2c1 Show InChI InChI=1S/C24H24N6O3/c1-16-3-4-17(23(31)26-22-7-12-33-27-22)13-21(16)30-15-25-20-6-5-18(14-19(20)24(30)32)29-10-8-28(2)9-11-29/h3-7,12-15H,8-11H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha kinase |
Bioorg Med Chem Lett 22: 3879-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.116
BindingDB Entry DOI: 10.7270/Q2VH5PWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402427
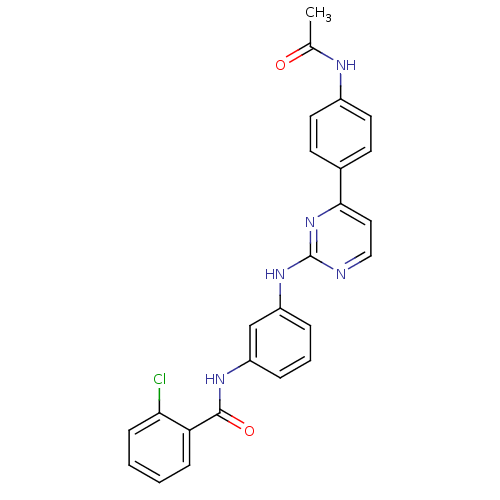
(CHEMBL2207766)Show SMILES CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2cccc(NC(=O)c3ccccc3Cl)c2)n1 Show InChI InChI=1S/C25H20ClN5O2/c1-16(32)28-18-11-9-17(10-12-18)23-13-14-27-25(31-23)30-20-6-4-5-19(15-20)29-24(33)21-7-2-3-8-22(21)26/h2-15H,1H3,(H,28,32)(H,29,33)(H,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50385079

(CHEMBL2035630)Show SMILES Brc1ccc2oc3c(nc([nH]c3=O)-c3ccc(NCC4CCNCC4)cc3)c2c1 Show InChI InChI=1S/C22H21BrN4O2/c23-15-3-6-18-17(11-15)19-20(29-18)22(28)27-21(26-19)14-1-4-16(5-2-14)25-12-13-7-9-24-10-8-13/h1-6,11,13,24-25H,7-10,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PIM1 expressed in Escherichia coli using AKRRRLSA as substrate after 1 to 2 hrs by luciferasse-luciferin-co... |
Bioorg Med Chem Lett 22: 3732-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.025
BindingDB Entry DOI: 10.7270/Q2X34ZHV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data