

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121552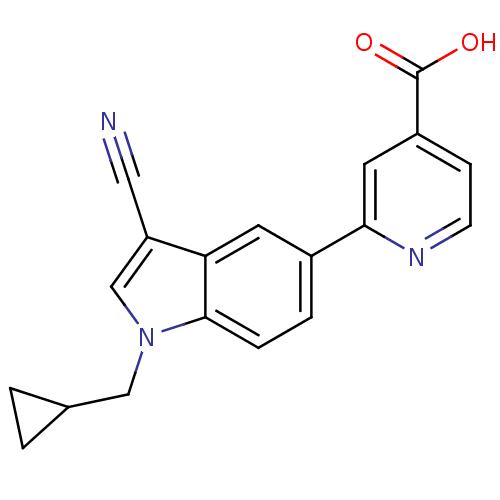 (US8729273, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121532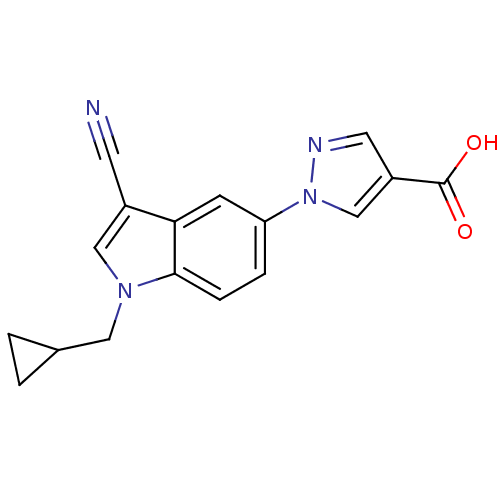 (US8729273, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001935 (CHEMBL3233601) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Danio rerio) | BDBM50525571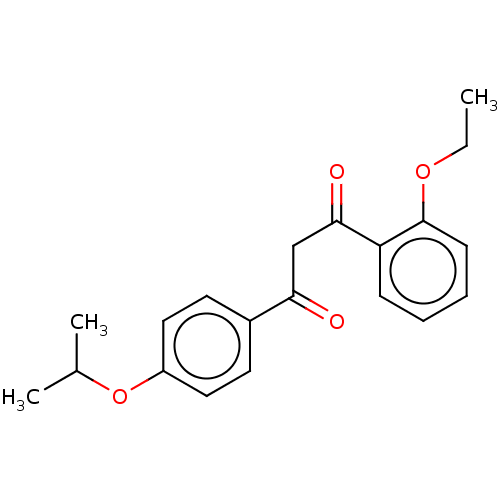 (CHEMBL4467659) | MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at zebrafish AhR2 expressed in COS7 cells transfected with ARNT-1c, Cyp1a-firefly luciferase and pRL-TK Renilla luciferase plasmi... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.07.030 BindingDB Entry DOI: 10.7270/Q25B05WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121531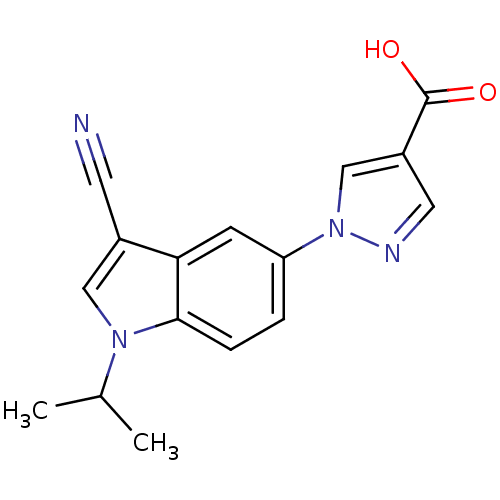 (US8729273, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121536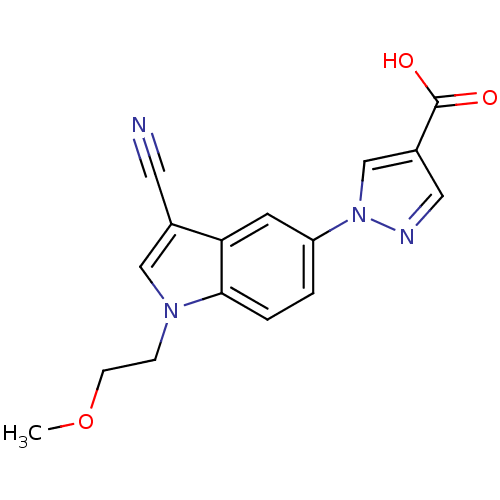 (US8729273, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121534 (US8729273, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121539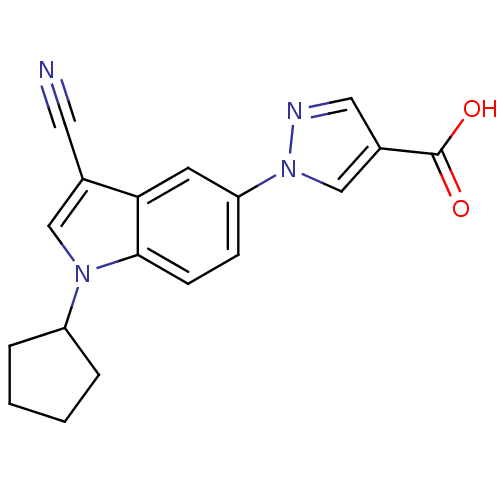 (US8729273, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121547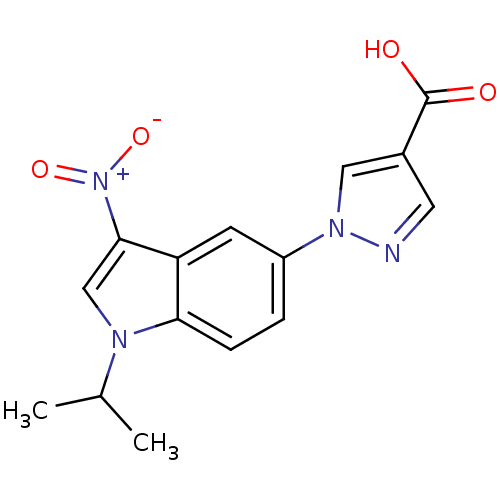 (US8729273, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121550 (US8729273, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121543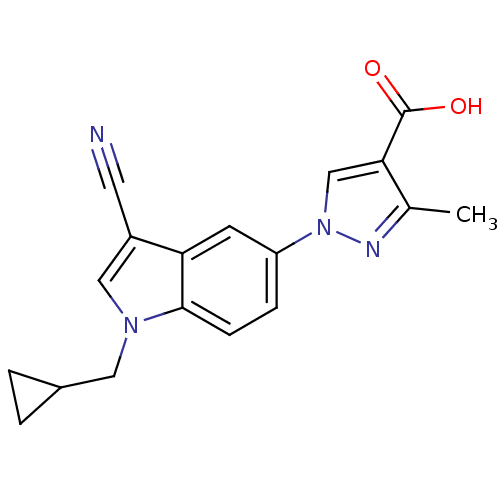 (US8729273, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121559 (US8729273, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121535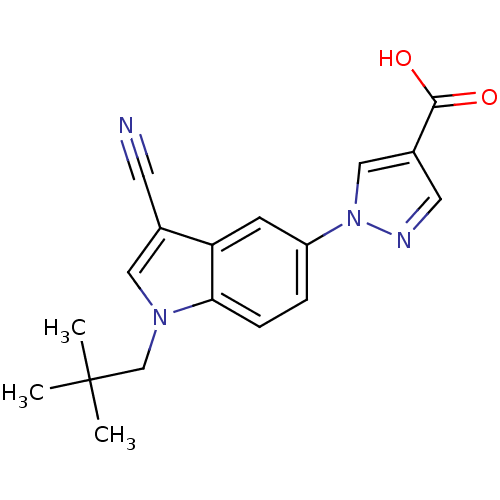 (US8729273, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121540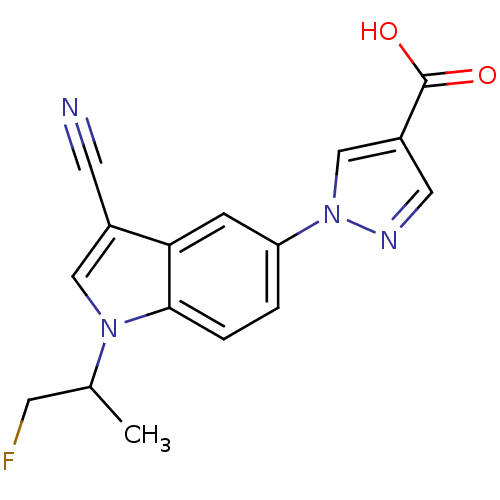 (US8729273, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121541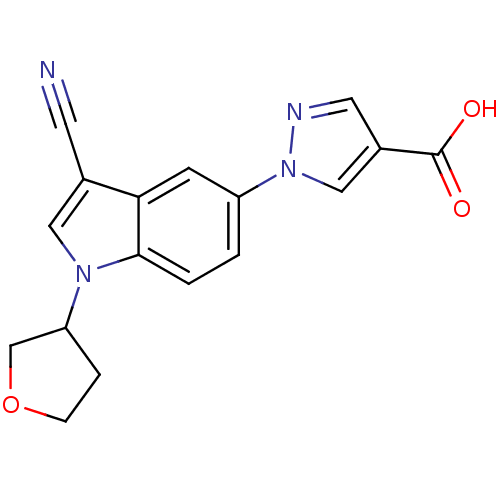 (US8729273, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001936 (CHEMBL3233602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121533 (US8729273, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121551 (US8729273, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121554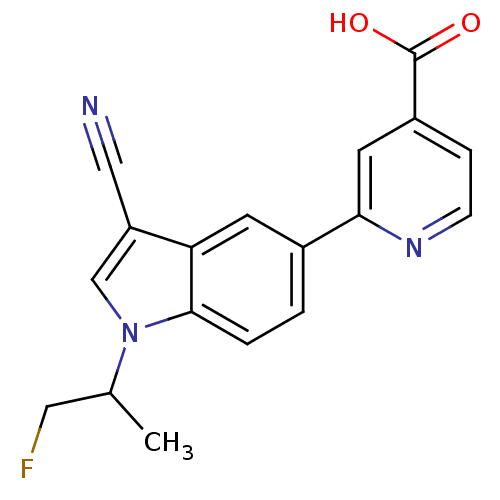 (US8729273, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121537 (US8729273, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121538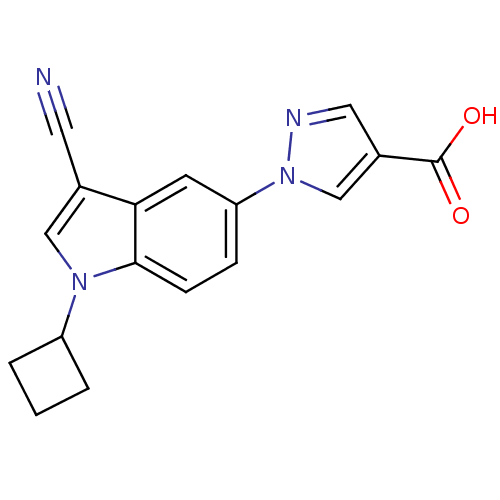 (US8729273, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50029593 (CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001934 (CHEMBL3233600) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Danio rerio) | BDBM50525574 (CHEMBL4546269) | MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at zebrafish AhR2 expressed in COS7 cells transfected with ARNT-1c, Cyp1a-firefly luciferase and pRL-TK Renilla luciferase plasmi... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.07.030 BindingDB Entry DOI: 10.7270/Q25B05WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121555 (US8729273, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001936 (CHEMBL3233602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121553 (US8729273, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121557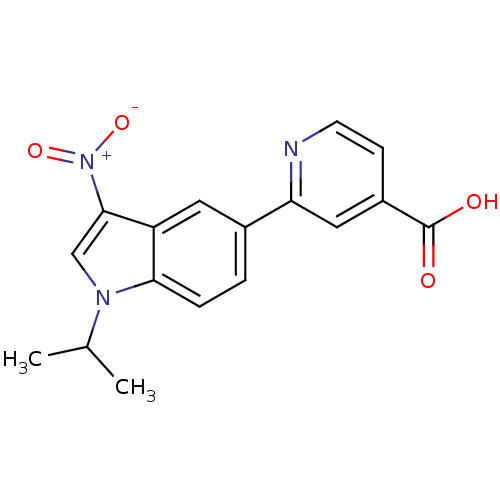 (US8729273, 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50001933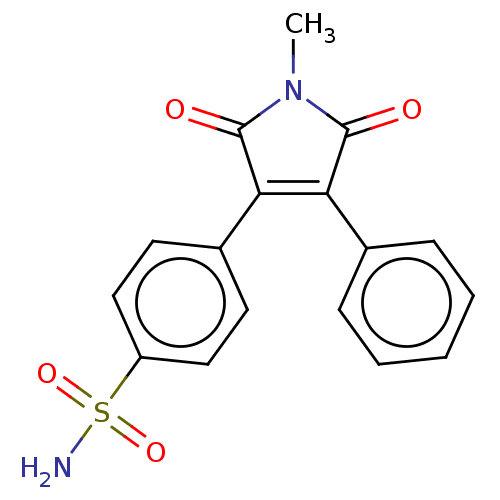 (CHEMBL3233599) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Danio rerio) | BDBM50525570 (CHEMBL4463944) | MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at zebrafish AhR2 expressed in COS7 cells transfected with ARNT-1c, Cyp1a-firefly luciferase and pRL-TK Renilla luciferase plasmi... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.07.030 BindingDB Entry DOI: 10.7270/Q25B05WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121542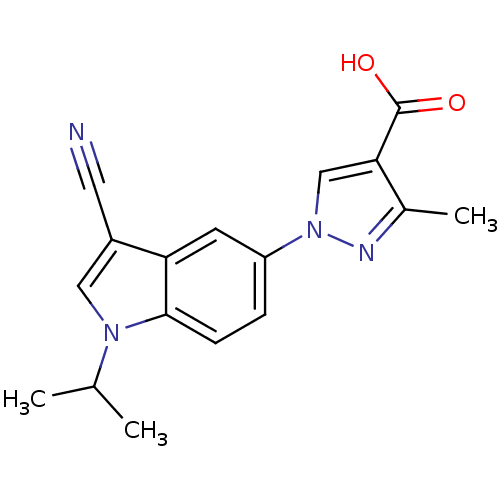 (US8729273, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121544 (US8729273, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121548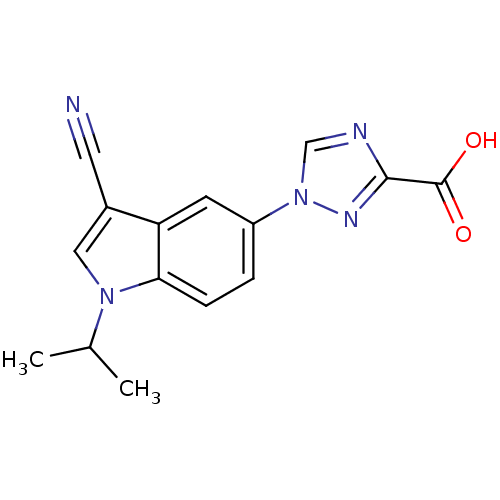 (US8729273, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Danio rerio) | BDBM50525573 (CHEMBL4470684) | MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at zebrafish AhR2 expressed in COS7 cells transfected with ARNT-1c, Cyp1a-firefly luciferase and pRL-TK Renilla luciferase plasmi... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.07.030 BindingDB Entry DOI: 10.7270/Q25B05WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121560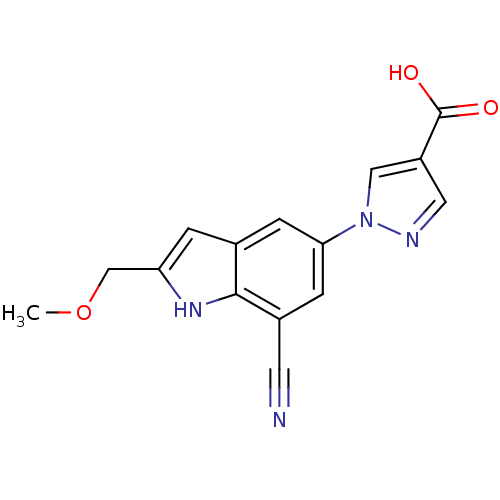 (US8729273, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121549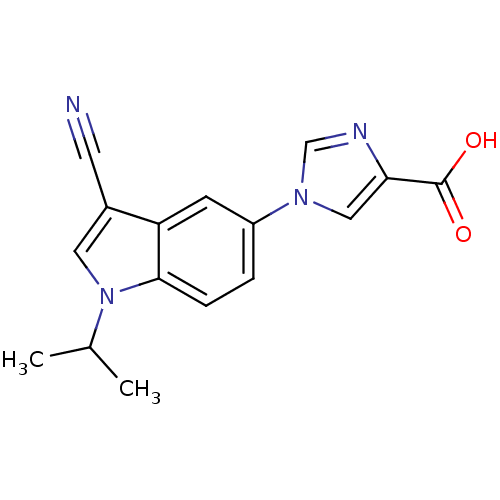 (US8729273, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121558 (US8729273, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001933 (CHEMBL3233599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM121561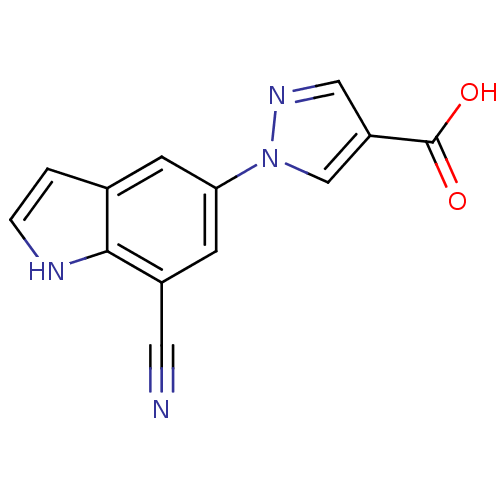 (US8729273, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. US Patent | Assay Description Xanthine oxidase originated from bovine milk was incubated for 3 min with test compounds, and the initial velocity of uric acid formation was determi... | US Patent US8729273 (2014) BindingDB Entry DOI: 10.7270/Q2D79937 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001935 (CHEMBL3233601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of HDAC6 after 30 mins by Fluor de Lys fluorescence assay | Bioorg Med Chem Lett 21: 6139-42 (2011) Article DOI: 10.1016/j.bmcl.2011.08.027 BindingDB Entry DOI: 10.7270/Q2X34XVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of HDAC6 using Fluor-de-Lys as substrate compound pretreated for 30 mins before substrate addition by fluorescence assay | Bioorg Med Chem Lett 22: 7084-6 (2012) Article DOI: 10.1016/j.bmcl.2012.09.093 BindingDB Entry DOI: 10.7270/Q2BV7HTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50001934 (CHEMBL3233600) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... | Bioorg Med Chem Lett 24: 1958-62 (2014) Article DOI: 10.1016/j.bmcl.2014.02.074 BindingDB Entry DOI: 10.7270/Q2SN0BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of HDAC1 using Fluor-de-Lys as substrate compound pretreated for 30 mins before substrate addition by fluorescence assay | Bioorg Med Chem Lett 22: 7084-6 (2012) Article DOI: 10.1016/j.bmcl.2012.09.093 BindingDB Entry DOI: 10.7270/Q2BV7HTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of HDAC1 after 30 mins by Fluor de Lys fluorescence assay | Bioorg Med Chem Lett 21: 6139-42 (2011) Article DOI: 10.1016/j.bmcl.2011.08.027 BindingDB Entry DOI: 10.7270/Q2X34XVX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aryl hydrocarbon receptor (Danio rerio) | BDBM50525572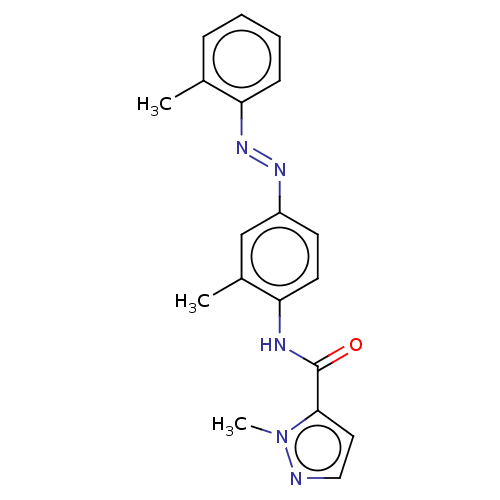 (CH-223191 | CHEMBL1743245) | MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at zebrafish AhR2 expressed in COS7 cells transfected with ARNT-1c, Cyp1a-firefly luciferase and pRL-TK Renilla luciferase plasmi... | Bioorg Med Chem 27: (2019) Article DOI: 10.1016/j.bmc.2019.07.030 BindingDB Entry DOI: 10.7270/Q25B05WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
KRIBB Curated by ChEMBL | Assay Description Inhibition of HDAC in HeLa cells by fluorescent activity assay | J Med Chem 50: 2737-41 (2007) Article DOI: 10.1021/jm0613828 BindingDB Entry DOI: 10.7270/Q2833VTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50476373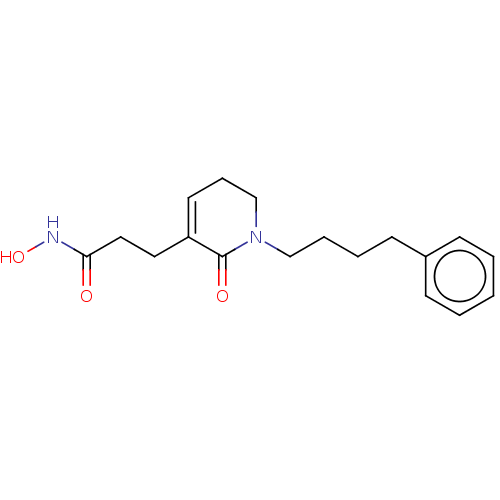 (CHEMBL226224) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
KRIBB Curated by ChEMBL | Assay Description Inhibition of HDAC in HeLa cells by fluorescent activity assay | J Med Chem 50: 2737-41 (2007) Article DOI: 10.1021/jm0613828 BindingDB Entry DOI: 10.7270/Q2833VTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1383 total ) | Next | Last >> |