

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305346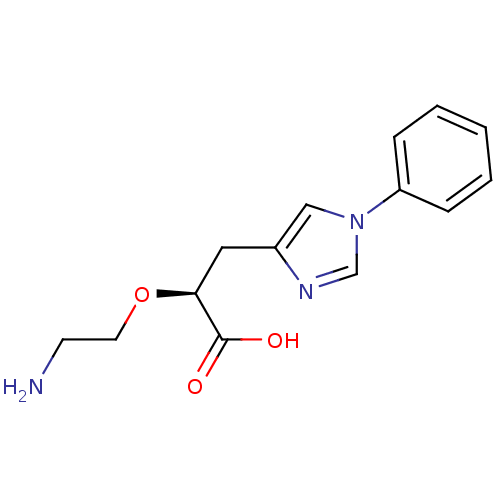 ((S)-2-(2-aminoethoxy)-3-(1-phenyl-1H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305347 ((S)-2-(2-aminoethoxy)-3-(1-(4-tert-butylphenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305351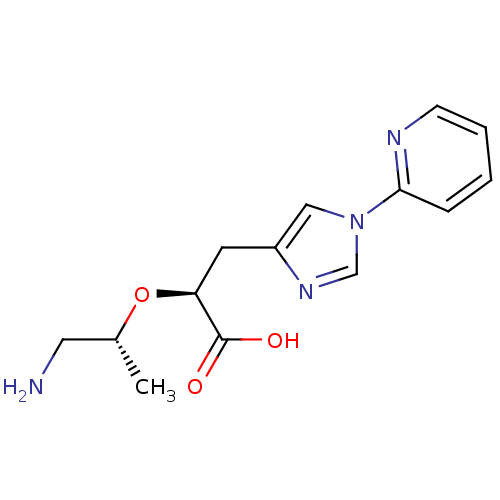 ((S)-2-((R)-1-aminopropan-2-yloxy)-3-(1-(pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305345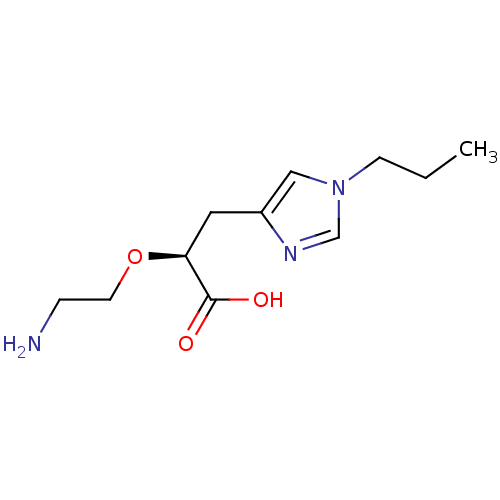 ((S)-2-(2-aminoethoxy)-3-(1-propyl-1H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305352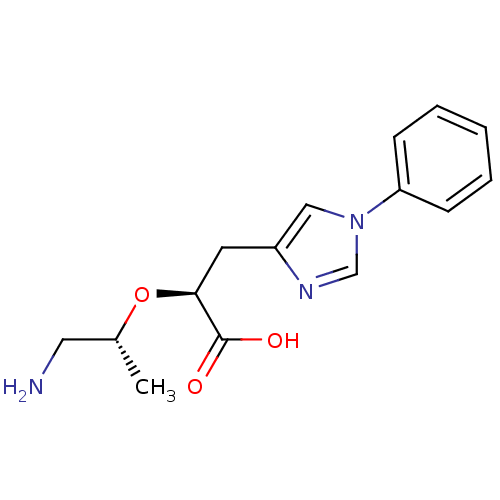 ((S)-2-((R)-1-aminopropan-2-yloxy)-3-(1-phenyl-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305348 ((S)-2-(2-aminoethoxy)-3-(1-(2-cyclohexylethyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305349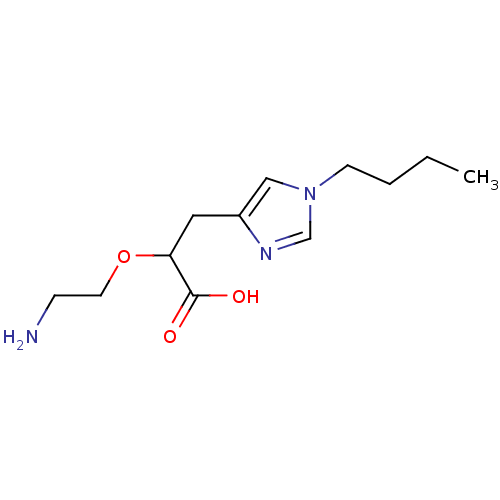 ((+/-)-2-(2-aminoethoxy)-3-(1-butyl-1H-imidazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50226610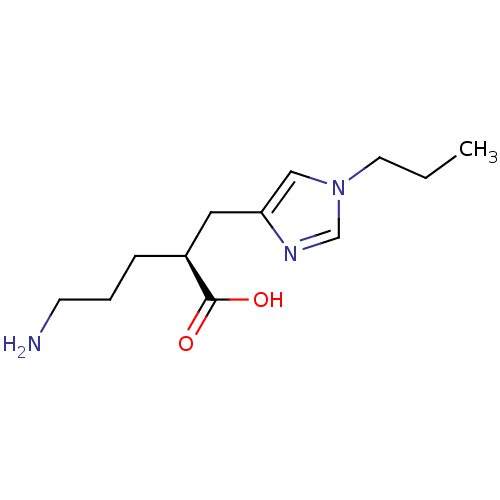 ((2S)-5-AMINO-2-[(1-PROPYL-1H-IMIDAZOL-4-YL)METHYL]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305346 ((S)-2-(2-aminoethoxy)-3-(1-phenyl-1H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305348 ((S)-2-(2-aminoethoxy)-3-(1-(2-cyclohexylethyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305347 ((S)-2-(2-aminoethoxy)-3-(1-(4-tert-butylphenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305350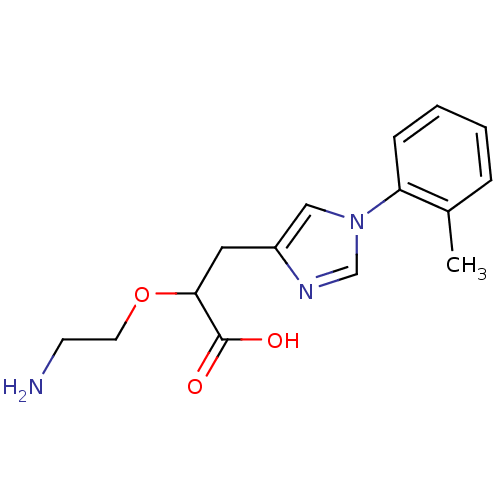 ((+/-)-2-(2-aminoethoxy)-3-(1-o-tolyl-1H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50226606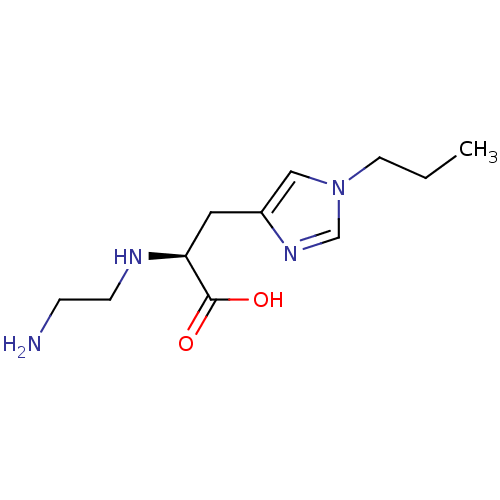 ((S)-2-(2-aminoethylamino)-3-(1-propyl-1H-imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50226610 ((2S)-5-AMINO-2-[(1-PROPYL-1H-IMIDAZOL-4-YL)METHYL]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305345 ((S)-2-(2-aminoethoxy)-3-(1-propyl-1H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305352 ((S)-2-((R)-1-aminopropan-2-yloxy)-3-(1-phenyl-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305351 ((S)-2-((R)-1-aminopropan-2-yloxy)-3-(1-(pyridin-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305349 ((+/-)-2-(2-aminoethoxy)-3-(1-butyl-1H-imidazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305353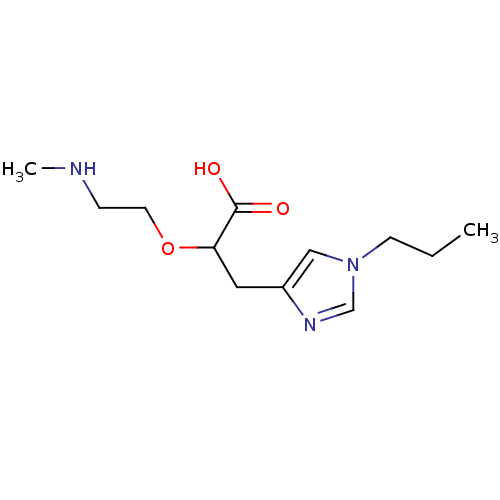 ((+/-)-2-(2-(methylamino)ethoxy)-3-(1-propyl-1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50305354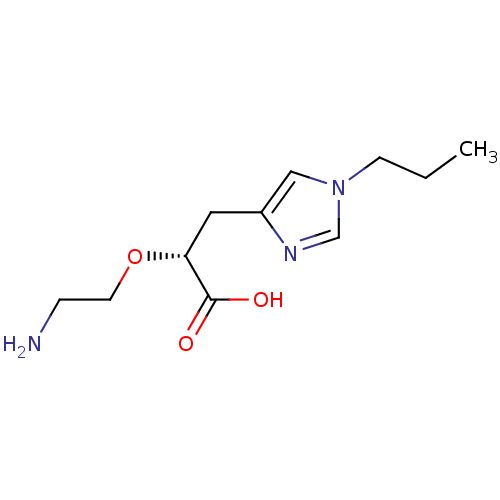 ((R)-2-(2-aminoethoxy)-3-(1-propyl-1H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of active form of human recombinant TAFI assessed as substrate turnover every 15 seconds for 30 mins | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50226606 ((S)-2-(2-aminoethylamino)-3-(1-propyl-1H-imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305350 ((+/-)-2-(2-aminoethoxy)-3-(1-o-tolyl-1H-imidazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305353 ((+/-)-2-(2-(methylamino)ethoxy)-3-(1-propyl-1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50305354 ((R)-2-(2-aminoethoxy)-3-(1-propyl-1H-imidazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human pancreatic carboxypeptidase B | Bioorg Med Chem Lett 20: 92-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.029 BindingDB Entry DOI: 10.7270/Q2J1038R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033236 (8,9-Dichloro-2-methyl-5-[4-(2-methyl-imidazo[4,5-c...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033242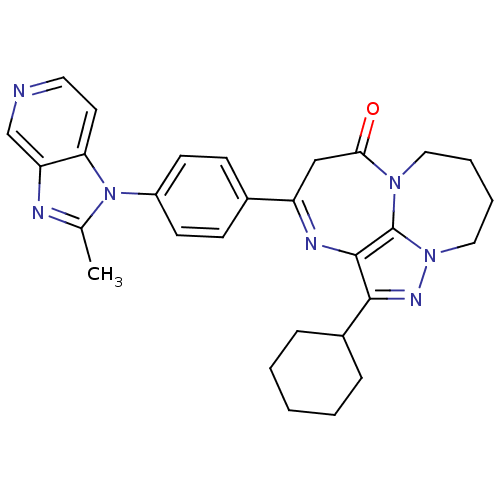 (3-Cyclohexyl-6-[4-(2-methyl-imidazo[4,5-c]pyridin-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033257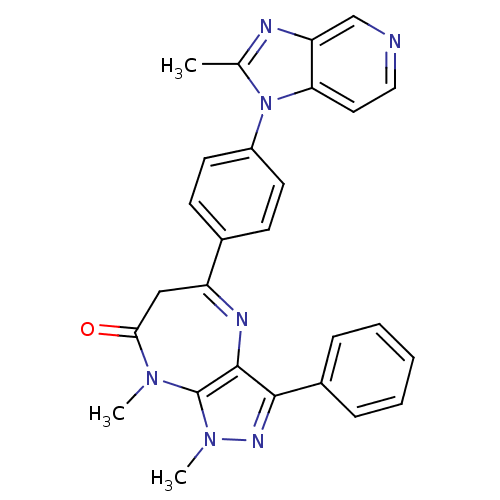 (1,8-Dimethyl-5-[4-(2-methyl-imidazo[4,5-c]pyridin-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033240 (1-Methyl-5-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033238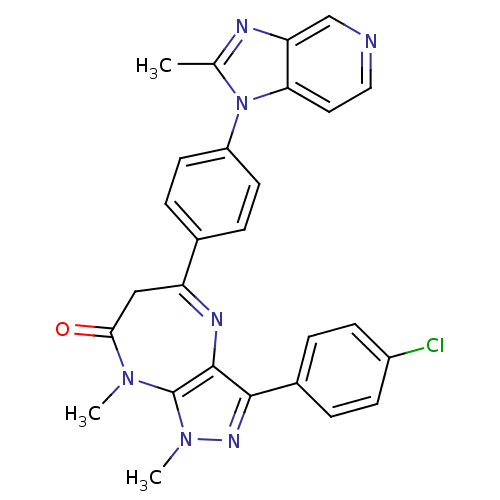 (3-(4-Chloro-phenyl)-1,8-dimethyl-5-[4-(2-methyl-im...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033264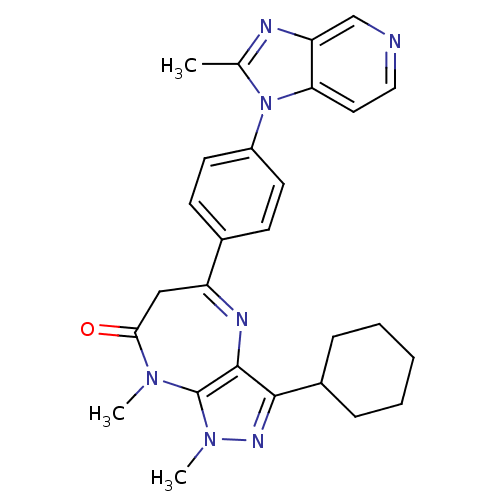 (3-Cyclohexyl-1,8-dimethyl-5-[4-(2-methyl-imidazo[4...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033260 (3-Isobutoxymethyl-1,8-dimethyl-5-[4-(2-methyl-imid...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033234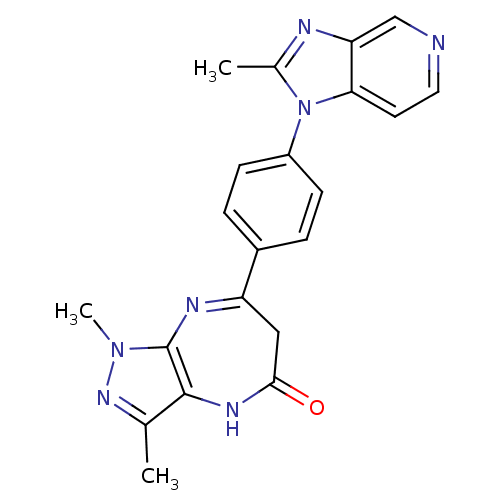 (1,3-Dimethyl-7-[4-(2-methyl-imidazo[4,5-c]pyridin-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033233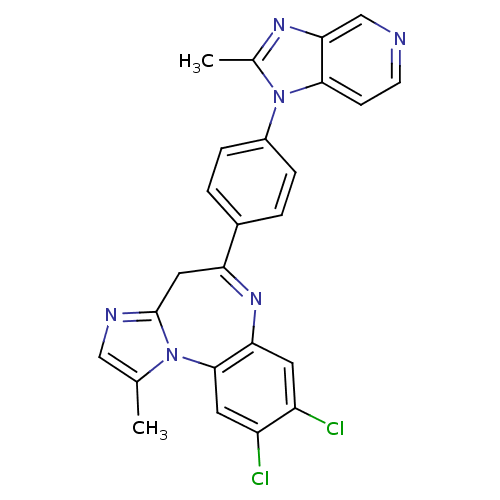 (8,9-Dichloro-1-methyl-5-[4-(2-methyl-imidazo[4,5-c...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033229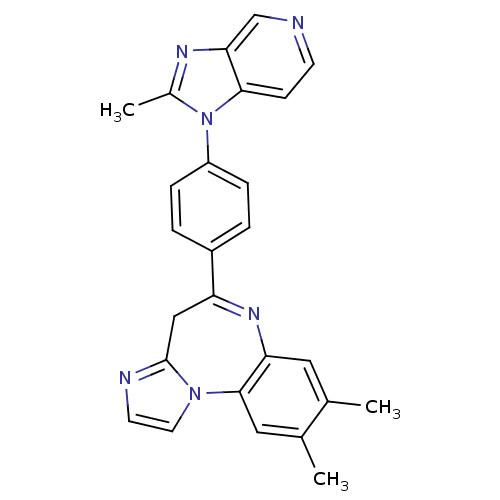 (8,9-Dimethyl-5-[4-(2-methyl-imidazo[4,5-c]pyridin-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033249 (5,7,9-Trimethyl-2-[4-(2-methyl-imidazo[4,5-c]pyrid...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033245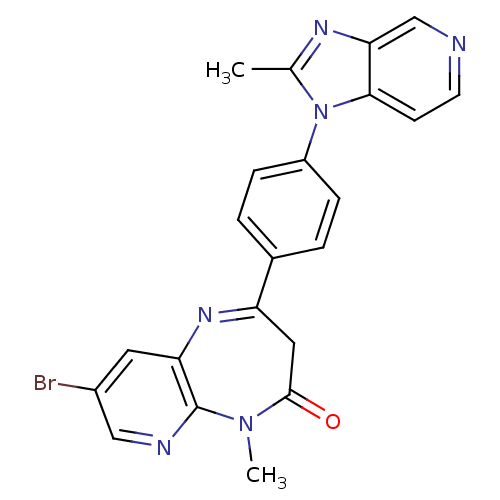 (8-Bromo-5-methyl-2-[4-(2-methyl-imidazo[4,5-c]pyri...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033255 (1,8-Dimethyl-5-[4-(2-methyl-imidazo[4,5-c]pyridin-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033232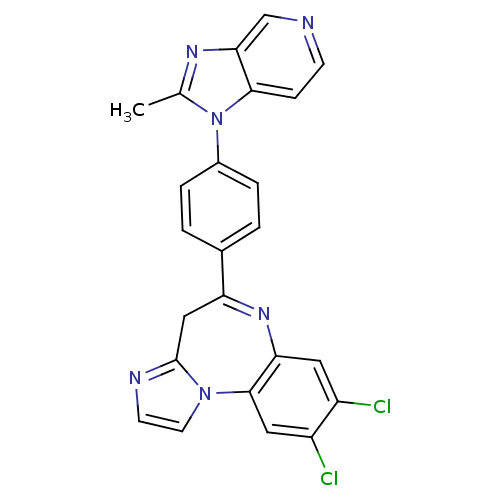 (8,9-Dichloro-5-[4-(2-methyl-imidazo[4,5-c]pyridin-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033263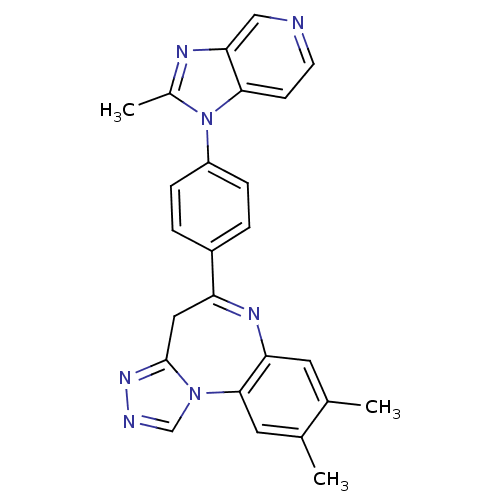 (8,9-Dimethyl-5-[4-(2-methyl-imidazo[4,5-c]pyridin-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033231 (1,8,9-Trimethyl-5-[4-(2-methyl-imidazo[4,5-c]pyrid...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033251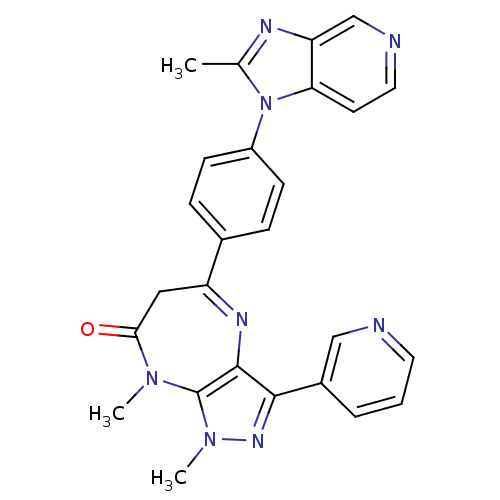 (1,8-Dimethyl-5-[4-(2-methyl-imidazo[4,5-c]pyridin-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033246 (5-Methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033243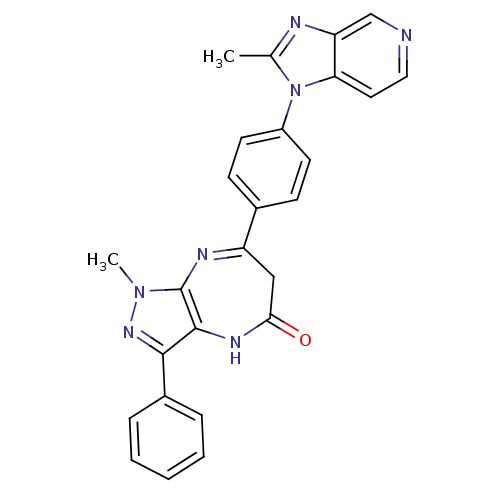 (1-Methyl-7-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033230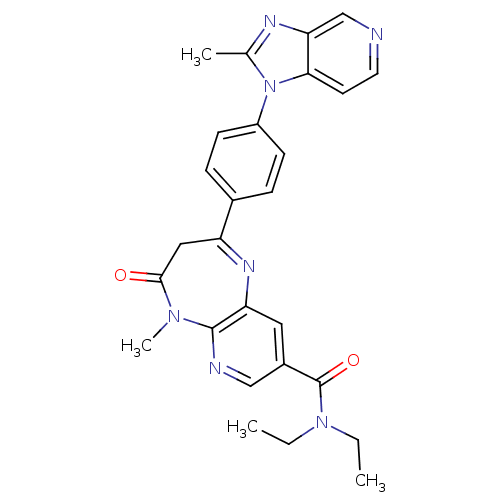 (5-Methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033241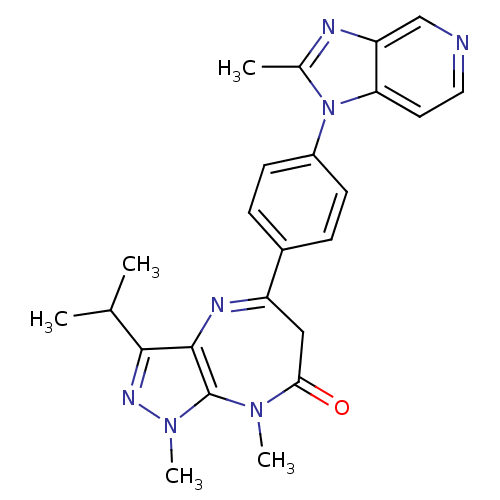 (3-Isopropyl-1,8-dimethyl-5-[4-(2-methyl-imidazo[4,...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033268 (5-Methyl-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033254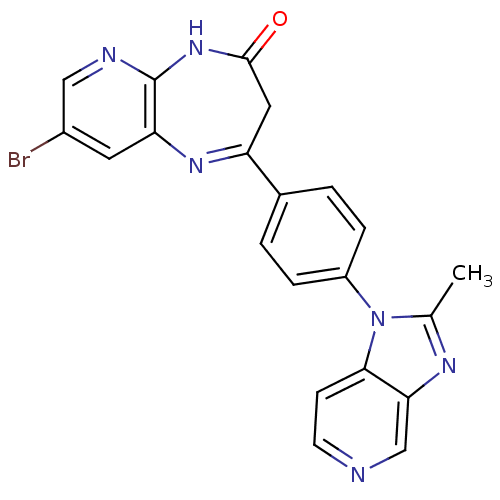 (8-Bromo-2-[4-(2-methyl-imidazo[4,5-c]pyridin-1-yl)...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033239 (8-Bromo-1-methyl-4-[4-(2-methyl-imidazo[4,5-c]pyri...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50033235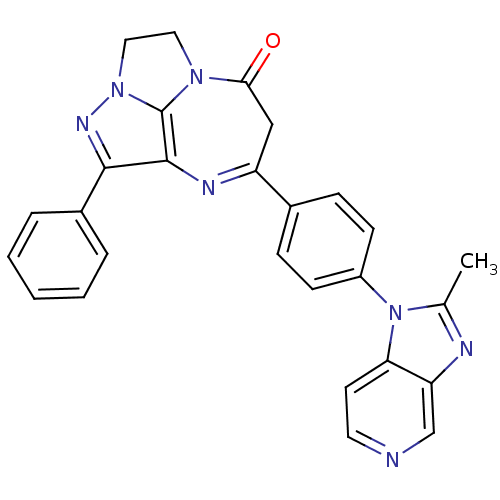 (7-[4-(2-Methyl-imidazo[4,5-c]pyridin-1-yl)-phenyl]...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50004633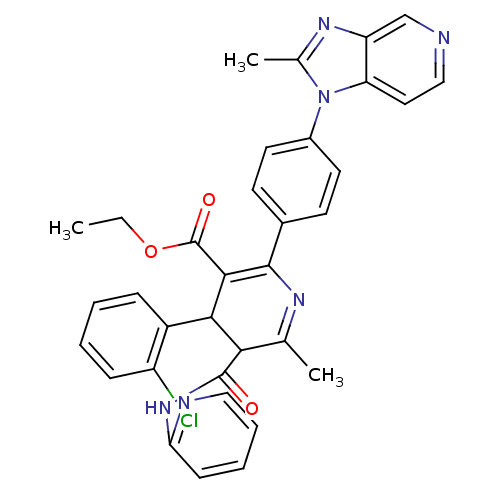 ((S)-4-(2-Chloro-phenyl)-6-methyl-2-[4-(2-methyl-im...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro antagonist activity against platelet activating factor (PAF) receptor, using washed rabbit platelets | J Med Chem 38: 3524-35 (1995) BindingDB Entry DOI: 10.7270/Q2JS9R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 67 total ) | Next | Last >> |