 Found 79 hits with Last Name = 'crist' and Initial = 'm'
Found 79 hits with Last Name = 'crist' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin-releasing peptide receptor
(RAT) | BDBM50005601
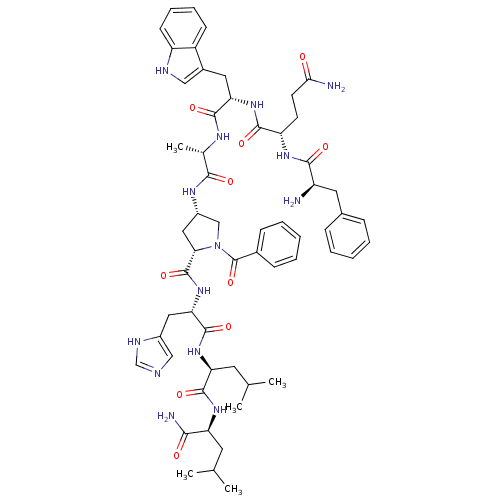
(CHEMBL2369819)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1C[C@@H](CN1C(=O)c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C58H76N14O10/c1-32(2)22-44(50(61)74)68-55(79)45(23-33(3)4)69-56(80)47(26-38-29-62-31-64-38)71-57(81)48-27-39(30-72(48)58(82)36-16-10-7-11-17-36)66-51(75)34(5)65-54(78)46(25-37-28-63-42-19-13-12-18-40(37)42)70-53(77)43(20-21-49(60)73)67-52(76)41(59)24-35-14-8-6-9-15-35/h6-19,28-29,31-34,39,41,43-48,63H,20-27,30,59H2,1-5H3,(H2,60,73)(H2,61,74)(H,62,64)(H,65,78)(H,66,75)(H,67,76)(H,68,79)(H,69,80)(H,70,77)(H,71,81)/t34-,39-,41+,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50408923
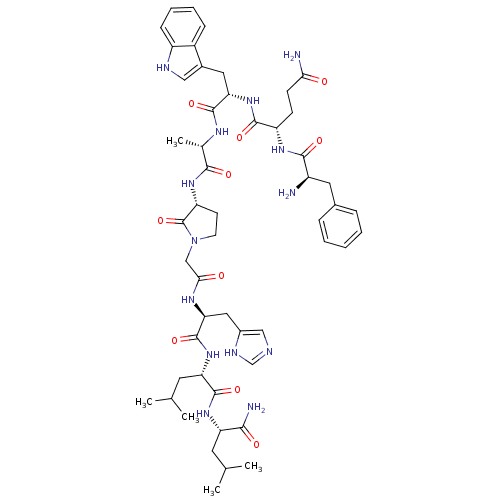
(CHEMBL2114155)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CN1CC[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C52H72N14O10/c1-28(2)19-39(45(55)69)63-50(74)40(20-29(3)4)64-51(75)42(23-33-25-56-27-58-33)60-44(68)26-66-18-17-38(52(66)76)62-46(70)30(5)59-49(73)41(22-32-24-57-36-14-10-9-13-34(32)36)65-48(72)37(15-16-43(54)67)61-47(71)35(53)21-31-11-7-6-8-12-31/h6-14,24-25,27-30,35,37-42,57H,15-23,26,53H2,1-5H3,(H2,54,67)(H2,55,69)(H,56,58)(H,59,73)(H,60,68)(H,61,71)(H,62,70)(H,63,74)(H,64,75)(H,65,72)/t30-,35+,37-,38+,39-,40-,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089280
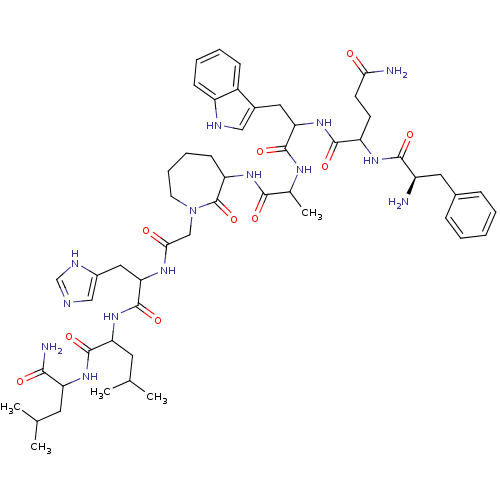
(CHEMBL437655 | JMV 1535)Show SMILES CC(C)CC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1CCCCC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C54H76N14O10/c1-30(2)21-41(47(57)71)65-52(76)42(22-31(3)4)66-53(77)44(25-35-27-58-29-60-35)62-46(70)28-68-20-12-11-17-40(54(68)78)64-48(72)32(5)61-51(75)43(24-34-26-59-38-16-10-9-15-36(34)38)67-50(74)39(18-19-45(56)69)63-49(73)37(55)23-33-13-7-6-8-14-33/h6-10,13-16,26-27,29-32,37,39-44,59H,11-12,17-25,28,55H2,1-5H3,(H2,56,69)(H2,57,71)(H,58,60)(H,61,75)(H,62,70)(H,63,73)(H,64,72)(H,65,76)(H,66,77)(H,67,74)/t32?,37-,39?,40?,41?,42?,43?,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089284
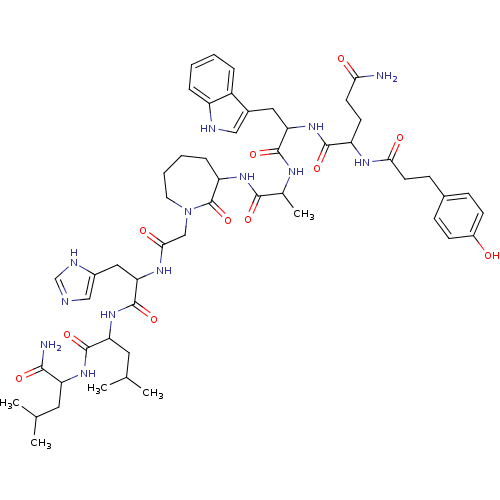
(CHEMBL265856 | JMV 1803)Show SMILES CC(C)CC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1CCCCC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)CCc2ccc(O)cc2)C1=O)C(N)=O Show InChI InChI=1S/C54H75N13O11/c1-30(2)22-41(48(56)72)64-52(76)42(23-31(3)4)65-53(77)44(25-35-27-57-29-59-35)62-47(71)28-67-21-9-8-12-40(54(67)78)63-49(73)32(5)60-51(75)43(24-34-26-58-38-11-7-6-10-37(34)38)66-50(74)39(18-19-45(55)69)61-46(70)20-15-33-13-16-36(68)17-14-33/h6-7,10-11,13-14,16-17,26-27,29-32,39-44,58,68H,8-9,12,15,18-25,28H2,1-5H3,(H2,55,69)(H2,56,72)(H,57,59)(H,60,75)(H,61,70)(H,62,71)(H,63,73)(H,64,76)(H,65,77)(H,66,74) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089285
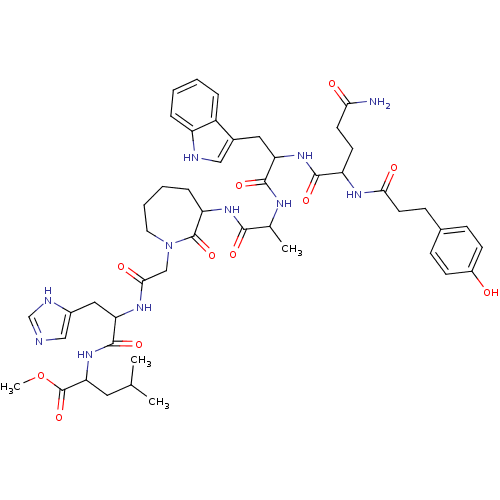
(CHEMBL415110 | JMV 1799)Show SMILES COC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1CCCCC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)CCc2ccc(O)cc2)C1=O Show InChI InChI=1S/C49H65N11O11/c1-28(2)21-40(49(70)71-4)59-47(68)39(23-32-25-51-27-53-32)56-43(64)26-60-20-8-7-11-37(48(60)69)57-44(65)29(3)54-46(67)38(22-31-24-52-35-10-6-5-9-34(31)35)58-45(66)36(17-18-41(50)62)55-42(63)19-14-30-12-15-33(61)16-13-30/h5-6,9-10,12-13,15-16,24-25,27-29,36-40,52,61H,7-8,11,14,17-23,26H2,1-4H3,(H2,50,62)(H,51,53)(H,54,67)(H,55,63)(H,56,64)(H,57,65)(H,58,66)(H,59,68) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089282
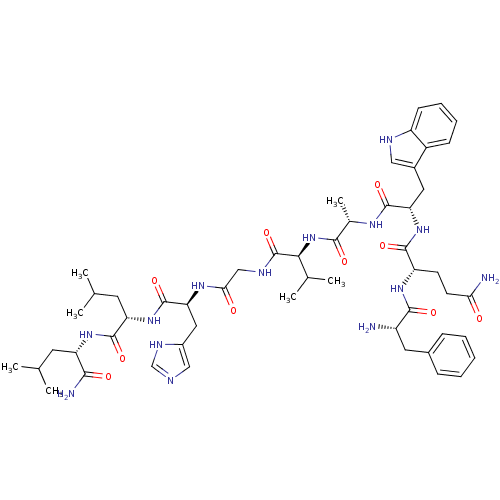
((D)Phe-Gln-Trp-Ala-Val-Gly-His-Leu-Leu-NH2 | CHEMB...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)C(N)=O Show InChI InChI=1S/C53H76N14O10/c1-28(2)19-39(46(56)70)64-51(75)40(20-29(3)4)65-52(76)42(23-34-25-57-27-60-34)62-44(69)26-59-53(77)45(30(5)6)67-47(71)31(7)61-50(74)41(22-33-24-58-37-16-12-11-15-35(33)37)66-49(73)38(17-18-43(55)68)63-48(72)36(54)21-32-13-9-8-10-14-32/h8-16,24-25,27-31,36,38-42,45,58H,17-23,26,54H2,1-7H3,(H2,55,68)(H2,56,70)(H,57,60)(H,59,77)(H,61,74)(H,62,69)(H,63,72)(H,64,75)(H,65,76)(H,66,73)(H,67,71)/t31-,36-,38-,39-,40-,41-,42-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089281
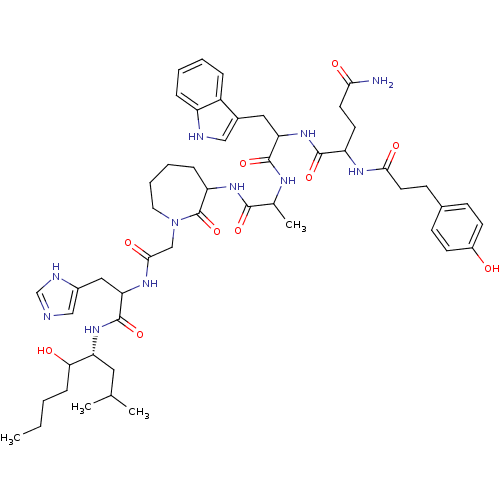
(CHEMBL386480 | JMV 1802)Show SMILES CCCCC(O)[C@@H](CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1CCCCC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)CCc2ccc(O)cc2)C1=O Show InChI InChI=1S/C52H73N11O10/c1-5-6-14-44(65)41(24-31(2)3)61-51(72)43(26-35-28-54-30-56-35)59-47(68)29-63-23-10-9-13-40(52(63)73)60-48(69)32(4)57-50(71)42(25-34-27-55-38-12-8-7-11-37(34)38)62-49(70)39(20-21-45(53)66)58-46(67)22-17-33-15-18-36(64)19-16-33/h7-8,11-12,15-16,18-19,27-28,30-32,39-44,55,64-65H,5-6,9-10,13-14,17,20-26,29H2,1-4H3,(H2,53,66)(H,54,56)(H,57,71)(H,58,67)(H,59,68)(H,60,69)(H,61,72)(H,62,70)/t32?,39?,40?,41-,42?,43?,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089291
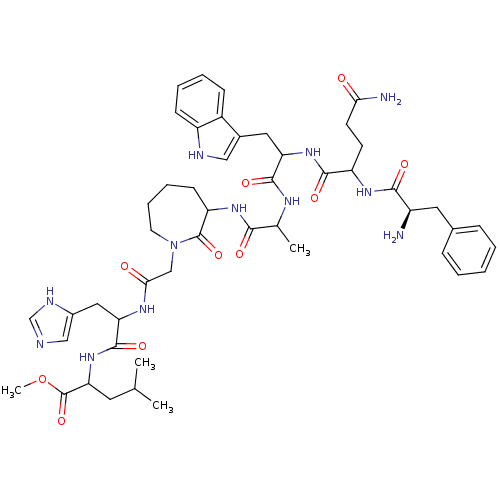
(CHEMBL415021 | JMV 1813)Show SMILES COC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1CCCCC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O Show InChI InChI=1S/C49H66N12O10/c1-28(2)20-40(49(70)71-4)60-47(68)39(23-32-25-52-27-54-32)56-42(63)26-61-19-11-10-16-37(48(61)69)58-43(64)29(3)55-46(67)38(22-31-24-53-35-15-9-8-14-33(31)35)59-45(66)36(17-18-41(51)62)57-44(65)34(50)21-30-12-6-5-7-13-30/h5-9,12-15,24-25,27-29,34,36-40,53H,10-11,16-23,26,50H2,1-4H3,(H2,51,62)(H,52,54)(H,55,67)(H,56,63)(H,57,65)(H,58,64)(H,59,66)(H,60,68)/t29?,34-,36?,37?,38?,39?,40?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089278
(CHEMBL411016 | JMV 1801)Show SMILES CCCCC(O)[C@@H](CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1CCCCC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O Show InChI InChI=1S/C52H74N12O9/c1-5-6-19-44(65)41(23-31(2)3)62-51(72)43(26-35-28-55-30-57-35)59-46(67)29-64-22-13-12-18-40(52(64)73)61-47(68)32(4)58-50(71)42(25-34-27-56-38-17-11-10-16-36(34)38)63-49(70)39(20-21-45(54)66)60-48(69)37(53)24-33-14-8-7-9-15-33/h7-11,14-17,27-28,30-32,37,39-44,56,65H,5-6,12-13,18-26,29,53H2,1-4H3,(H2,54,66)(H,55,57)(H,58,71)(H,59,67)(H,60,69)(H,61,68)(H,62,72)(H,63,70)/t32?,37-,39?,40?,41-,42?,43?,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089279
(CHEMBL406013 | JMV 1719)Show SMILES CC(C)C[C@@H](NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1CCCCC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)CCc2ccc(O)cc2)C1=O)C(=O)NO Show InChI InChI=1S/C48H64N12O11/c1-27(2)20-37(47(69)59-71)57-46(68)39(22-31-24-50-26-52-31)55-42(64)25-60-19-7-6-10-36(48(60)70)56-43(65)28(3)53-45(67)38(21-30-23-51-34-9-5-4-8-33(30)34)58-44(66)35(16-17-40(49)62)54-41(63)18-13-29-11-14-32(61)15-12-29/h4-5,8-9,11-12,14-15,23-24,26-28,35-39,51,61,71H,6-7,10,13,16-22,25H2,1-3H3,(H2,49,62)(H,50,52)(H,53,67)(H,54,63)(H,55,64)(H,56,65)(H,57,68)(H,58,66)(H,59,69)/t28?,35?,36?,37-,38?,39?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089286
(CHEMBL407022 | JMV 1693)Show SMILES CC(C)C[C@@H](NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1CCCCC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O)C(=O)NO Show InChI InChI=1S/C48H65N13O10/c1-27(2)19-37(47(69)60-71)58-46(68)39(22-31-24-51-26-53-31)55-41(63)25-61-18-10-9-15-36(48(61)70)57-42(64)28(3)54-45(67)38(21-30-23-52-34-14-8-7-13-32(30)34)59-44(66)35(16-17-40(50)62)56-43(65)33(49)20-29-11-5-4-6-12-29/h4-8,11-14,23-24,26-28,33,35-39,52,71H,9-10,15-22,25,49H2,1-3H3,(H2,50,62)(H,51,53)(H,54,67)(H,55,63)(H,56,65)(H,57,64)(H,58,68)(H,59,66)(H,60,69)/t28?,33-,35?,36?,37-,38?,39?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50408922
(CHEMBL2115179)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CN1CC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C52H72N14O10/c1-28(2)19-39(45(55)69)63-50(74)40(20-29(3)4)64-51(75)42(23-33-25-56-27-58-33)60-44(68)26-66-18-17-38(52(66)76)62-46(70)30(5)59-49(73)41(22-32-24-57-36-14-10-9-13-34(32)36)65-48(72)37(15-16-43(54)67)61-47(71)35(53)21-31-11-7-6-8-12-31/h6-14,24-25,27-30,35,37-42,57H,15-23,26,53H2,1-5H3,(H2,54,67)(H2,55,69)(H,56,58)(H,59,73)(H,60,68)(H,61,71)(H,62,70)(H,63,74)(H,64,75)(H,65,72)/t30-,35+,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089288
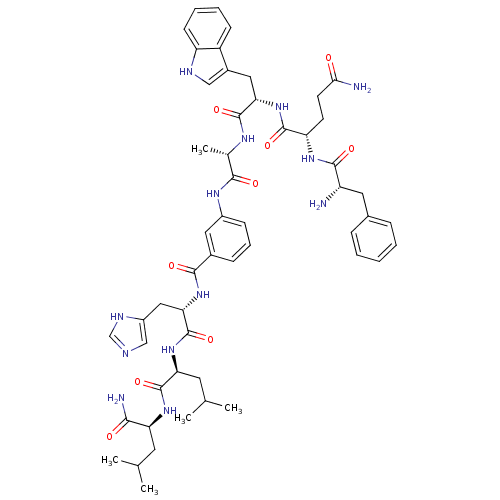
(Bombesin analogues | CHEMBL415022)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)c1cccc(NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)Cc2ccccc2)c1)C(N)=O Show InChI InChI=1S/C53H69N13O9/c1-29(2)20-41(46(56)68)63-52(74)42(21-30(3)4)65-53(75)44(25-36-27-57-28-59-36)64-48(70)33-14-11-15-35(23-33)61-47(69)31(5)60-51(73)43(24-34-26-58-39-17-10-9-16-37(34)39)66-50(72)40(18-19-45(55)67)62-49(71)38(54)22-32-12-7-6-8-13-32/h6-17,23,26-31,38,40-44,58H,18-22,24-25,54H2,1-5H3,(H2,55,67)(H2,56,68)(H,57,59)(H,60,73)(H,61,69)(H,62,71)(H,63,74)(H,64,70)(H,65,75)(H,66,72)/t31-,38-,40-,41-,42-,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089292
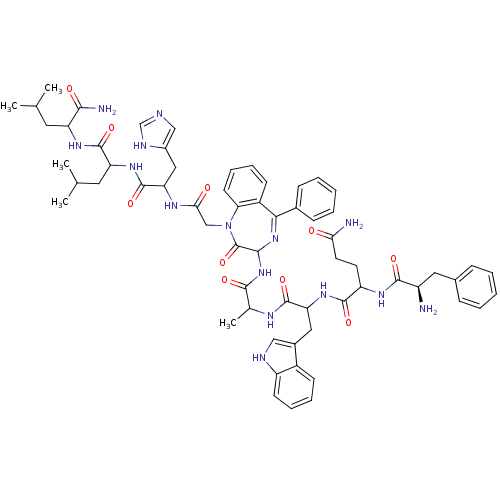
(Bombesin analogues | CHEMBL439440)Show SMILES CC(C)CC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CN1c2ccccc2C(=NC(NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O)c1ccccc1)C(N)=O |c:36| Show InChI InChI=1S/C63H77N15O10/c1-35(2)26-47(55(66)81)73-61(86)48(27-36(3)4)74-62(87)50(30-41-32-67-34-69-41)71-53(80)33-78-51-23-15-13-21-43(51)54(39-18-10-7-11-19-39)76-56(63(78)88)77-57(82)37(5)70-60(85)49(29-40-31-68-45-22-14-12-20-42(40)45)75-59(84)46(24-25-52(65)79)72-58(83)44(64)28-38-16-8-6-9-17-38/h6-23,31-32,34-37,44,46-50,56,68H,24-30,33,64H2,1-5H3,(H2,65,79)(H2,66,81)(H,67,69)(H,70,85)(H,71,80)(H,72,83)(H,73,86)(H,74,87)(H,75,84)(H,77,82)/t37?,44-,46?,47?,48?,49?,50?,56?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089290
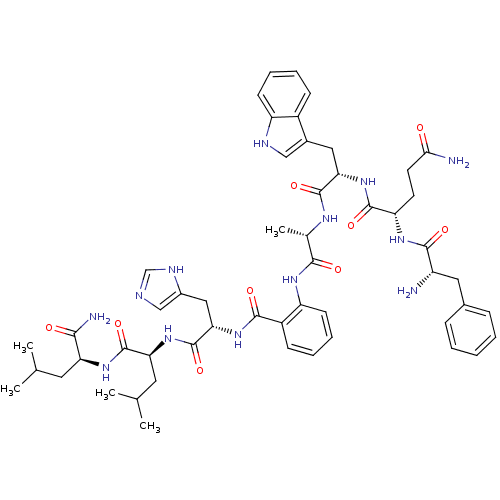
(Bombesin analogues | CHEMBL413938)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)c1ccccc1NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H69N13O9/c1-29(2)21-41(46(56)68)63-52(74)42(22-30(3)4)65-53(75)44(25-34-27-57-28-59-34)64-48(70)36-16-10-12-18-39(36)61-47(69)31(5)60-51(73)43(24-33-26-58-38-17-11-9-15-35(33)38)66-50(72)40(19-20-45(55)67)62-49(71)37(54)23-32-13-7-6-8-14-32/h6-18,26-31,37,40-44,58H,19-25,54H2,1-5H3,(H2,55,67)(H2,56,68)(H,57,59)(H,60,73)(H,61,69)(H,62,71)(H,63,74)(H,64,70)(H,65,75)(H,66,72)/t31-,37-,40-,41-,42-,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089293
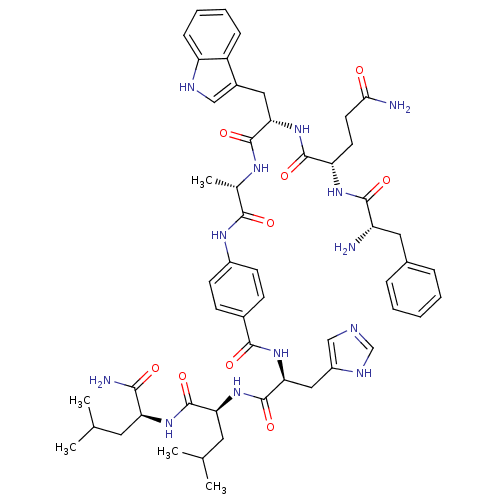
(Bombesin analogues | CHEMBL407481)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)c1ccc(NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)Cc2ccccc2)cc1)C(N)=O Show InChI InChI=1S/C53H69N13O9/c1-29(2)21-41(46(56)68)63-52(74)42(22-30(3)4)65-53(75)44(25-36-27-57-28-59-36)64-48(70)33-15-17-35(18-16-33)61-47(69)31(5)60-51(73)43(24-34-26-58-39-14-10-9-13-37(34)39)66-50(72)40(19-20-45(55)67)62-49(71)38(54)23-32-11-7-6-8-12-32/h6-18,26-31,38,40-44,58H,19-25,54H2,1-5H3,(H2,55,67)(H2,56,68)(H,57,59)(H,60,73)(H,61,69)(H,62,71)(H,63,74)(H,64,70)(H,65,75)(H,66,72)/t31-,38-,40-,41-,42-,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089287
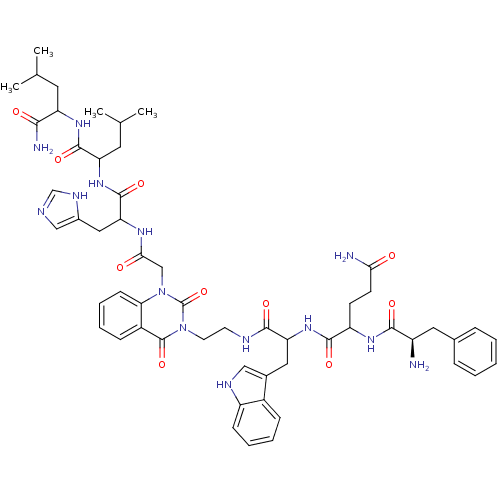
(Bombesin analogues | CHEMBL384242)Show SMILES CC(C)CC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)Cn1c2ccccc2c(=O)n(CCNC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)c1=O)C(N)=O Show InChI InChI=1S/C55H70N14O10/c1-31(2)22-41(48(58)72)65-52(76)42(23-32(3)4)66-53(77)44(26-35-28-59-30-62-35)63-47(71)29-69-45-17-11-9-15-37(45)54(78)68(55(69)79)21-20-60-50(74)43(25-34-27-61-39-16-10-8-14-36(34)39)67-51(75)40(18-19-46(57)70)64-49(73)38(56)24-33-12-6-5-7-13-33/h5-17,27-28,30-32,38,40-44,61H,18-26,29,56H2,1-4H3,(H2,57,70)(H2,58,72)(H,59,62)(H,60,74)(H,63,71)(H,64,73)(H,65,76)(H,66,77)(H,67,75)/t38-,40?,41?,42?,43?,44?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50089289
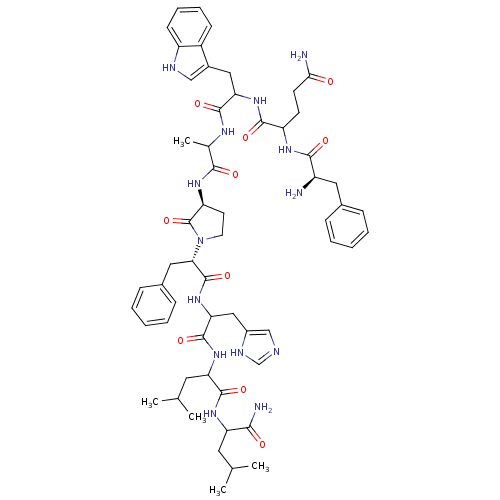
(Bombesin analogues | CHEMBL384241)Show SMILES CC(C)CC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)N1CC[C@H](NC(=O)C(C)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C59H78N14O10/c1-33(2)24-45(51(62)75)69-56(80)46(25-34(3)4)70-57(81)48(29-39-31-63-32-65-39)72-58(82)49(27-37-16-10-7-11-17-37)73-23-22-44(59(73)83)68-52(76)35(5)66-55(79)47(28-38-30-64-42-19-13-12-18-40(38)42)71-54(78)43(20-21-50(61)74)67-53(77)41(60)26-36-14-8-6-9-15-36/h6-19,30-35,41,43-49,64H,20-29,60H2,1-5H3,(H2,61,74)(H2,62,75)(H,63,65)(H,66,79)(H,67,77)(H,68,76)(H,69,80)(H,70,81)(H,71,78)(H,72,82)/t35?,41-,43?,44+,45?,46?,47?,48?,49+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50299583

((S)-N-(3-bromophenyl)-N'-cyano-2-methyl-4-(5-methy...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(Br)c1)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C20H21BrN8/c1-13-9-23-18-17(13)19(26-12-25-18)28-6-7-29(14(2)10-28)20(24-11-22)27-16-5-3-4-15(21)8-16/h3-5,8-9,12,14H,6-7,10H2,1-2H3,(H,24,27)(H,23,25,26)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of LIMK1 |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299619
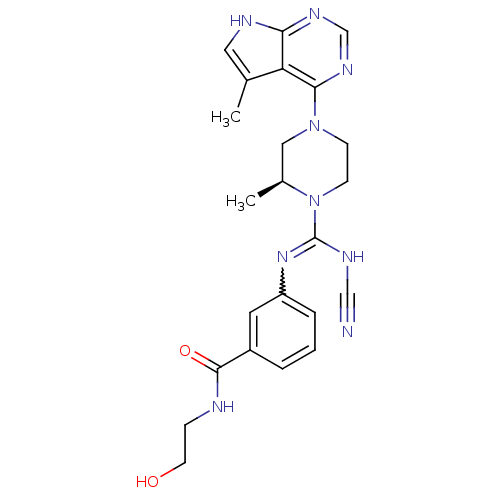
((S)-3-(N'-cyano-2-methyl-4-(5-methyl-7H-pyrrolo[2,...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(c1)C(=O)NCCO)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C23H27N9O2/c1-15-11-26-20-19(15)21(29-14-28-20)31-7-8-32(16(2)12-31)23(27-13-24)30-18-5-3-4-17(10-18)22(34)25-6-9-33/h3-5,10-11,14,16,33H,6-9,12H2,1-2H3,(H,25,34)(H,27,30)(H,26,28,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299583

((S)-N-(3-bromophenyl)-N'-cyano-2-methyl-4-(5-methy...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(Br)c1)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C20H21BrN8/c1-13-9-23-18-17(13)19(26-12-25-18)28-6-7-29(14(2)10-28)20(24-11-22)27-16-5-3-4-15(21)8-16/h3-5,8-9,12,14H,6-7,10H2,1-2H3,(H,24,27)(H,23,25,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299616
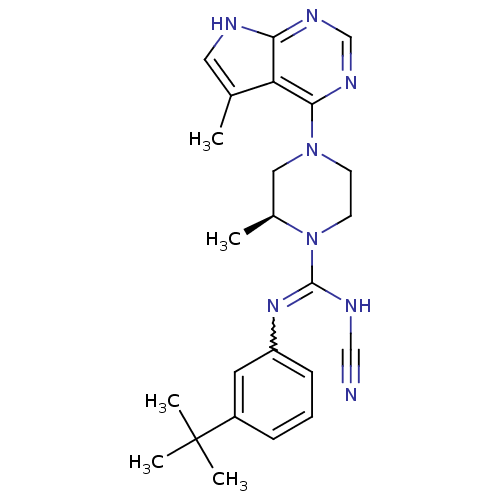
((S)-N-(3-tert-butylphenyl)-N'-cyano-2-methyl-4-(5-...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(c1)C(C)(C)C)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C24H30N8/c1-16-12-26-21-20(16)22(29-15-28-21)31-9-10-32(17(2)13-31)23(27-14-25)30-19-8-6-7-18(11-19)24(3,4)5/h6-8,11-12,15,17H,9-10,13H2,1-5H3,(H,27,30)(H,26,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299607

((S)-N-(3-bromophenyl)-4-(5-chloro-7H-pyrrolo[2,3-d...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(Br)c1)c1ncnc2[nH]cc(Cl)c12 |r,w:11.12| Show InChI InChI=1S/C19H18BrClN8/c1-12-9-28(18-16-15(21)8-23-17(16)25-11-26-18)5-6-29(12)19(24-10-22)27-14-4-2-3-13(20)7-14/h2-4,7-8,11-12H,5-6,9H2,1H3,(H,24,27)(H,23,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299586
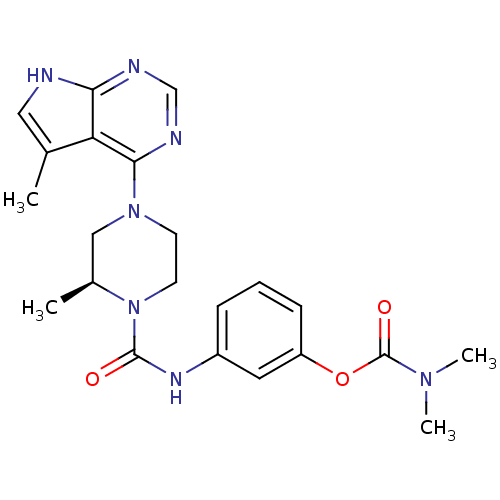
((S)-3-(2-methyl-4-(5-methyl-7H-pyrrolo[2,3-d]pyrim...)Show SMILES C[C@H]1CN(CCN1C(=O)Nc1cccc(OC(=O)N(C)C)c1)c1ncnc2[nH]cc(C)c12 |r| Show InChI InChI=1S/C22H27N7O3/c1-14-11-23-19-18(14)20(25-13-24-19)28-8-9-29(15(2)12-28)21(30)26-16-6-5-7-17(10-16)32-22(31)27(3)4/h5-7,10-11,13,15H,8-9,12H2,1-4H3,(H,26,30)(H,23,24,25)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299615

((S)-N'-cyano-N-(3-cyanophenyl)-2-methyl-4-(5-methy...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(c1)C#N)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C21H21N9/c1-14-10-24-19-18(14)20(27-13-26-19)29-6-7-30(15(2)11-29)21(25-12-23)28-17-5-3-4-16(8-17)9-22/h3-5,8,10,13,15H,6-7,11H2,1-2H3,(H,25,28)(H,24,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299584

((S)-N-(3-bromo-4-fluorophenyl)-N'-cyano-2-methyl-4...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1ccc(F)c(Br)c1)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C20H20BrFN8/c1-12-8-24-18-17(12)19(27-11-26-18)29-5-6-30(13(2)9-29)20(25-10-23)28-14-3-4-16(22)15(21)7-14/h3-4,7-8,11,13H,5-6,9H2,1-2H3,(H,25,28)(H,24,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299611
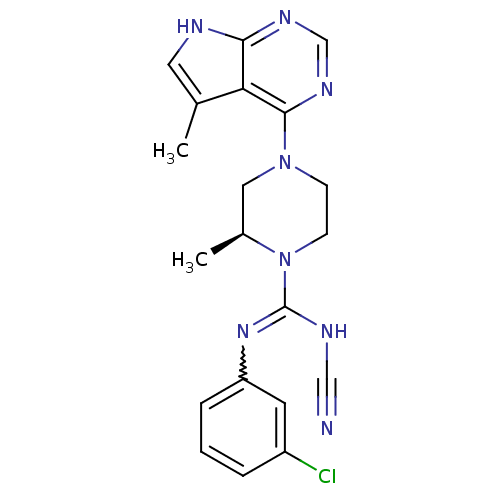
((S)-N-(3-chlorophenyl)-N'-cyano-2-methyl-4-(5-meth...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(Cl)c1)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C20H21ClN8/c1-13-9-23-18-17(13)19(26-12-25-18)28-6-7-29(14(2)10-28)20(24-11-22)27-16-5-3-4-15(21)8-16/h3-5,8-9,12,14H,6-7,10H2,1-2H3,(H,24,27)(H,23,25,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299623

((S)-N'-cyano-N-(3-(N-isopropylsulfamoyl)phenyl)-2-...)Show SMILES CC(C)NS(=O)(=O)c1cccc(c1)N=C(NC#N)N1CCN(C[C@@H]1C)c1ncnc2[nH]cc(C)c12 |r,w:13.13| Show InChI InChI=1S/C23H29N9O2S/c1-15(2)30-35(33,34)19-7-5-6-18(10-19)29-23(26-13-24)32-9-8-31(12-17(32)4)22-20-16(3)11-25-21(20)27-14-28-22/h5-7,10-11,14-15,17,30H,8-9,12H2,1-4H3,(H,26,29)(H,25,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299613
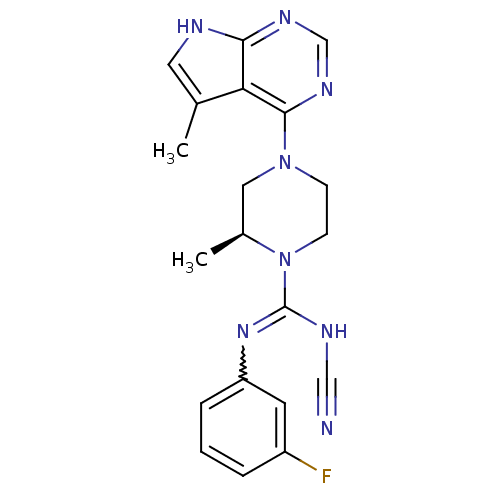
((S)-N'-cyano-N-(3-fluorophenyl)-2-methyl-4-(5-meth...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(F)c1)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C20H21FN8/c1-13-9-23-18-17(13)19(26-12-25-18)28-6-7-29(14(2)10-28)20(24-11-22)27-16-5-3-4-15(21)8-16/h3-5,8-9,12,14H,6-7,10H2,1-2H3,(H,24,27)(H,23,25,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299614
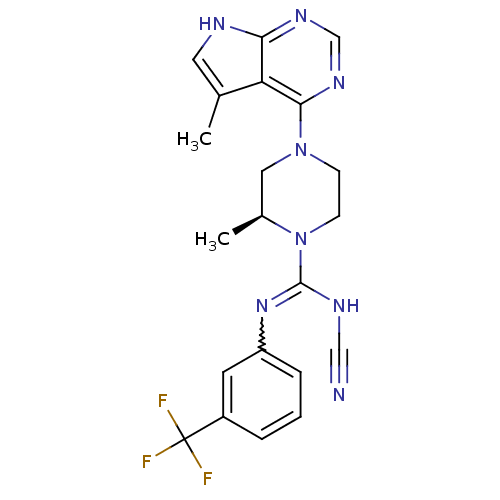
((S)-N'-cyano-2-methyl-4-(5-methyl-7H-pyrrolo[2,3-d...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(c1)C(F)(F)F)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C21H21F3N8/c1-13-9-26-18-17(13)19(29-12-28-18)31-6-7-32(14(2)10-31)20(27-11-25)30-16-5-3-4-15(8-16)21(22,23)24/h3-5,8-9,12,14H,6-7,10H2,1-2H3,(H,27,30)(H,26,28,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50243232

(CHEMBL486232 | GNF-PF-5434 | N-((S)-4-methyl-1-oxo...)Show SMILES CC(C)C[C@H](NC(=O)N1CCOCC1)C(=O)N[C@@H](CCc1ccccc1)C=CS(=O)(=O)c1ccccc1 |r,w:27.29| Show InChI InChI=1S/C28H37N3O5S/c1-22(2)21-26(30-28(33)31-16-18-36-19-17-31)27(32)29-24(14-13-23-9-5-3-6-10-23)15-20-37(34,35)25-11-7-4-8-12-25/h3-12,15,20,22,24,26H,13-14,16-19,21H2,1-2H3,(H,29,32)(H,30,33)/t24-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Algarve
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum recombinant falcipain 2 |
Bioorg Med Chem Lett 18: 4210-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.068
BindingDB Entry DOI: 10.7270/Q27H1JDB |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299612
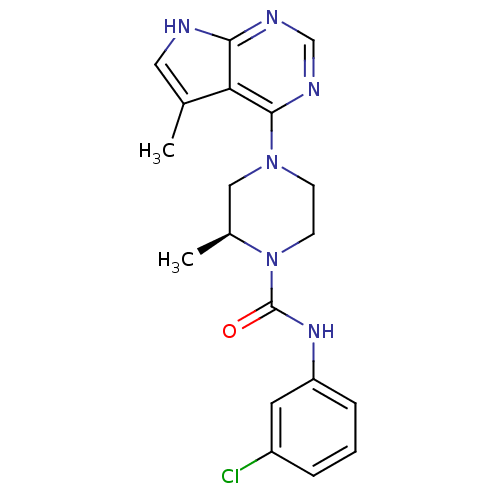
((S)-N-(3-chlorophenyl)-2-methyl-4-(5-methyl-7H-pyr...)Show SMILES C[C@H]1CN(CCN1C(=O)Nc1cccc(Cl)c1)c1ncnc2[nH]cc(C)c12 |r| Show InChI InChI=1S/C19H21ClN6O/c1-12-9-21-17-16(12)18(23-11-22-17)25-6-7-26(13(2)10-25)19(27)24-15-5-3-4-14(20)8-15/h3-5,8-9,11,13H,6-7,10H2,1-2H3,(H,24,27)(H,21,22,23)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299624
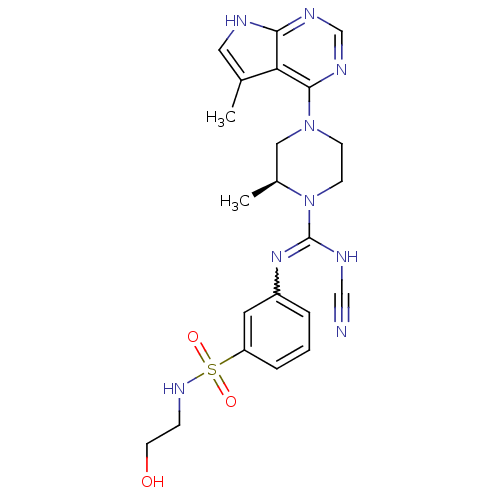
((S)-N'-cyano-N-(3-(N-(2-hydroxyethyl)sulfamoyl)phe...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(c1)S(=O)(=O)NCCO)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C22H27N9O3S/c1-15-11-24-20-19(15)21(27-14-26-20)30-7-8-31(16(2)12-30)22(25-13-23)29-17-4-3-5-18(10-17)35(33,34)28-6-9-32/h3-5,10-11,14,16,28,32H,6-9,12H2,1-2H3,(H,25,29)(H,24,26,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299620

((S)-N-(3-(2-hydroxyethylcarbamoyl)phenyl)-2-methyl...)Show SMILES C[C@H]1CN(CCN1C(=O)Nc1cccc(c1)C(=O)NCCO)c1ncnc2[nH]cc(C)c12 |r| Show InChI InChI=1S/C22H27N7O3/c1-14-11-24-19-18(14)20(26-13-25-19)28-7-8-29(15(2)12-28)22(32)27-17-5-3-4-16(10-17)21(31)23-6-9-30/h3-5,10-11,13,15,30H,6-9,12H2,1-2H3,(H,23,31)(H,27,32)(H,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299621
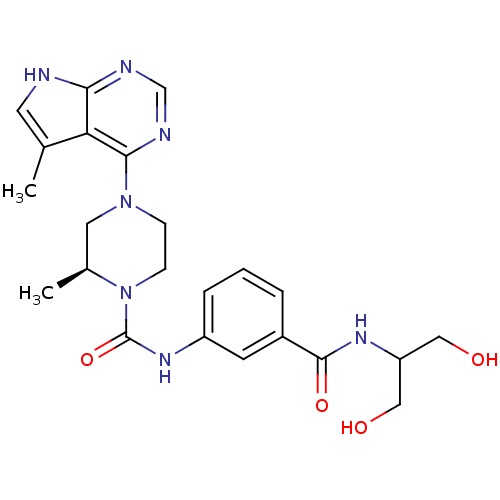
((S)-N-(3-(1,3-dihydroxypropan-2-ylcarbamoyl)phenyl...)Show SMILES C[C@H]1CN(CCN1C(=O)Nc1cccc(c1)C(=O)NC(CO)CO)c1ncnc2[nH]cc(C)c12 |r| Show InChI InChI=1S/C23H29N7O4/c1-14-9-24-20-19(14)21(26-13-25-20)29-6-7-30(15(2)10-29)23(34)28-17-5-3-4-16(8-17)22(33)27-18(11-31)12-32/h3-5,8-9,13,15,18,31-32H,6-7,10-12H2,1-2H3,(H,27,33)(H,28,34)(H,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50299586

((S)-3-(2-methyl-4-(5-methyl-7H-pyrrolo[2,3-d]pyrim...)Show SMILES C[C@H]1CN(CCN1C(=O)Nc1cccc(OC(=O)N(C)C)c1)c1ncnc2[nH]cc(C)c12 |r| Show InChI InChI=1S/C22H27N7O3/c1-14-11-23-19-18(14)20(25-13-24-19)28-8-9-29(15(2)12-28)21(30)26-16-6-5-7-17(10-16)32-22(31)27(3)4/h5-7,10-11,13,15H,8-9,12H2,1-4H3,(H,26,30)(H,23,24,25)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of LIMK1 |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299585
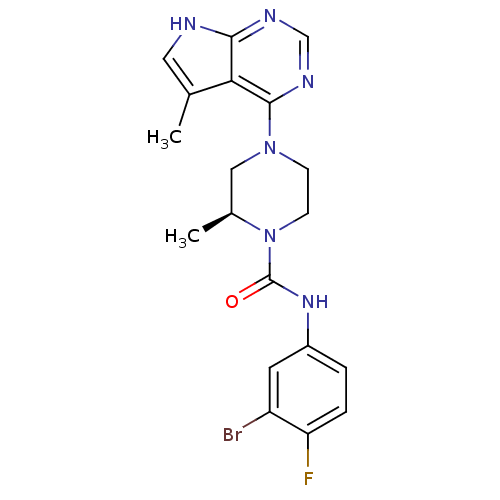
((S)-N-(3-bromo-4-fluorophenyl)-2-methyl-4-(5-methy...)Show SMILES C[C@H]1CN(CCN1C(=O)Nc1ccc(F)c(Br)c1)c1ncnc2[nH]cc(C)c12 |r| Show InChI InChI=1S/C19H20BrFN6O/c1-11-8-22-17-16(11)18(24-10-23-17)26-5-6-27(12(2)9-26)19(28)25-13-3-4-15(21)14(20)7-13/h3-4,7-8,10,12H,5-6,9H2,1-2H3,(H,25,28)(H,22,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299622
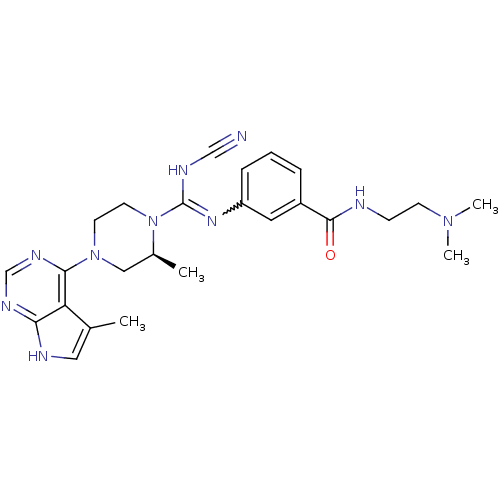
((S)-3-(N'-cyano-2-methyl-4-(5-methyl-7H-pyrrolo[2,...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(c1)C(=O)NCCN(C)C)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C25H32N10O/c1-17-13-28-22-21(17)23(31-16-30-22)34-10-11-35(18(2)14-34)25(29-15-26)32-20-7-5-6-19(12-20)24(36)27-8-9-33(3)4/h5-7,12-13,16,18H,8-11,14H2,1-4H3,(H,27,36)(H,29,32)(H,28,30,31)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299617

((S)-3-(N'-cyano-2-methyl-4-(5-methyl-7H-pyrrolo[2,...)Show SMILES CC(C)NC(=O)c1cccc(c1)N=C(NC#N)N1CCN(C[C@@H]1C)c1ncnc2[nH]cc(C)c12 |r,w:12.12| Show InChI InChI=1S/C24H29N9O/c1-15(2)30-23(34)18-6-5-7-19(10-18)31-24(27-13-25)33-9-8-32(12-17(33)4)22-20-16(3)11-26-21(20)28-14-29-22/h5-7,10-11,14-15,17H,8-9,12H2,1-4H3,(H,27,31)(H,30,34)(H,26,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50299584

((S)-N-(3-bromo-4-fluorophenyl)-N'-cyano-2-methyl-4...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1ccc(F)c(Br)c1)c1ncnc2[nH]cc(C)c12 |r,w:11.12| Show InChI InChI=1S/C20H20BrFN8/c1-12-8-24-18-17(12)19(27-11-26-18)29-5-6-30(13(2)9-29)20(25-10-23)28-14-3-4-16(22)15(21)7-14/h3-4,7-8,11,13H,5-6,9H2,1-2H3,(H,25,28)(H,24,26,27)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of LIMK1 |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299599
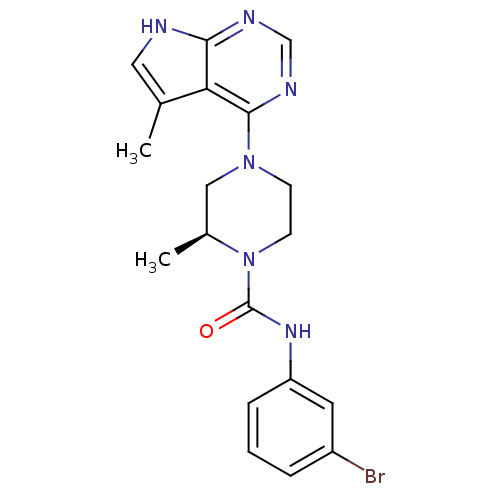
((S)-N-(3-bromophenyl)-2-methyl-4-(5-methyl-7H-pyrr...)Show SMILES C[C@H]1CN(CCN1C(=O)Nc1cccc(Br)c1)c1ncnc2[nH]cc(C)c12 |r| Show InChI InChI=1S/C19H21BrN6O/c1-12-9-21-17-16(12)18(23-11-22-17)25-6-7-26(13(2)10-25)19(27)24-15-5-3-4-14(20)8-15/h3-5,8-9,11,13H,6-7,10H2,1-2H3,(H,24,27)(H,21,22,23)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299602
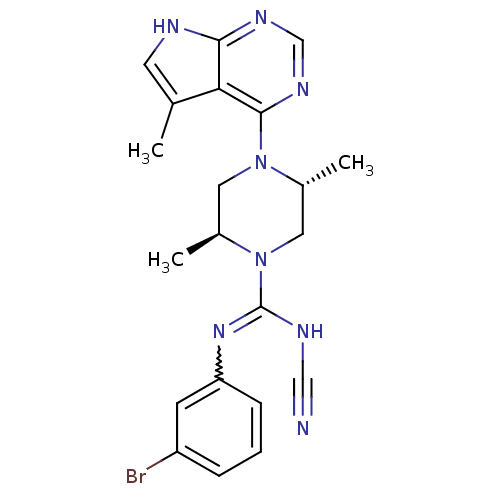
(CHEMBL570586 | Trans-N-(3-bromophenyl)-N'-cyano-2,...)Show SMILES C[C@H]1CN([C@H](C)CN1C(NC#N)=Nc1cccc(Br)c1)c1ncnc2[nH]cc(C)c12 |r,w:12.13| Show InChI InChI=1S/C21H23BrN8/c1-13-8-24-19-18(13)20(27-12-26-19)29-9-15(3)30(10-14(29)2)21(25-11-23)28-17-6-4-5-16(22)7-17/h4-8,12,14-15H,9-10H2,1-3H3,(H,25,28)(H,24,26,27)/t14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299601
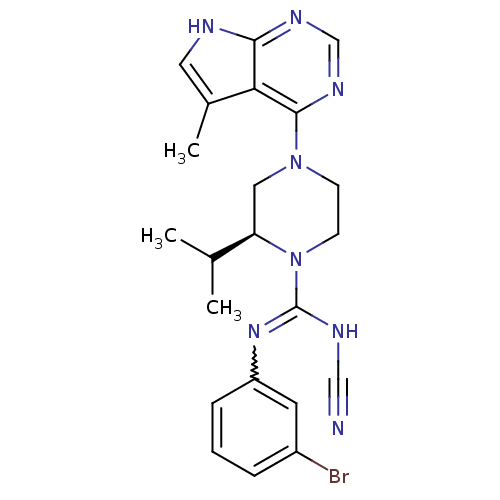
((S)-N-(3-bromophenyl)-N'-cyano-2-isopropyl-4-(5-me...)Show SMILES CC(C)[C@H]1CN(CCN1C(NC#N)=Nc1cccc(Br)c1)c1ncnc2[nH]cc(C)c12 |r,w:13.14| Show InChI InChI=1S/C22H25BrN8/c1-14(2)18-11-30(21-19-15(3)10-25-20(19)27-13-28-21)7-8-31(18)22(26-12-24)29-17-6-4-5-16(23)9-17/h4-6,9-10,13-14,18H,7-8,11H2,1-3H3,(H,26,29)(H,25,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299608

((S)-N-(3-bromophenyl)-N'-cyano-2-methyl-4-(6-methy...)Show SMILES C[C@H]1CN(CCN1C(NC#N)=Nc1cccc(Br)c1)c1ncnc2[nH]c(C)cc12 |r,w:11.12| Show InChI InChI=1S/C20H21BrN8/c1-13-8-17-18(26-13)24-12-25-19(17)28-6-7-29(14(2)10-28)20(23-11-22)27-16-5-3-4-15(21)9-16/h3-5,8-9,12,14H,6-7,10H2,1-2H3,(H,23,27)(H,24,25,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299618
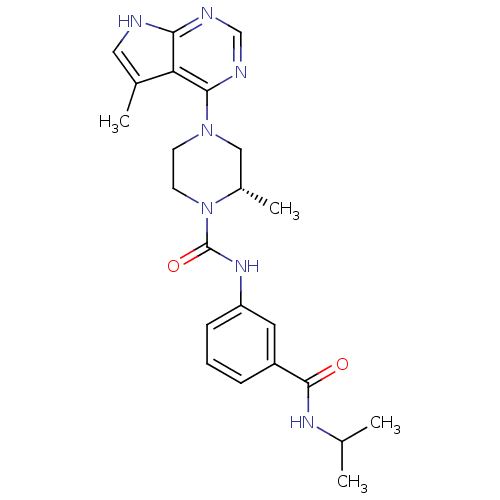
((S)-N-(3-(isopropylcarbamoyl)phenyl)-2-methyl-4-(5...)Show SMILES CC(C)NC(=O)c1cccc(NC(=O)N2CCN(C[C@@H]2C)c2ncnc3[nH]cc(C)c23)c1 |r| Show InChI InChI=1S/C23H29N7O2/c1-14(2)27-22(31)17-6-5-7-18(10-17)28-23(32)30-9-8-29(12-16(30)4)21-19-15(3)11-24-20(19)25-13-26-21/h5-7,10-11,13-14,16H,8-9,12H2,1-4H3,(H,27,31)(H,28,32)(H,24,25,26)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50299585

((S)-N-(3-bromo-4-fluorophenyl)-2-methyl-4-(5-methy...)Show SMILES C[C@H]1CN(CCN1C(=O)Nc1ccc(F)c(Br)c1)c1ncnc2[nH]cc(C)c12 |r| Show InChI InChI=1S/C19H20BrFN6O/c1-11-8-22-17-16(11)18(24-10-23-17)26-5-6-27(12(2)9-26)19(28)25-13-3-4-15(21)14(20)7-13/h3-4,7-8,10,12H,5-6,9H2,1-2H3,(H,25,28)(H,22,23,24)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of LIMK1 |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299610
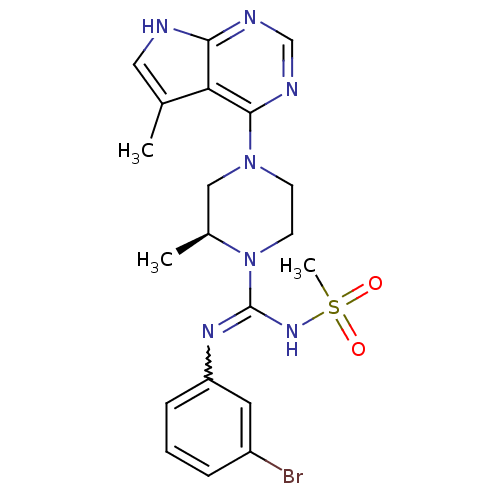
((S)-N-(3-bromophenyl)-2-methyl-4-(5-methyl-7H-pyrr...)Show SMILES C[C@H]1CN(CCN1C(NS(C)(=O)=O)=Nc1cccc(Br)c1)c1ncnc2[nH]cc(C)c12 |r,w:13.14| Show InChI InChI=1S/C20H24BrN7O2S/c1-13-10-22-18-17(13)19(24-12-23-18)27-7-8-28(14(2)11-27)20(26-31(3,29)30)25-16-6-4-5-15(21)9-16/h4-6,9-10,12,14H,7-8,11H2,1-3H3,(H,25,26)(H,22,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299606

((+/-)-N-(3-bromophenyl)-N'-cyano-2-methyl-4-(7H-py...)Show SMILES CC1CN(CCN1C(NC#N)=Nc1cccc(Br)c1)c1ncnc2[nH]ccc12 |w:11.12| Show InChI InChI=1S/C19H19BrN8/c1-13-10-27(18-16-5-6-22-17(16)24-12-25-18)7-8-28(13)19(23-11-21)26-15-4-2-3-14(20)9-15/h2-6,9,12-13H,7-8,10H2,1H3,(H,23,26)(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299598

(CHEMBL568484 | N-(3-bromophenyl)-N'-cyano-4-(5-met...)Show SMILES Cc1c[nH]c2ncnc(N3CCN(CC3)C(NC#N)=Nc3cccc(Br)c3)c12 |w:19.20| Show InChI InChI=1S/C19H19BrN8/c1-13-10-22-17-16(13)18(25-12-24-17)27-5-7-28(8-6-27)19(23-11-21)26-15-4-2-3-14(20)9-15/h2-4,9-10,12H,5-8H2,1H3,(H,23,26)(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50299604

(CHEMBL584968 | N-(3-bromophenyl)-3-(5-methyl-7H-py...)Show SMILES Cc1c[nH]c2ncnc(N3CC4CCC(C3)N4C(=O)Nc3cccc(Br)c3)c12 |TLB:8:9:16:12.13| Show InChI InChI=1S/C20H21BrN6O/c1-12-8-22-18-17(12)19(24-11-23-18)26-9-15-5-6-16(10-26)27(15)20(28)25-14-4-2-3-13(21)7-14/h2-4,7-8,11,15-16H,5-6,9-10H2,1H3,(H,25,28)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LIMK2 expressed in baculovirus-Sf9 system by scintillation counting |
J Med Chem 52: 6515-8 (2009)
Article DOI: 10.1021/jm901226j
BindingDB Entry DOI: 10.7270/Q2HH6K4J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data