 Found 125 hits with Last Name = 'cebula' and Initial = 'r'
Found 125 hits with Last Name = 'cebula' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372752
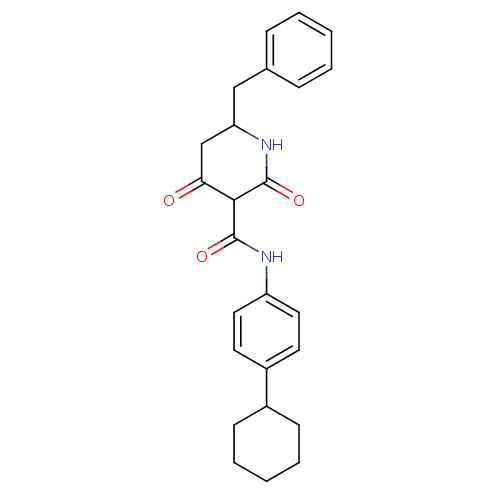
(CHEMBL272547)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)CC(Cc2ccccc2)NC1=O Show InChI InChI=1S/C25H28N2O3/c28-22-16-21(15-17-7-3-1-4-8-17)27-25(30)23(22)24(29)26-20-13-11-19(12-14-20)18-9-5-2-6-10-18/h1,3-4,7-8,11-14,18,21,23H,2,5-6,9-10,15-16H2,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372771
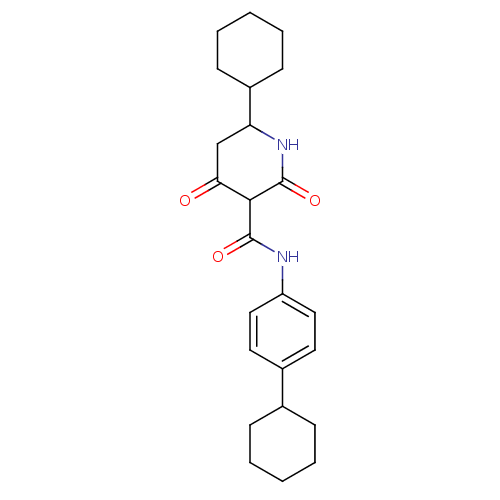
(CHEMBL404127)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)CC(NC1=O)C1CCCCC1 Show InChI InChI=1S/C24H32N2O3/c27-21-15-20(18-9-5-2-6-10-18)26-24(29)22(21)23(28)25-19-13-11-17(12-14-19)16-7-3-1-4-8-16/h11-14,16,18,20,22H,1-10,15H2,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372766
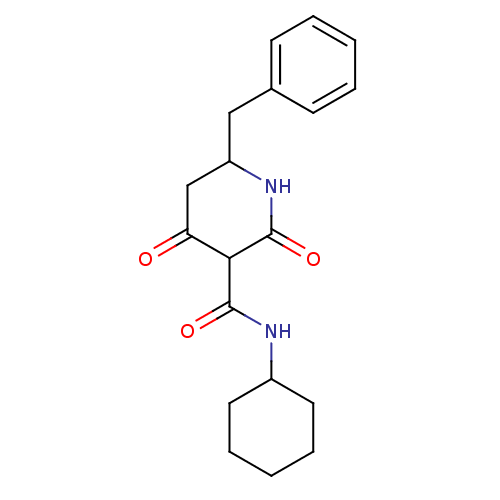
(CHEMBL271482)Show InChI InChI=1S/C19H24N2O3/c22-16-12-15(11-13-7-3-1-4-8-13)21-19(24)17(16)18(23)20-14-9-5-2-6-10-14/h1,3-4,7-8,14-15,17H,2,5-6,9-12H2,(H,20,23)(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372754
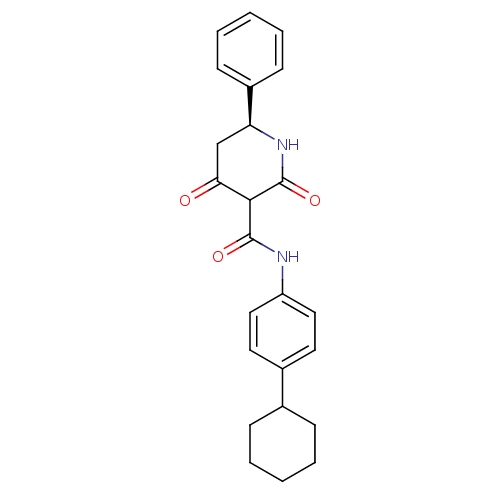
(CHEMBL272054)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)C[C@H](NC1=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O3/c27-21-15-20(18-9-5-2-6-10-18)26-24(29)22(21)23(28)25-19-13-11-17(12-14-19)16-7-3-1-4-8-16/h2,5-6,9-14,16,20,22H,1,3-4,7-8,15H2,(H,25,28)(H,26,29)/t20-,22?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372751

(CHEMBL256222)Show SMILES O=C(Nc1ccc(Oc2ccccc2)cc1)C1C(=O)CC(Cc2ccccc2)NC1=O Show InChI InChI=1S/C25H22N2O4/c28-22-16-19(15-17-7-3-1-4-8-17)27-25(30)23(22)24(29)26-18-11-13-21(14-12-18)31-20-9-5-2-6-10-20/h1-14,19,23H,15-16H2,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372750

(CHEMBL272914)Show SMILES O=C(Nc1ccc(cc1)N1CCCCC1)C1C(=O)CC(Cc2ccccc2)NC1=O Show InChI InChI=1S/C24H27N3O3/c28-21-16-19(15-17-7-3-1-4-8-17)26-24(30)22(21)23(29)25-18-9-11-20(12-10-18)27-13-5-2-6-14-27/h1,3-4,7-12,19,22H,2,5-6,13-16H2,(H,25,29)(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372760

(CHEMBL257948)Show SMILES O=C(Nc1ccc(cc1)-c1ccccc1)C1C(=O)NC(CCc2ccccc2)C1=O Show InChI InChI=1S/C25H22N2O3/c28-23-21(16-11-17-7-3-1-4-8-17)27-25(30)22(23)24(29)26-20-14-12-19(13-15-20)18-9-5-2-6-10-18/h1-10,12-15,21-22H,11,16H2,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372767
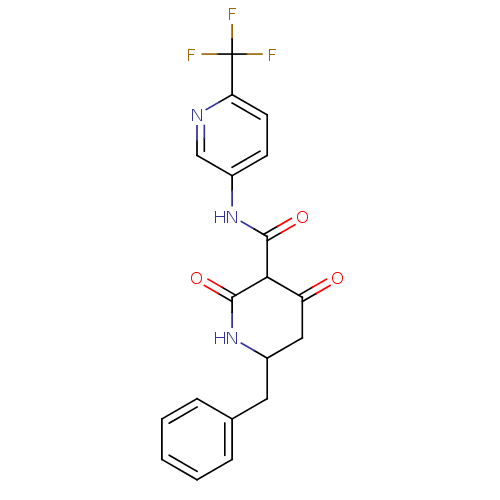
(CHEMBL272913)Show SMILES FC(F)(F)c1ccc(NC(=O)C2C(=O)CC(Cc3ccccc3)NC2=O)cn1 Show InChI InChI=1S/C19H16F3N3O3/c20-19(21,22)15-7-6-12(10-23-15)24-17(27)16-14(26)9-13(25-18(16)28)8-11-4-2-1-3-5-11/h1-7,10,13,16H,8-9H2,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372762
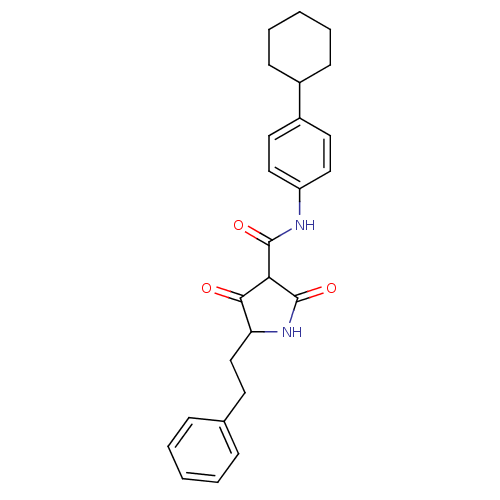
(CHEMBL272162)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)NC(CCc2ccccc2)C1=O Show InChI InChI=1S/C25H28N2O3/c28-23-21(16-11-17-7-3-1-4-8-17)27-25(30)22(23)24(29)26-20-14-12-19(13-15-20)18-9-5-2-6-10-18/h1,3-4,7-8,12-15,18,21-22H,2,5-6,9-11,16H2,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372753
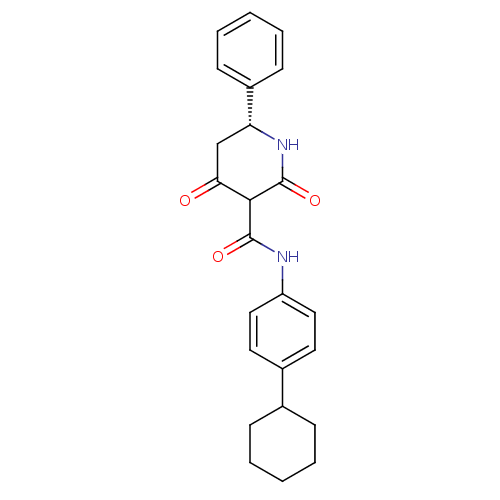
(CHEMBL271845)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)C[C@@H](NC1=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O3/c27-21-15-20(18-9-5-2-6-10-18)26-24(29)22(21)23(28)25-19-13-11-17(12-14-19)16-7-3-1-4-8-16/h2,5-6,9-14,16,20,22H,1,3-4,7-8,15H2,(H,25,28)(H,26,29)/t20-,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372780
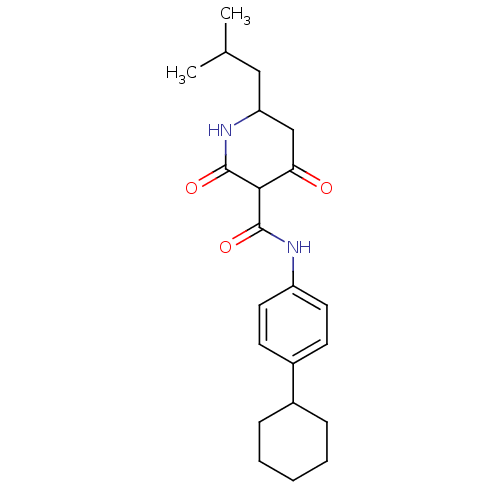
(CHEMBL404126)Show SMILES CC(C)CC1CC(=O)C(C(=O)Nc2ccc(cc2)C2CCCCC2)C(=O)N1 Show InChI InChI=1S/C22H30N2O3/c1-14(2)12-18-13-19(25)20(22(27)24-18)21(26)23-17-10-8-16(9-11-17)15-6-4-3-5-7-15/h8-11,14-15,18,20H,3-7,12-13H2,1-2H3,(H,23,26)(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372753

(CHEMBL271845)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)C[C@@H](NC1=O)c1ccccc1 Show InChI InChI=1S/C24H26N2O3/c27-21-15-20(18-9-5-2-6-10-18)26-24(29)22(21)23(28)25-19-13-11-17(12-14-19)16-7-3-1-4-8-16/h2,5-6,9-14,16,20,22H,1,3-4,7-8,15H2,(H,25,28)(H,26,29)/t20-,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372758
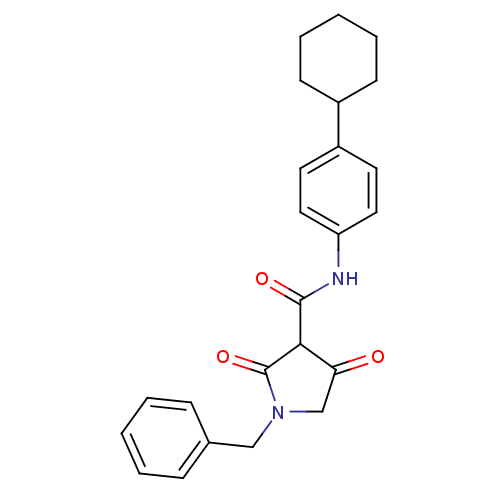
(CHEMBL269960)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)CN(Cc2ccccc2)C1=O Show InChI InChI=1S/C24H26N2O3/c27-21-16-26(15-17-7-3-1-4-8-17)24(29)22(21)23(28)25-20-13-11-19(12-14-20)18-9-5-2-6-10-18/h1,3-4,7-8,11-14,18,22H,2,5-6,9-10,15-16H2,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372755
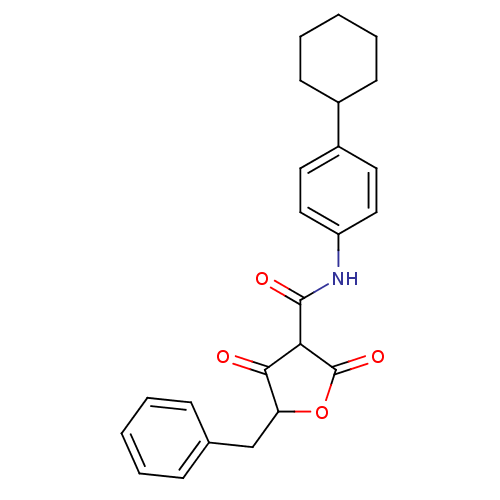
(CHEMBL255828)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)OC(Cc2ccccc2)C1=O Show InChI InChI=1S/C24H25NO4/c26-22-20(15-16-7-3-1-4-8-16)29-24(28)21(22)23(27)25-19-13-11-18(12-14-19)17-9-5-2-6-10-17/h1,3-4,7-8,11-14,17,20-21H,2,5-6,9-10,15H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372768
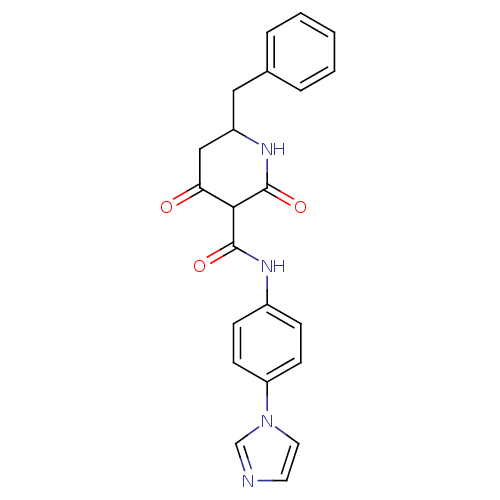
(CHEMBL272696)Show SMILES O=C(Nc1ccc(cc1)-n1ccnc1)C1C(=O)CC(Cc2ccccc2)NC1=O Show InChI InChI=1S/C22H20N4O3/c27-19-13-17(12-15-4-2-1-3-5-15)25-22(29)20(19)21(28)24-16-6-8-18(9-7-16)26-11-10-23-14-26/h1-11,14,17,20H,12-13H2,(H,24,28)(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372759
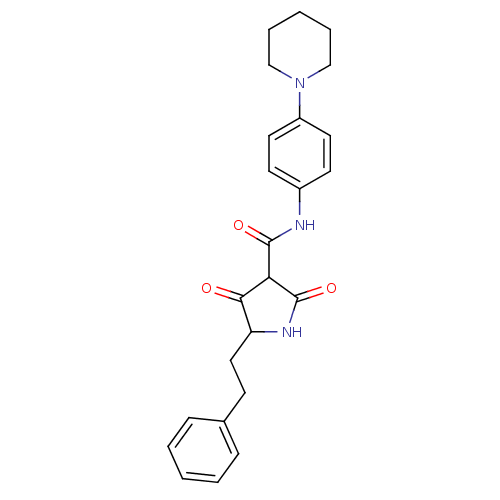
(CHEMBL256476)Show SMILES O=C(Nc1ccc(cc1)N1CCCCC1)C1C(=O)NC(CCc2ccccc2)C1=O Show InChI InChI=1S/C24H27N3O3/c28-22-20(14-9-17-7-3-1-4-8-17)26-24(30)21(22)23(29)25-18-10-12-19(13-11-18)27-15-5-2-6-16-27/h1,3-4,7-8,10-13,20-21H,2,5-6,9,14-16H2,(H,25,29)(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372770
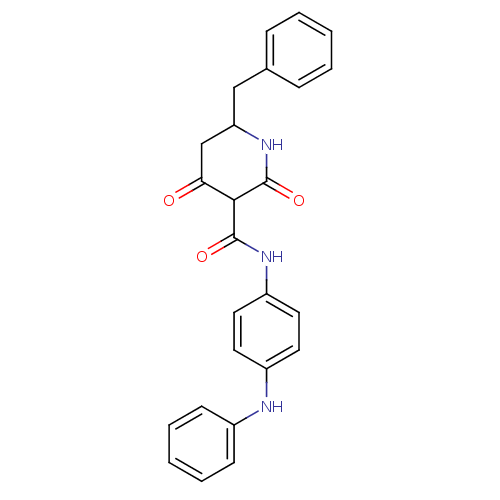
(CHEMBL256223)Show SMILES O=C(Nc1ccc(Nc2ccccc2)cc1)C1C(=O)CC(Cc2ccccc2)NC1=O Show InChI InChI=1S/C25H23N3O3/c29-22-16-21(15-17-7-3-1-4-8-17)28-25(31)23(22)24(30)27-20-13-11-19(12-14-20)26-18-9-5-2-6-10-18/h1-14,21,23,26H,15-16H2,(H,27,30)(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372772
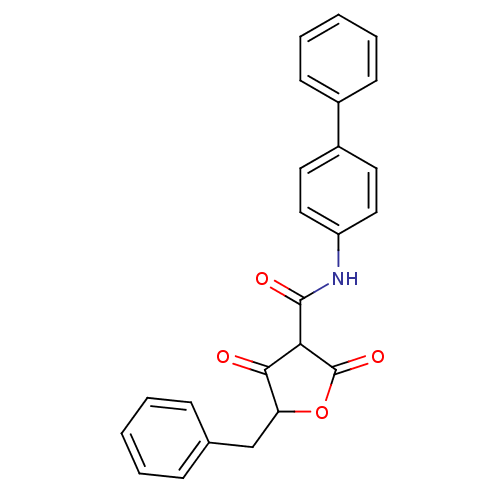
(CHEMBL271293)Show SMILES O=C(Nc1ccc(cc1)-c1ccccc1)C1C(=O)OC(Cc2ccccc2)C1=O Show InChI InChI=1S/C24H19NO4/c26-22-20(15-16-7-3-1-4-8-16)29-24(28)21(22)23(27)25-19-13-11-18(12-14-19)17-9-5-2-6-10-17/h1-14,20-21H,15H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372761
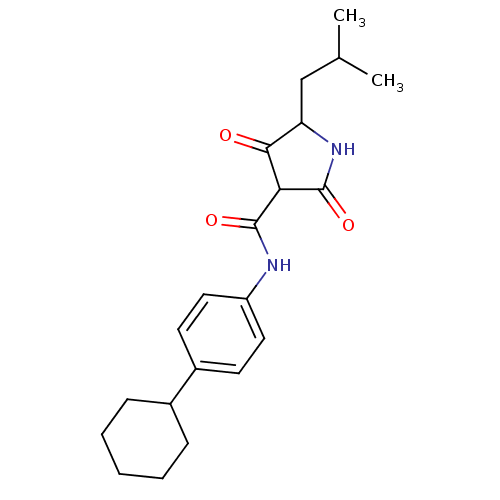
(CHEMBL272163)Show SMILES CC(C)CC1NC(=O)C(C(=O)Nc2ccc(cc2)C2CCCCC2)C1=O Show InChI InChI=1S/C21H28N2O3/c1-13(2)12-17-19(24)18(21(26)23-17)20(25)22-16-10-8-15(9-11-16)14-6-4-3-5-7-14/h8-11,13-14,17-18H,3-7,12H2,1-2H3,(H,22,25)(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372778
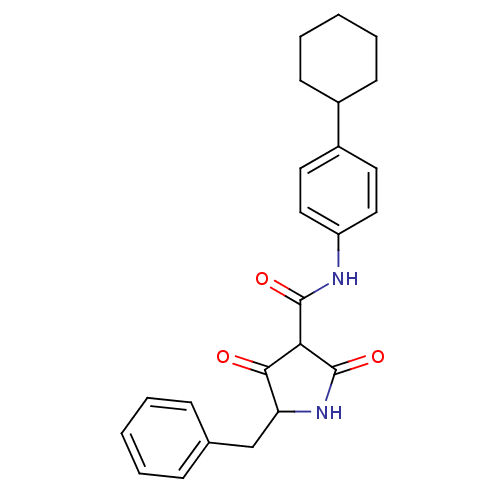
(CHEMBL403226)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)NC(Cc2ccccc2)C1=O Show InChI InChI=1S/C24H26N2O3/c27-22-20(15-16-7-3-1-4-8-16)26-24(29)21(22)23(28)25-19-13-11-18(12-14-19)17-9-5-2-6-10-17/h1,3-4,7-8,11-14,17,20-21H,2,5-6,9-10,15H2,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372756
(CHEMBL404337)Show SMILES Clc1cc(Cl)cc(NC(=O)C2C(=O)OC(C2=O)c2ccccc2)c1 Show InChI InChI=1S/C17H11Cl2NO4/c18-10-6-11(19)8-12(7-10)20-16(22)13-14(21)15(24-17(13)23)9-4-2-1-3-5-9/h1-8,13,15H,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372765
(CHEMBL256483)Show SMILES O=C(Nc1ccc(cc1)-n1ccnc1)C1C(=O)NCC(CC2CCCCC2)C1=O Show InChI InChI=1S/C22H26N4O3/c27-20-16(12-15-4-2-1-3-5-15)13-24-21(28)19(20)22(29)25-17-6-8-18(9-7-17)26-11-10-23-14-26/h6-11,14-16,19H,1-5,12-13H2,(H,24,28)(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372774
(CHEMBL270170)Show InChI InChI=1S/C19H17FN2O3/c20-13-7-9-14(10-8-13)21-18(24)16-17(23)15(22-19(16)25)11-6-12-4-2-1-3-5-12/h1-5,7-10,15-16H,6,11H2,(H,21,24)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372763
(CHEMBL272377)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)NC(C1=O)c1ccccc1 Show InChI InChI=1S/C23H24N2O3/c26-21-19(23(28)25-20(21)17-9-5-2-6-10-17)22(27)24-18-13-11-16(12-14-18)15-7-3-1-4-8-15/h2,5-6,9-15,19-20H,1,3-4,7-8H2,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289899
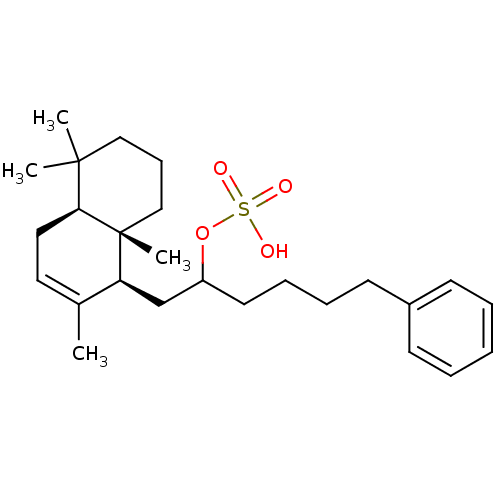
(CHEMBL292556 | Sulfuric acid mono-[5-phenyl-1-((1S...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C26H40O4S/c1-20-15-16-24-25(2,3)17-10-18-26(24,4)23(20)19-22(30-31(27,28)29)14-9-8-13-21-11-6-5-7-12-21/h5-7,11-12,15,22-24H,8-10,13-14,16-19H2,1-4H3,(H,27,28,29)/t22?,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289900
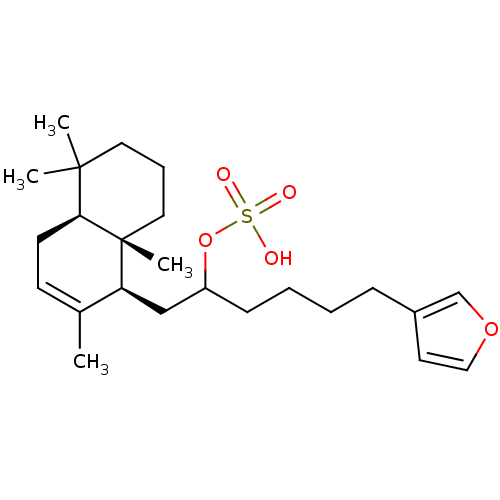
(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372776
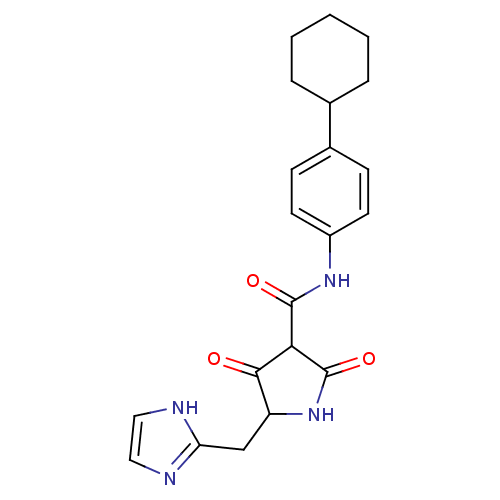
(CHEMBL428250)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)NC(Cc2ncc[nH]2)C1=O Show InChI InChI=1S/C21H24N4O3/c26-19-16(12-17-22-10-11-23-17)25-21(28)18(19)20(27)24-15-8-6-14(7-9-15)13-4-2-1-3-5-13/h6-11,13,16,18H,1-5,12H2,(H,22,23)(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289899

(CHEMBL292556 | Sulfuric acid mono-[5-phenyl-1-((1S...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C26H40O4S/c1-20-15-16-24-25(2,3)17-10-18-26(24,4)23(20)19-22(30-31(27,28)29)14-9-8-13-21-11-6-5-7-12-21/h5-7,11-12,15,22-24H,8-10,13-14,16-19H2,1-4H3,(H,27,28,29)/t22?,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50286608

(CHEMBL60072 | Sulfircin analogue | Sulfuric acid m...)Show SMILES C[C@H](CCCc1ccoc1)[C@H](C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS([O-])(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/p-1/t19-,21+,22+,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50289899

(CHEMBL292556 | Sulfuric acid mono-[5-phenyl-1-((1S...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C26H40O4S/c1-20-15-16-24-25(2,3)17-10-18-26(24,4)23(20)19-22(30-31(27,28)29)14-9-8-13-21-11-6-5-7-12-21/h5-7,11-12,15,22-24H,8-10,13-14,16-19H2,1-4H3,(H,27,28,29)/t22?,23-,24-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289906
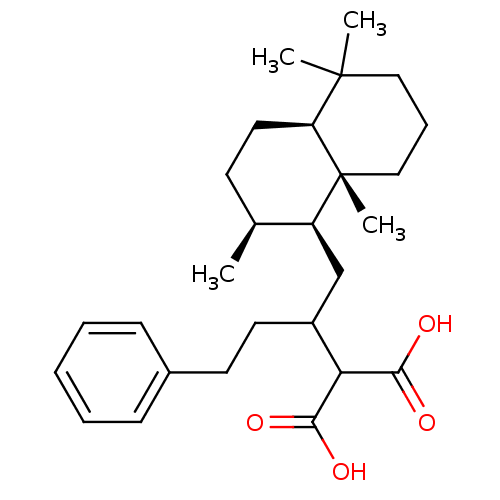
(2-[3-Phenyl-1-((1S,2S,4aS,8aR)-2,5,5,8a-tetramethy...)Show SMILES C[C@H]1CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCc1ccccc1)C(C(O)=O)C(O)=O Show InChI InChI=1S/C27H40O4/c1-18-11-14-22-26(2,3)15-8-16-27(22,4)21(18)17-20(23(24(28)29)25(30)31)13-12-19-9-6-5-7-10-19/h5-7,9-10,18,20-23H,8,11-17H2,1-4H3,(H,28,29)(H,30,31)/t18-,20?,21-,22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289906

(2-[3-Phenyl-1-((1S,2S,4aS,8aR)-2,5,5,8a-tetramethy...)Show SMILES C[C@H]1CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCc1ccccc1)C(C(O)=O)C(O)=O Show InChI InChI=1S/C27H40O4/c1-18-11-14-22-26(2,3)15-8-16-27(22,4)21(18)17-20(23(24(28)29)25(30)31)13-12-19-9-6-5-7-10-19/h5-7,9-10,18,20-23H,8,11-17H2,1-4H3,(H,28,29)(H,30,31)/t18-,20?,21-,22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50286608

(CHEMBL60072 | Sulfircin analogue | Sulfuric acid m...)Show SMILES C[C@H](CCCc1ccoc1)[C@H](C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS([O-])(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/p-1/t19-,21+,22+,23+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289897
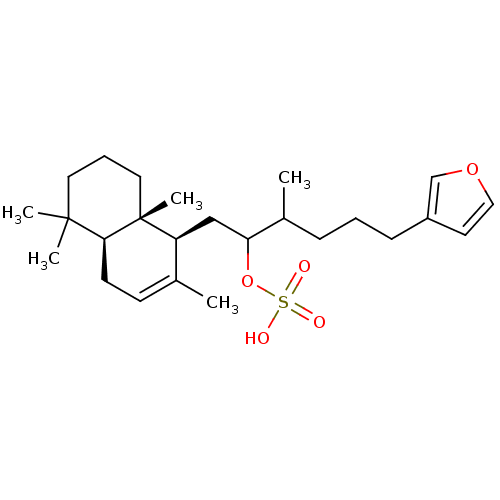
(CHEMBL60297 | Sulfuric acid mono-[5-furan-3-yl-2-m...)Show SMILES CC(CCCc1ccoc1)C(C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS(O)(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/t19?,21-,22?,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50372758

(CHEMBL269960)Show SMILES O=C(Nc1ccc(cc1)C1CCCCC1)C1C(=O)CN(Cc2ccccc2)C1=O Show InChI InChI=1S/C24H26N2O3/c27-21-16-26(15-17-7-3-1-4-8-17)24(29)22(21)23(28)25-20-13-11-19(12-14-20)18-9-5-2-6-10-18/h1,3-4,7-8,11-14,18,22H,2,5-6,9-10,15-16H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289897

(CHEMBL60297 | Sulfuric acid mono-[5-furan-3-yl-2-m...)Show SMILES CC(CCCc1ccoc1)C(C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS(O)(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/t19?,21-,22?,23-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372769
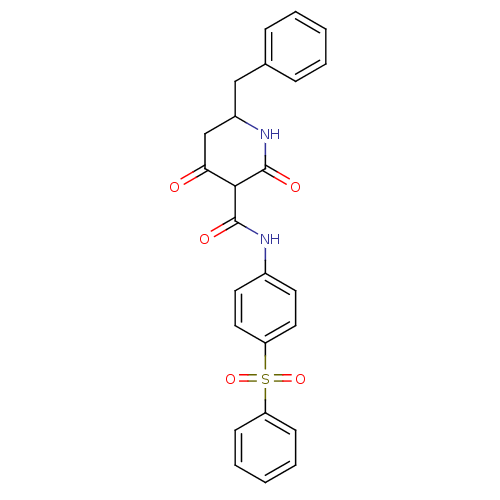
(CHEMBL256017)Show SMILES O=C(Nc1ccc(cc1)S(=O)(=O)c1ccccc1)C1C(=O)CC(Cc2ccccc2)NC1=O Show InChI InChI=1S/C25H22N2O5S/c28-22-16-19(15-17-7-3-1-4-8-17)27-25(30)23(22)24(29)26-18-11-13-21(14-12-18)33(31,32)20-9-5-2-6-10-20/h1-14,19,23H,15-16H2,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289902
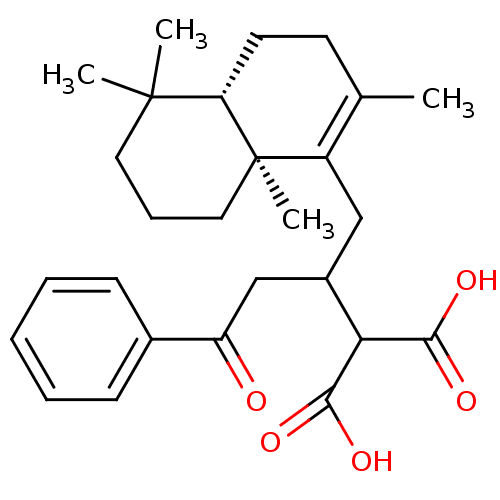
(2-[3-Oxo-3-phenyl-1-((4aS,8aS)-2,5,5,8a-tetramethy...)Show SMILES CC1=C(CC(CC(=O)c2ccccc2)C(C(O)=O)C(O)=O)[C@@]2(C)CCCC(C)(C)[C@@H]2CC1 |c:1| Show InChI InChI=1S/C27H36O5/c1-17-11-12-22-26(2,3)13-8-14-27(22,4)20(17)15-19(23(24(29)30)25(31)32)16-21(28)18-9-6-5-7-10-18/h5-7,9-10,19,22-23H,8,11-16H2,1-4H3,(H,29,30)(H,31,32)/t19?,22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372779

(CHEMBL271282)Show SMILES O=C(Nc1ccc(cc1)-n1ccnc1)C1C(=O)NCC(Cc2ccccc2)C1=O Show InChI InChI=1S/C22H20N4O3/c27-20-16(12-15-4-2-1-3-5-15)13-24-21(28)19(20)22(29)25-17-6-8-18(9-7-17)26-11-10-23-14-26/h1-11,14,16,19H,12-13H2,(H,24,28)(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against VHR phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50289900

(CHEMBL303581 | Sulfuric acid mono-[5-furan-3-yl-1-...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCCCc1ccoc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H38O5S/c1-18-10-11-22-23(2,3)13-7-14-24(22,4)21(18)16-20(29-30(25,26)27)9-6-5-8-19-12-15-28-17-19/h10,12,15,17,20-22H,5-9,11,13-14,16H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289903
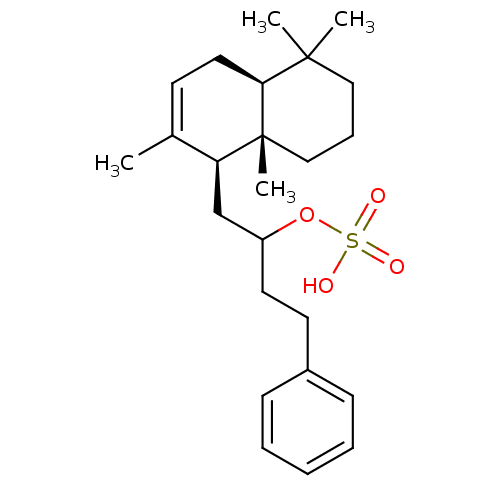
(CHEMBL64690 | Sulfuric acid mono-[3-phenyl-1-((1S,...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H36O4S/c1-18-11-14-22-23(2,3)15-8-16-24(22,4)21(18)17-20(28-29(25,26)27)13-12-19-9-6-5-7-10-19/h5-7,9-11,20-22H,8,12-17H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50289903

(CHEMBL64690 | Sulfuric acid mono-[3-phenyl-1-((1S,...)Show SMILES CC1=CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCc1ccccc1)OS(O)(=O)=O |t:1| Show InChI InChI=1S/C24H36O4S/c1-18-11-14-22-23(2,3)15-8-16-24(22,4)21(18)17-20(28-29(25,26)27)13-12-19-9-6-5-7-10-19/h5-7,9-11,20-22H,8,12-17H2,1-4H3,(H,25,26,27)/t20?,21-,22-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against Cdc25A phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50289897

(CHEMBL60297 | Sulfuric acid mono-[5-furan-3-yl-2-m...)Show SMILES CC(CCCc1ccoc1)C(C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS(O)(=O)=O |c:15| Show InChI InChI=1S/C25H40O5S/c1-18-10-11-23-24(3,4)13-7-14-25(23,5)21(18)16-22(30-31(26,27)28)19(2)8-6-9-20-12-15-29-17-20/h10,12,15,17,19,21-23H,6-9,11,13-14,16H2,1-5H3,(H,26,27,28)/t19?,21-,22?,23-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372764
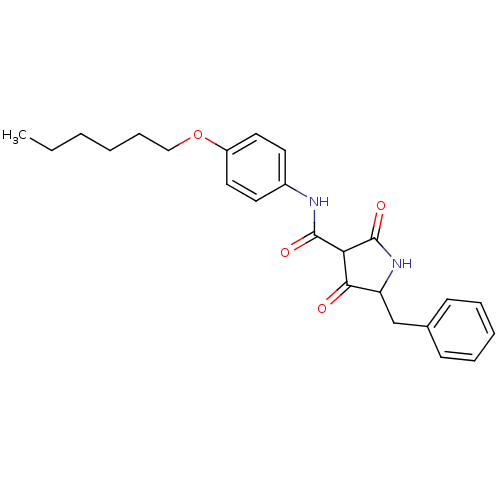
(CHEMBL272703)Show SMILES CCCCCCOc1ccc(NC(=O)C2C(=O)NC(Cc3ccccc3)C2=O)cc1 Show InChI InChI=1S/C24H28N2O4/c1-2-3-4-8-15-30-19-13-11-18(12-14-19)25-23(28)21-22(27)20(26-24(21)29)16-17-9-6-5-7-10-17/h5-7,9-14,20-21H,2-4,8,15-16H2,1H3,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50289906

(2-[3-Phenyl-1-((1S,2S,4aS,8aR)-2,5,5,8a-tetramethy...)Show SMILES C[C@H]1CC[C@H]2C(C)(C)CCC[C@]2(C)[C@H]1CC(CCc1ccccc1)C(C(O)=O)C(O)=O Show InChI InChI=1S/C27H40O4/c1-18-11-14-22-26(2,3)15-8-16-27(22,4)21(18)17-20(23(24(28)29)25(30)31)13-12-19-9-6-5-7-10-19/h5-7,9-10,18,20-23H,8,11-17H2,1-4H3,(H,28,29)(H,30,31)/t18-,20?,21-,22-,27+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration required for its inhibitory activity against PTB1B phosphatase |
Bioorg Med Chem Lett 7: 2015-2020 (1997)
Article DOI: 10.1016/S0960-894X(97)00357-0
BindingDB Entry DOI: 10.7270/Q28S4PXX |
More data for this
Ligand-Target Pair | |
Isoprenyl transferase
(Streptococcus pneumoniae) | BDBM50372757
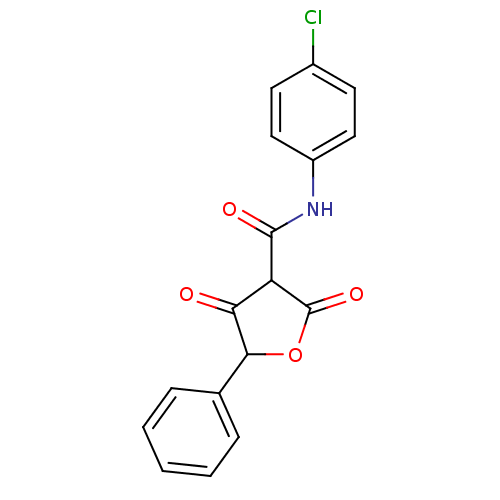
(CHEMBL256033)Show InChI InChI=1S/C17H12ClNO4/c18-11-6-8-12(9-7-11)19-16(21)13-14(20)15(23-17(13)22)10-4-2-1-3-5-10/h1-9,13,15H,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae UPPS |
Bioorg Med Chem Lett 18: 1840-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.009
BindingDB Entry DOI: 10.7270/Q2CF9QZ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data