

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160907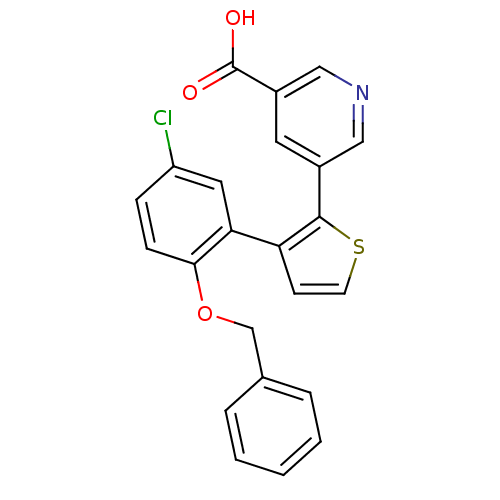 (5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160912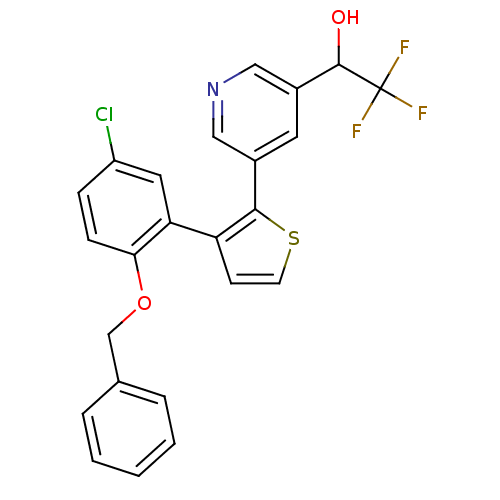 (1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160917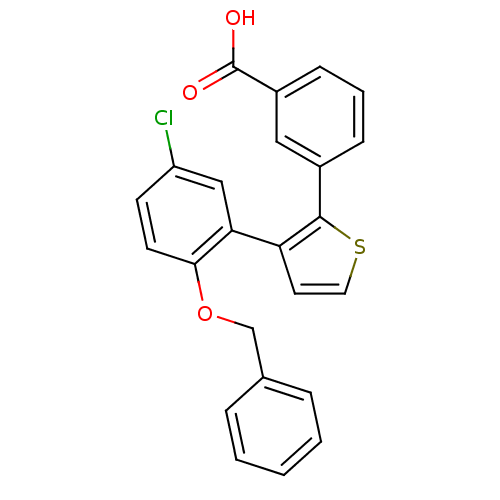 (3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160915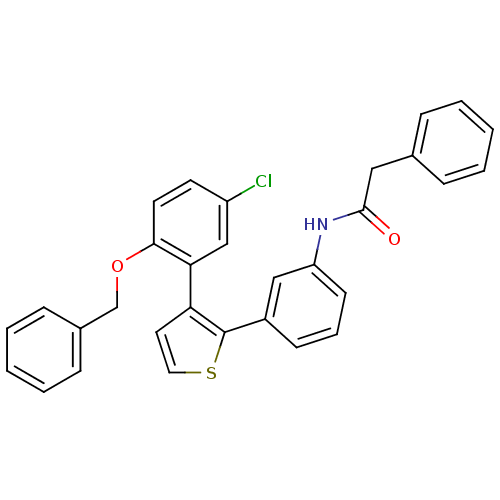 (CHEMBL180046 | N-{3-[3-(2-Benzyloxy-5-chloro-pheny...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160909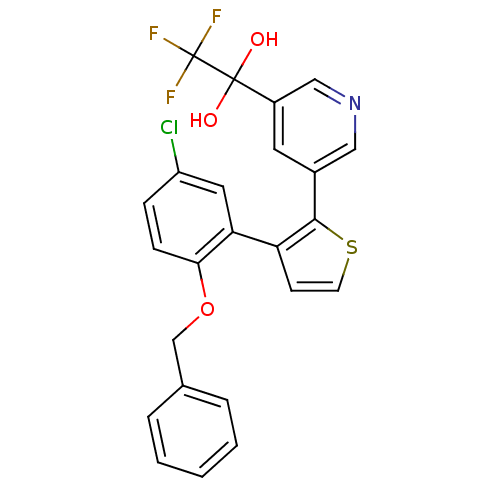 (1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160915 (CHEMBL180046 | N-{3-[3-(2-Benzyloxy-5-chloro-pheny...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160909 (1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160913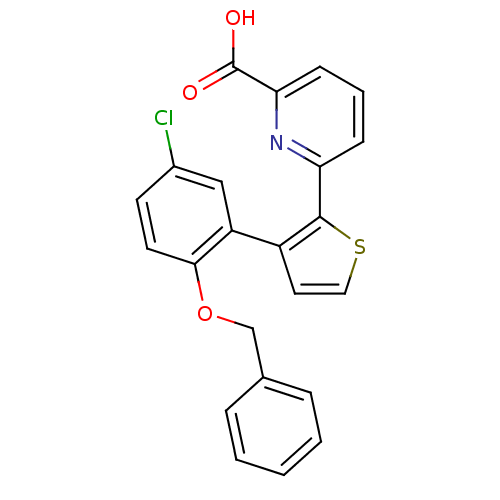 (6-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160912 (1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50410087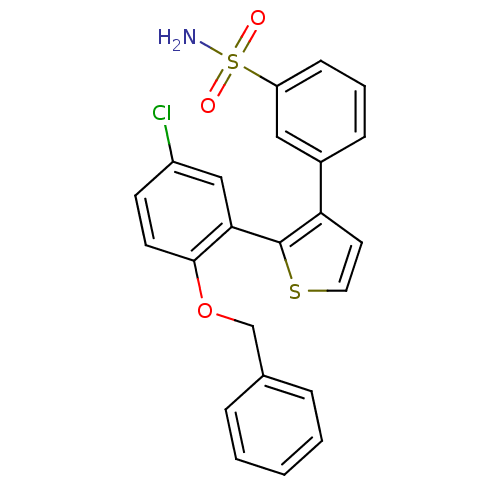 (CHEMBL2113029) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160914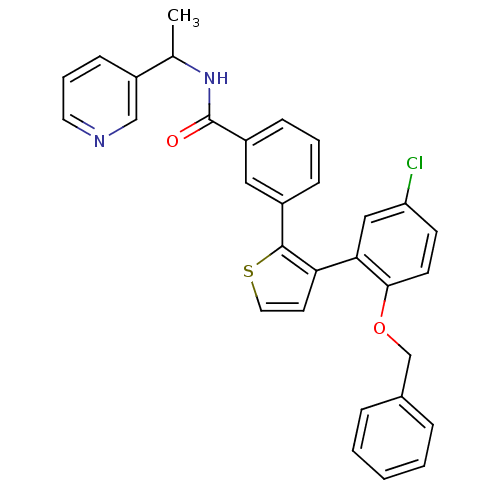 (3-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Rattus norvegicus (Rat)) | BDBM50160913 (6-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity towards Prostaglandin E receptor was determined in rat | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160910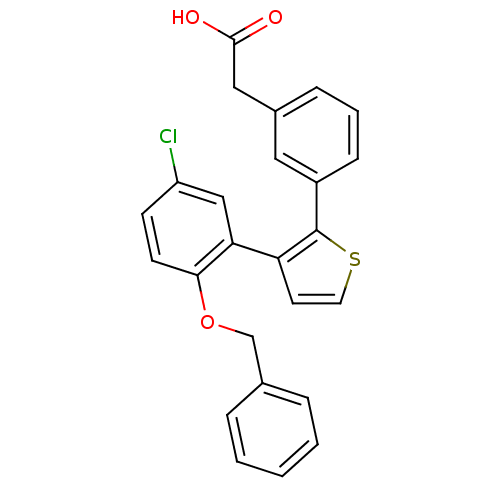 (CHEMBL184779 | {3-[3-(2-Benzyloxy-5-chloro-phenyl)...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160914 (3-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50410087 (CHEMBL2113029) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160908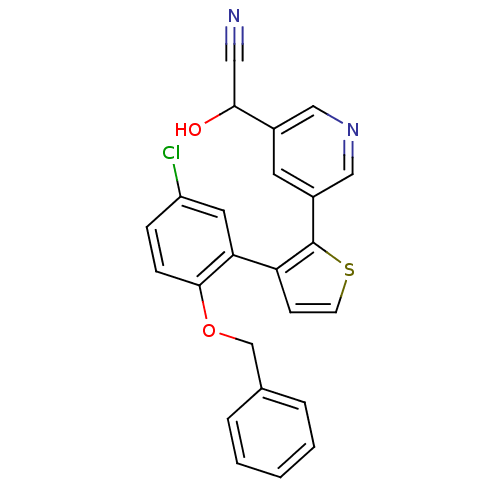 (CHEMBL182623 | {5-[3-(2-Benzyloxy-5-chloro-phenyl)...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160918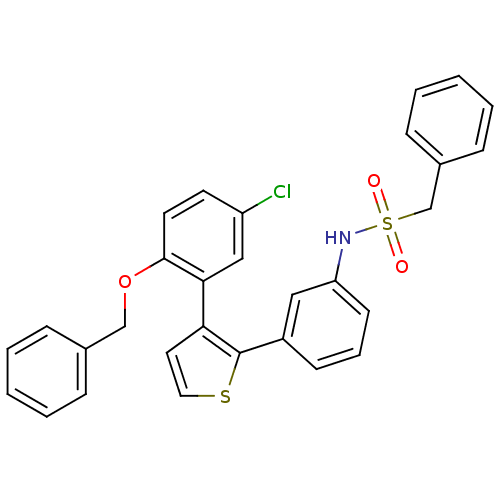 (CHEMBL182662 | N-{3-[3-(2-Benzyloxy-5-chloro-pheny...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160917 (3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160918 (CHEMBL182662 | N-{3-[3-(2-Benzyloxy-5-chloro-pheny...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160907 (5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160917 (3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160912 (1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160913 (6-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160910 (CHEMBL184779 | {3-[3-(2-Benzyloxy-5-chloro-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160908 (CHEMBL182623 | {5-[3-(2-Benzyloxy-5-chloro-phenyl)...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160916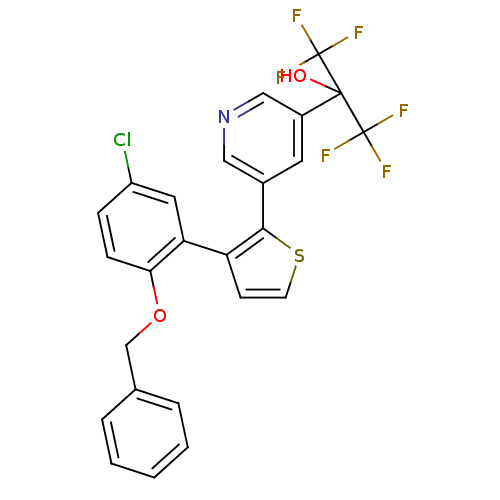 (2-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160909 (1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160910 (CHEMBL184779 | {3-[3-(2-Benzyloxy-5-chloro-phenyl)...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160907 (5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160916 (2-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor was determined in human | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160913 (6-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160908 (CHEMBL182623 | {5-[3-(2-Benzyloxy-5-chloro-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50410087 (CHEMBL2113029) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128419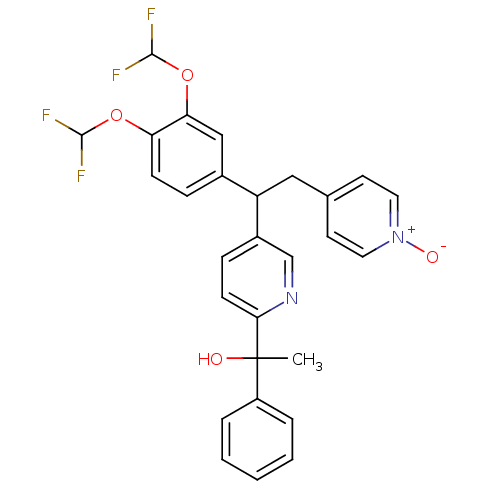 (1-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160914 (3-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50409702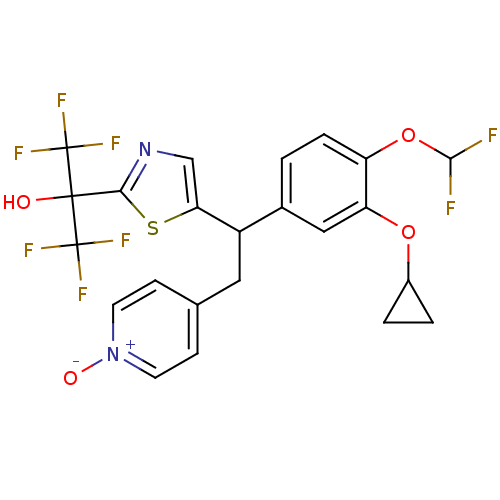 (CHEMBL2112296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160915 (CHEMBL180046 | N-{3-[3-(2-Benzyloxy-5-chloro-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50160918 (CHEMBL182662 | N-{3-[3-(2-Benzyloxy-5-chloro-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity against human Prostanoid TP receptor | Bioorg Med Chem Lett 15: 1155-60 (2005) Article DOI: 10.1016/j.bmcl.2004.12.005 BindingDB Entry DOI: 10.7270/Q2SF2VNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50409699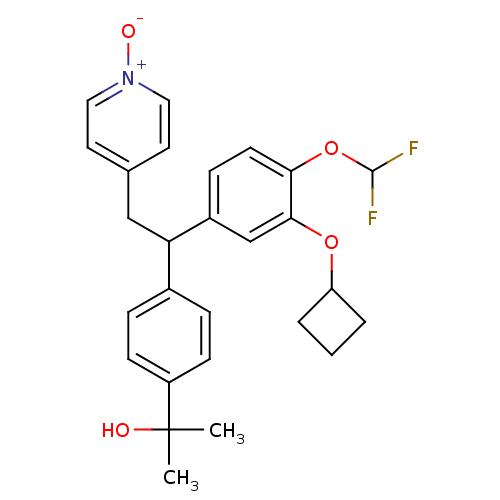 (CHEMBL2112294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128424 (2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128424 (2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128686 (2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128689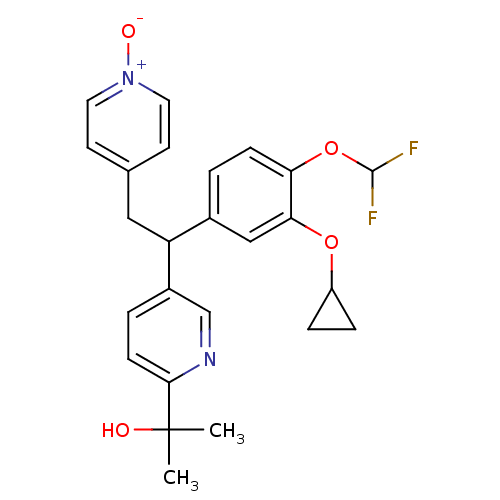 (2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128689 (2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50409700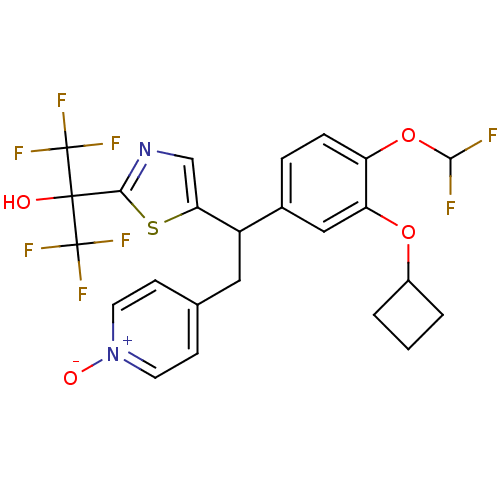 (CHEMBL2112295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50409701 (CHEMBL383762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128683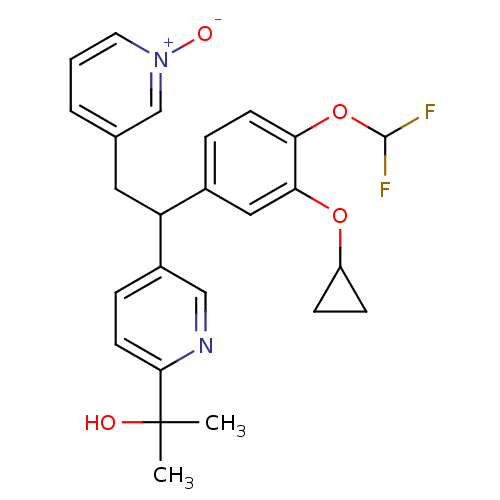 (2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128683 (2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128692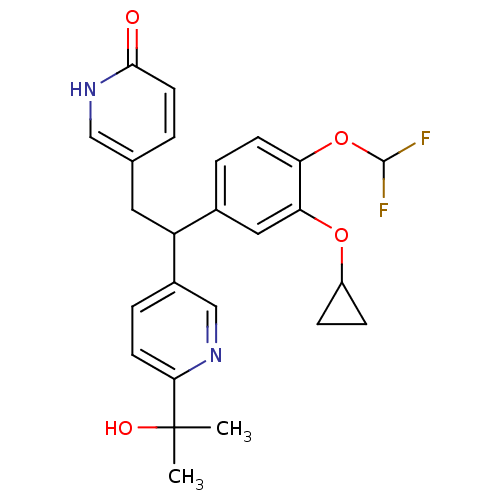 (5-{2-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50128685 (2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 | J Med Chem 46: 2413-26 (2003) Article DOI: 10.1021/jm0204542 BindingDB Entry DOI: 10.7270/Q24X5756 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 275 total ) | Next | Last >> |