

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092233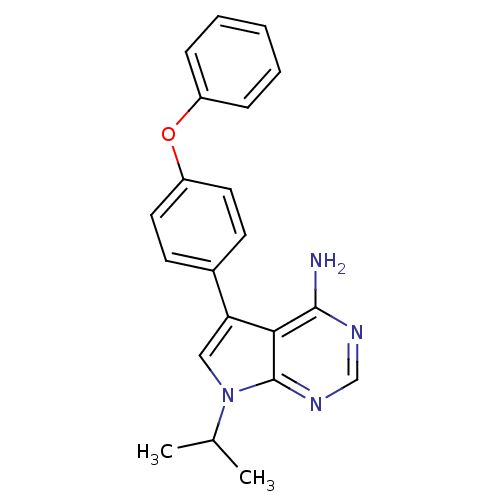 (7-Isopropyl-5-(4-phenoxy-phenyl)-7H-pyrrolo[2,3-d]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092249 (4-[4-(4-Amino-7-tert-butyl-7H-pyrrolo[2,3-d]pyrimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092241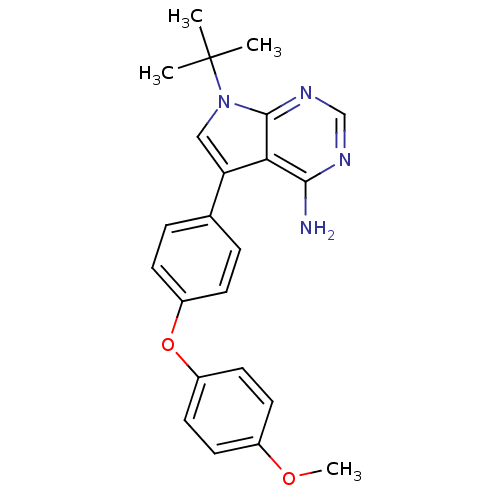 (7-tert-Butyl-5-[4-(4-methoxy-phenoxy)-phenyl]-7H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092228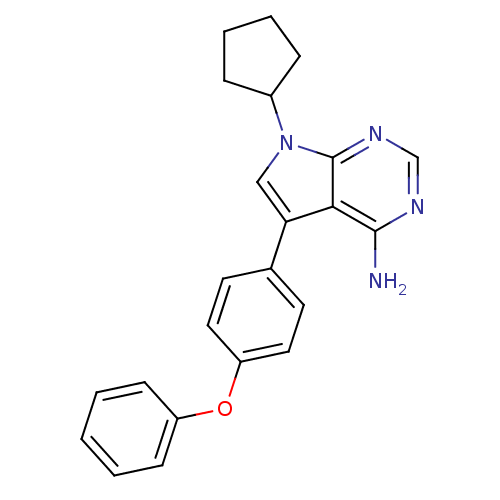 (4-Amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092228 (4-Amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092225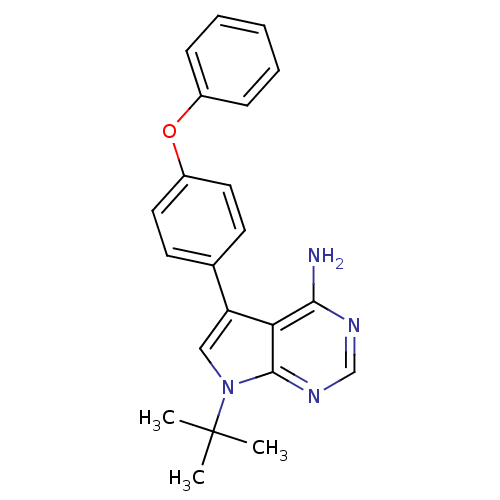 (7-tert-Butyl-5-(4-phenoxy-phenyl)-7H-pyrrolo[2,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092256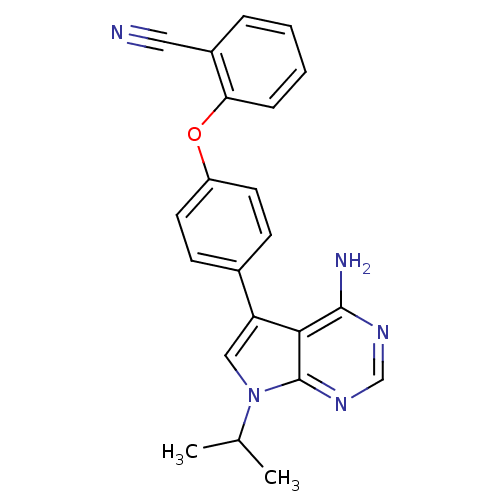 (2-[4-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092244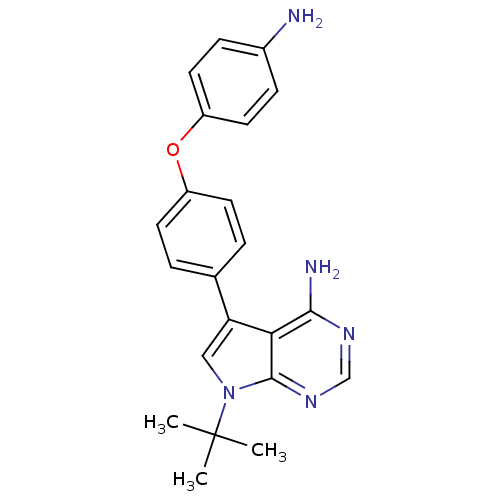 (5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092240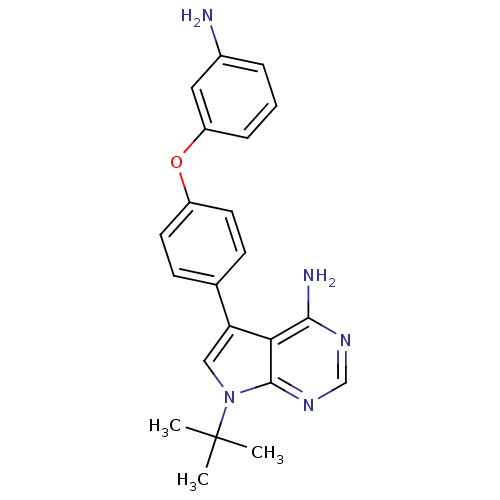 (5-[4-(3-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092236 (CHEMBL71541 | {2-[4-(4-Amino-7-isopropyl-7H-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092254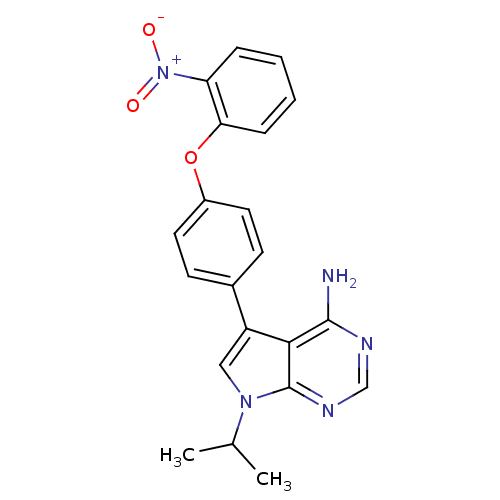 (7-Isopropyl-5-[4-(2-nitro-phenoxy)-phenyl]-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092243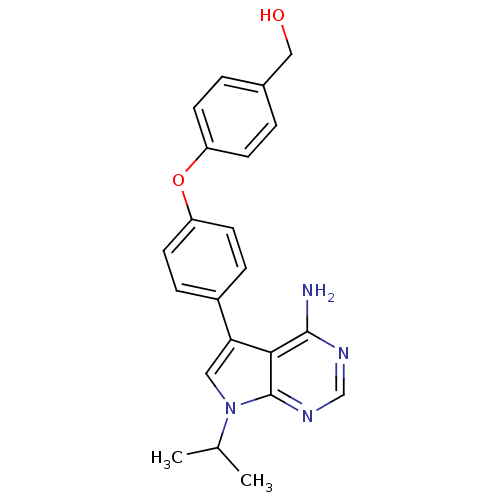 (CHEMBL70034 | {4-[4-(4-Amino-7-isopropyl-7H-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM8798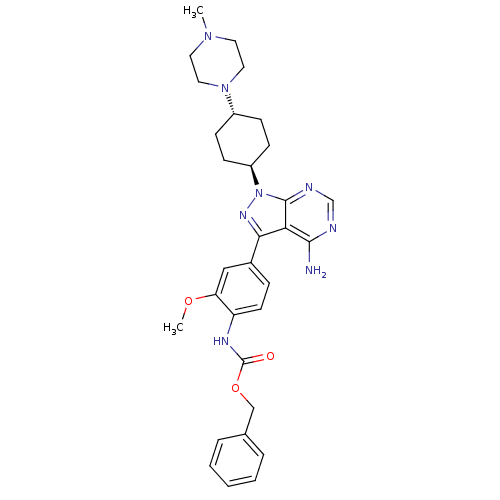 (CHEMBL47787 | Pyrazolo[3,4-d]pyrimidine 6 | benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Bioresearch Center | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | Bioorg Med Chem Lett 12: 1687-90 (2002) Article DOI: 10.1016/s0960-894x(02)00196-8 BindingDB Entry DOI: 10.7270/Q2154F84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235829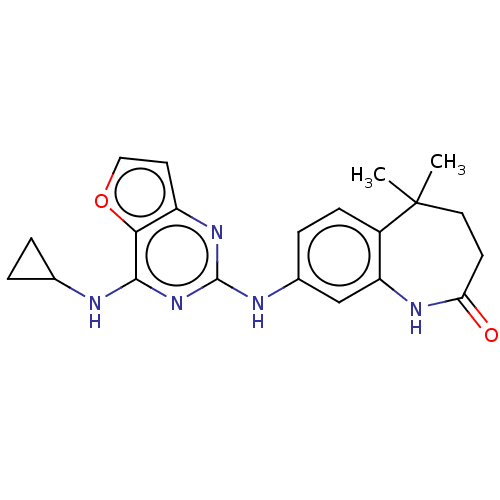 (CHEMBL4098474) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235828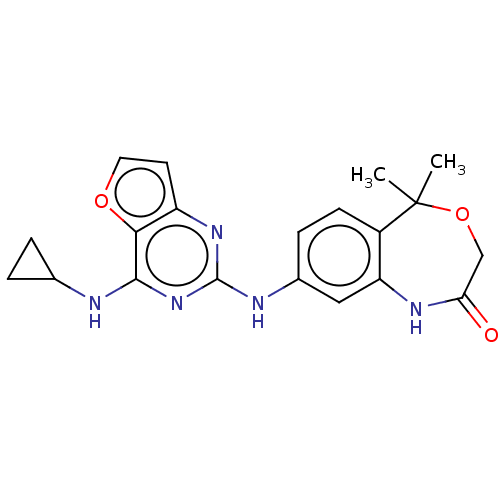 (CHEMBL4090753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092235 (5-[4-(2-Amino-phenoxy)-phenyl]-7-isopropyl-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092239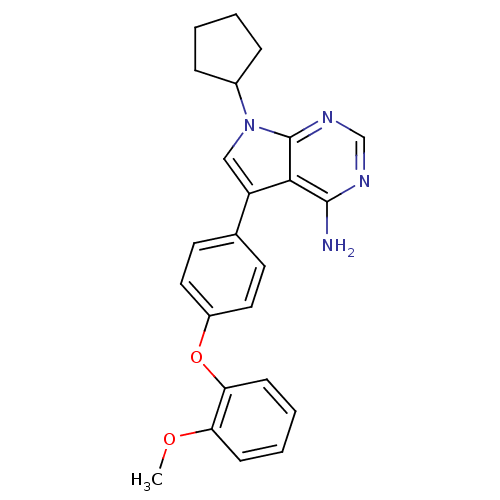 (7-Cyclopentyl-5-[4-(2-methoxy-phenoxy)-phenyl]-7H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235831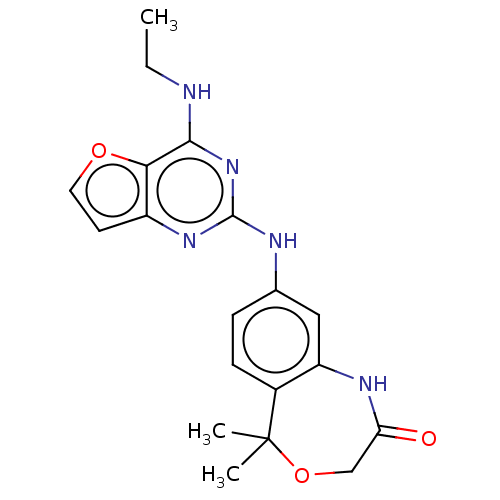 (CHEMBL4093260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235816 (CHEMBL4069365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092234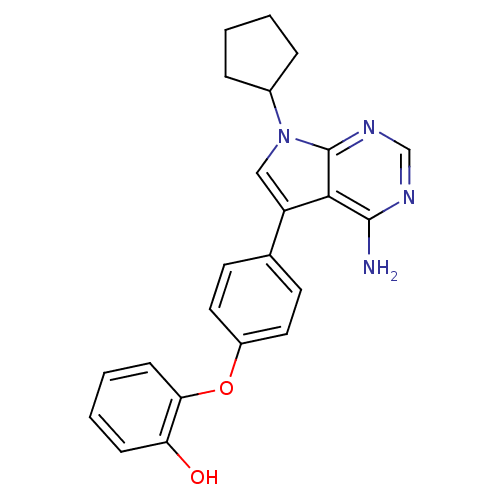 (2-[4-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235830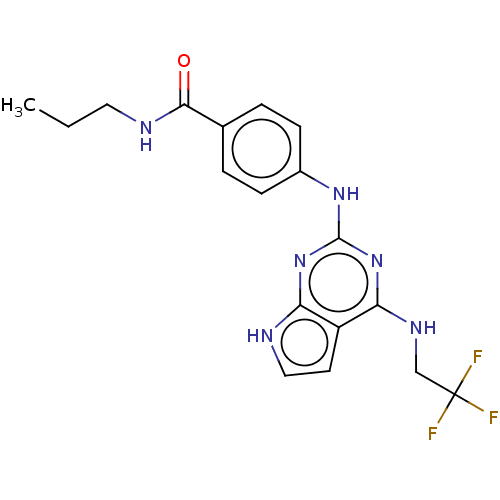 (CHEMBL2006765) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235816 (CHEMBL4069365) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM8807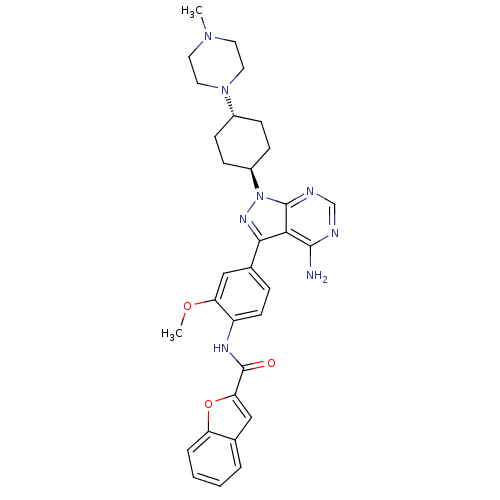 (CHEMBL43773 | N-(4-{4-amino-1-[4-(4-methylpiperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Bioresearch Center | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | Bioorg Med Chem Lett 12: 1687-90 (2002) Article DOI: 10.1016/s0960-894x(02)00196-8 BindingDB Entry DOI: 10.7270/Q2154F84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50338159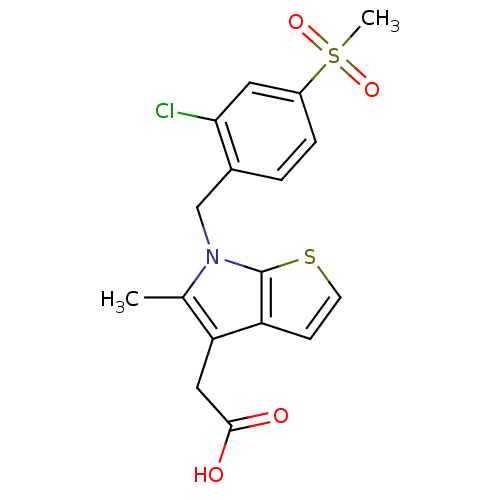 (2-(6-(2-chloro-4-(methylsulfonyl)benzyl)-5-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cell membranes | Bioorg Med Chem Lett 21: 1861-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.008 BindingDB Entry DOI: 10.7270/Q2BV7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092233 (7-Isopropyl-5-(4-phenoxy-phenyl)-7H-pyrrolo[2,3-d]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092252 (6-Chloro-7-cyclopentyl-5-(4-phenoxy-phenyl)-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235818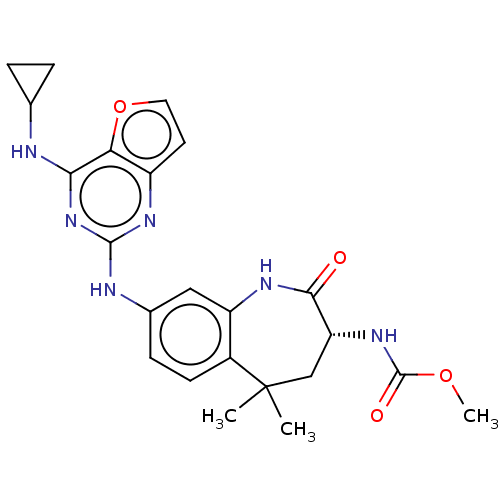 (CHEMBL4071441) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM8798 (CHEMBL47787 | Pyrazolo[3,4-d]pyrimidine 6 | benzyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | Bioorg Med Chem Lett 12: 1687-90 (2002) Article DOI: 10.1016/s0960-894x(02)00196-8 BindingDB Entry DOI: 10.7270/Q2154F84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235821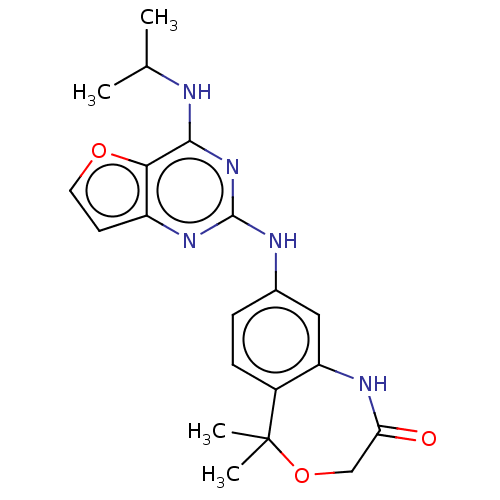 (CHEMBL4100939) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50175210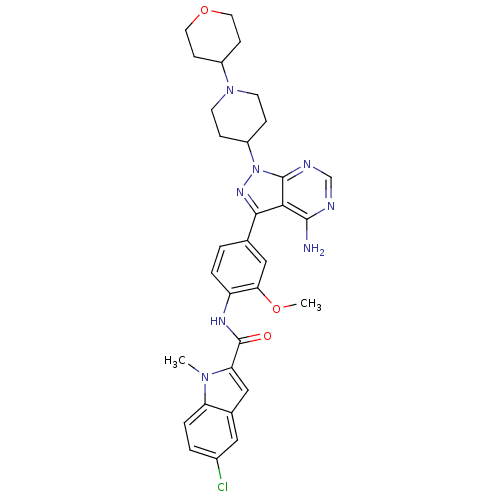 (CHEMBL199438 | N-(4-(4-amino-1-(1-(tetrahydro-2H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center Curated by ChEMBL | Assay Description Inhibitory activity against anti-CD3 mAb-induced IL2 production in human whole blood | Bioorg Med Chem Lett 16: 118-22 (2005) Article DOI: 10.1016/j.bmcl.2005.09.039 BindingDB Entry DOI: 10.7270/Q22F7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092225 (7-tert-Butyl-5-(4-phenoxy-phenyl)-7H-pyrrolo[2,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM8793 (7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Bioresearch Center | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | Bioorg Med Chem Lett 12: 1687-90 (2002) Article DOI: 10.1016/s0960-894x(02)00196-8 BindingDB Entry DOI: 10.7270/Q2154F84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092240 (5-[4-(3-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092249 (4-[4-(4-Amino-7-tert-butyl-7H-pyrrolo[2,3-d]pyrimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235830 (CHEMBL2006765) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092250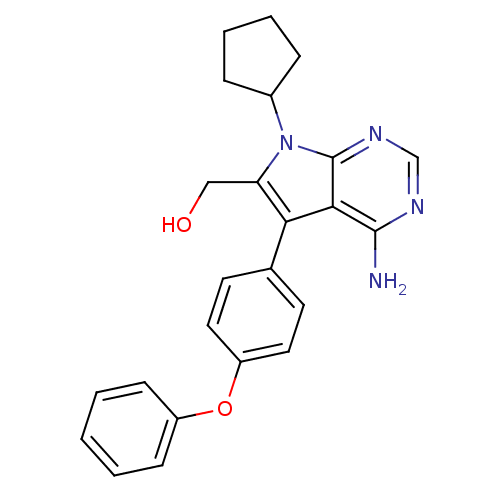 (CHEMBL302279 | [4-Amino-7-cyclopentyl-5-(4-phenoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50305006 (5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of MK2 | Bioorg Med Chem Lett 20: 334-7 (2010) Article DOI: 10.1016/j.bmcl.2009.10.103 BindingDB Entry DOI: 10.7270/Q2VD6ZJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM8806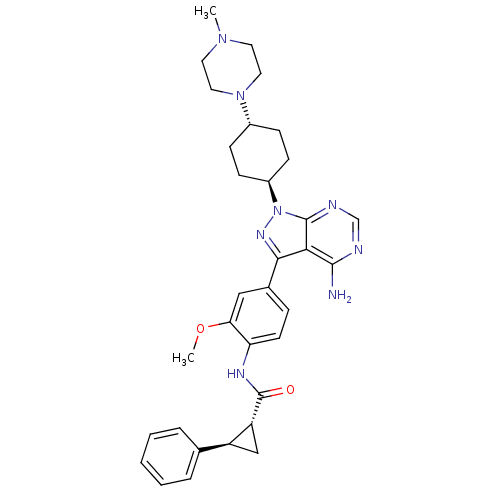 ((1R,2R)-N-(4-{4-amino-1-[4-(4-methylpiperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Bioresearch Center | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | Bioorg Med Chem Lett 12: 1687-90 (2002) Article DOI: 10.1016/s0960-894x(02)00196-8 BindingDB Entry DOI: 10.7270/Q2154F84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092244 (5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50305013 (3-(2-amino-7-(7-(benzo[b]thiophen-2-yl)-1H-indazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of MK2 | Bioorg Med Chem Lett 20: 334-7 (2010) Article DOI: 10.1016/j.bmcl.2009.10.103 BindingDB Entry DOI: 10.7270/Q2VD6ZJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092239 (7-Cyclopentyl-5-[4-(2-methoxy-phenoxy)-phenyl]-7H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50175200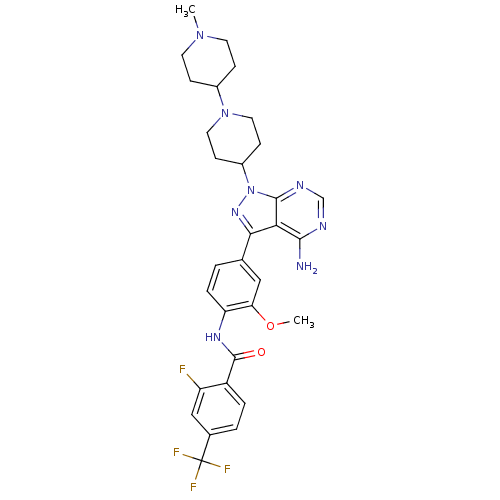 (CHEMBL371295 | N-(4-(4-amino-1-(1-(1-methylpiperid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center Curated by ChEMBL | Assay Description Inhibitory activity against anti-CD3 mAb-induced IL2 production in human whole blood | Bioorg Med Chem Lett 16: 118-22 (2005) Article DOI: 10.1016/j.bmcl.2005.09.039 BindingDB Entry DOI: 10.7270/Q22F7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092246 (4-Amino-7-cyclopentyl-5-(4-phenoxy-phenyl)-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50338157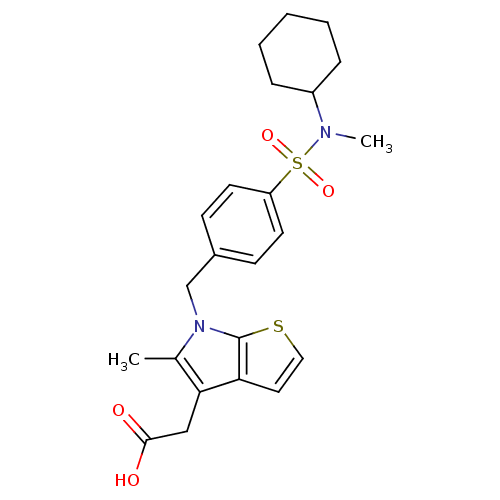 (2-(6-(4-(N-cyclohexyl-N-methylsulfamoyl)benzyl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTH2 receptor expressed in HEK293 cell membranes | Bioorg Med Chem Lett 21: 1861-4 (2011) Article DOI: 10.1016/j.bmcl.2011.01.008 BindingDB Entry DOI: 10.7270/Q2BV7GXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235827 (CHEMBL4081974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibitory concentration required against 5-lipoxygenase activity in cytosolic fractions of human neutrophils | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50175200 (CHEMBL371295 | N-(4-(4-amino-1-(1-(1-methylpiperid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center Curated by ChEMBL | Assay Description Inhibitory activity against lck | Bioorg Med Chem Lett 16: 118-22 (2005) Article DOI: 10.1016/j.bmcl.2005.09.039 BindingDB Entry DOI: 10.7270/Q22F7N07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235833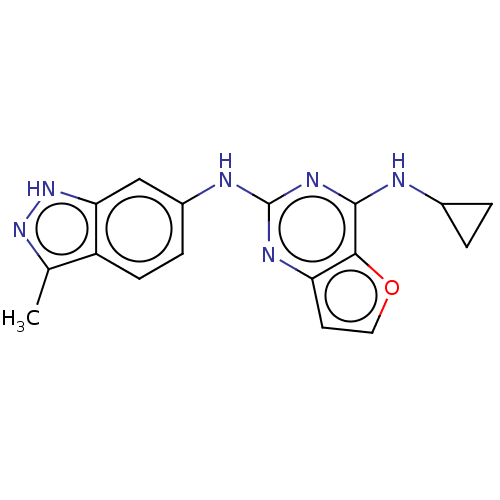 (CHEMBL4062803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50235833 (CHEMBL4062803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) after 60 mins by TR-FRET assay | Bioorg Med Chem Lett 26: 5562-5567 (2016) Article DOI: 10.1016/j.bmcl.2016.09.077 BindingDB Entry DOI: 10.7270/Q2WQ061V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM8806 ((1R,2R)-N-(4-{4-amino-1-[4-(4-methylpiperazin-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | Bioorg Med Chem Lett 12: 1687-90 (2002) Article DOI: 10.1016/s0960-894x(02)00196-8 BindingDB Entry DOI: 10.7270/Q2154F84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM8803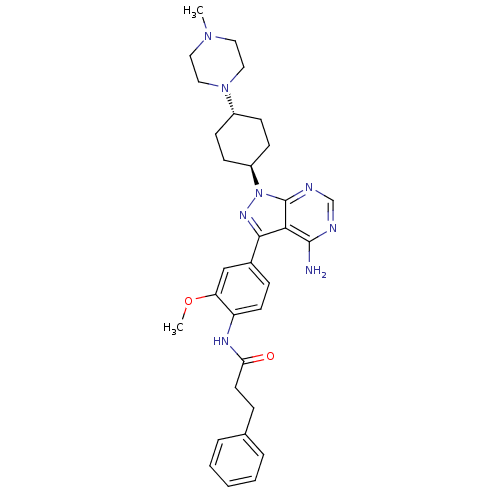 (CHEMBL295601 | N-(4-{4-amino-1-[4-(4-methylpiperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Bioresearch Center | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | Bioorg Med Chem Lett 12: 1687-90 (2002) Article DOI: 10.1016/s0960-894x(02)00196-8 BindingDB Entry DOI: 10.7270/Q2154F84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 796 total ) | Next | Last >> |