 Found 466 hits with Last Name = 'critchlow' and Initial = 'se'
Found 466 hits with Last Name = 'critchlow' and Initial = 'se' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081185
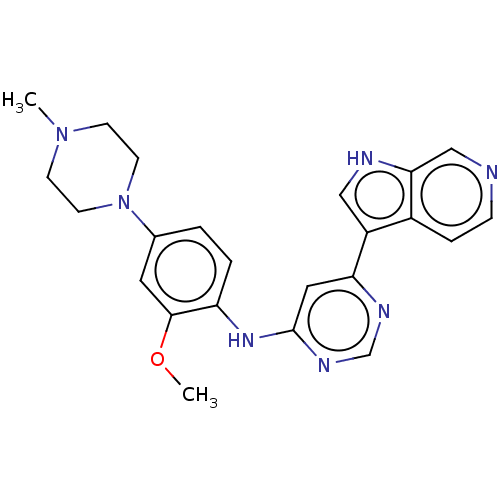
(CHEMBL3421963)Show SMILES COc1cc(ccc1Nc1cc(ncn1)-c1c[nH]c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C23H25N7O/c1-29-7-9-30(10-8-29)16-3-4-19(22(11-16)31-2)28-23-12-20(26-15-27-23)18-13-25-21-14-24-6-5-17(18)21/h3-6,11-15,25H,7-10H2,1-2H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081186
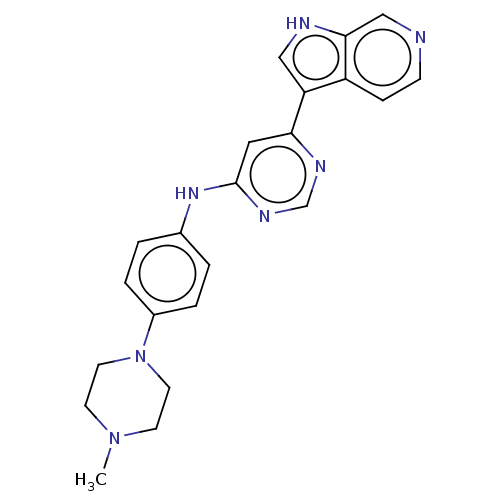
(CHEMBL3421962)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(ncn2)-c2c[nH]c3cnccc23)cc1 Show InChI InChI=1S/C22H23N7/c1-28-8-10-29(11-9-28)17-4-2-16(3-5-17)27-22-12-20(25-15-26-22)19-13-24-21-14-23-7-6-18(19)21/h2-7,12-15,24H,8-11H2,1H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081188
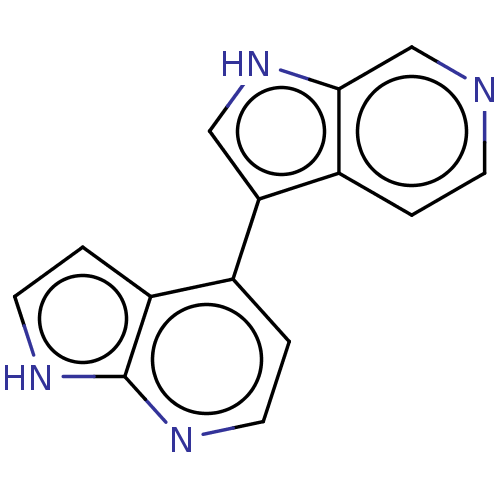
(CHEMBL3421981)Show InChI InChI=1S/C14H10N4/c1-4-15-8-13-10(1)12(7-18-13)9-2-5-16-14-11(9)3-6-17-14/h1-8,18H,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553937
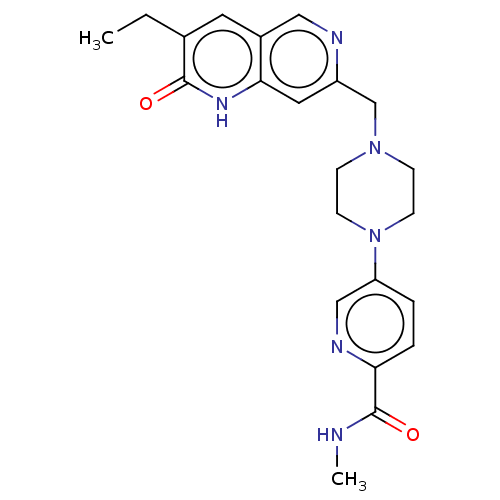
(US11325906, Example 1)Show SMILES CCc1cc2cnc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082118
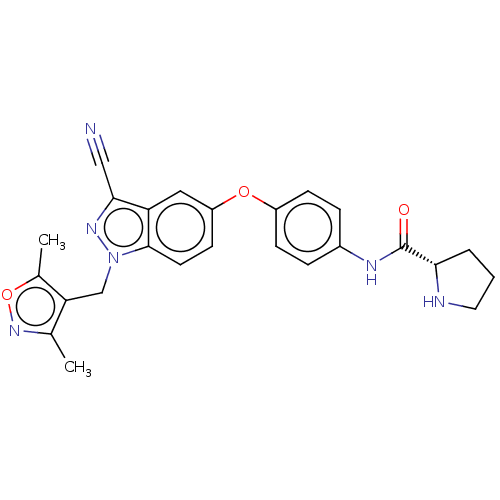
(CHEMBL3422678)Show SMILES Cc1noc(C)c1Cn1nc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C25H24N6O3/c1-15-21(16(2)34-30-15)14-31-24-10-9-19(12-20(24)23(13-26)29-31)33-18-7-5-17(6-8-18)28-25(32)22-4-3-11-27-22/h5-10,12,22,27H,3-4,11,14H2,1-2H3,(H,28,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553940
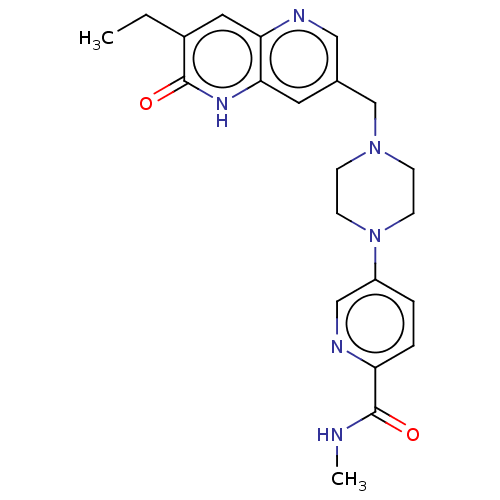
(US11325906, Example 4)Show SMILES CCc1cc2ncc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081174
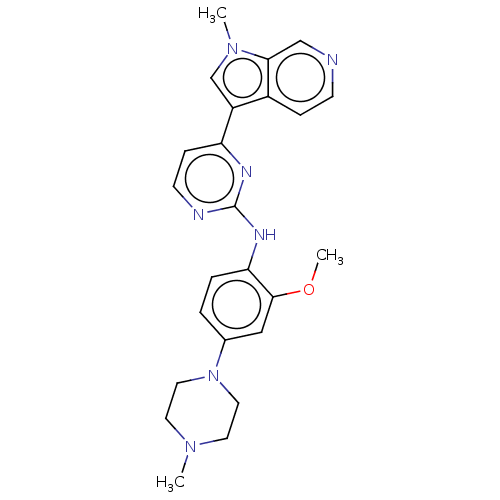
(CHEMBL3421968)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-29-10-12-31(13-11-29)17-4-5-21(23(14-17)32-3)28-24-26-9-7-20(27-24)19-16-30(2)22-15-25-8-6-18(19)22/h4-9,14-16H,10-13H2,1-3H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601780
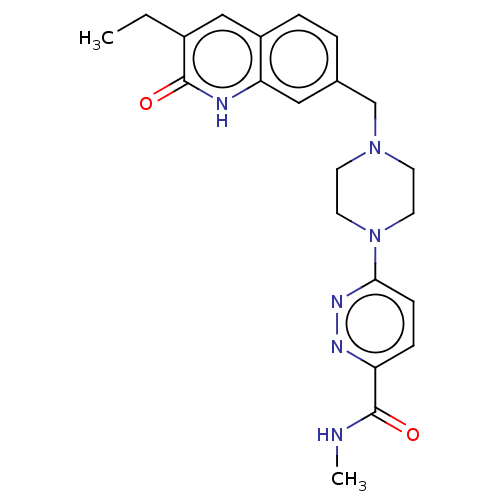
(CHEMBL5202060)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(nn3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081180
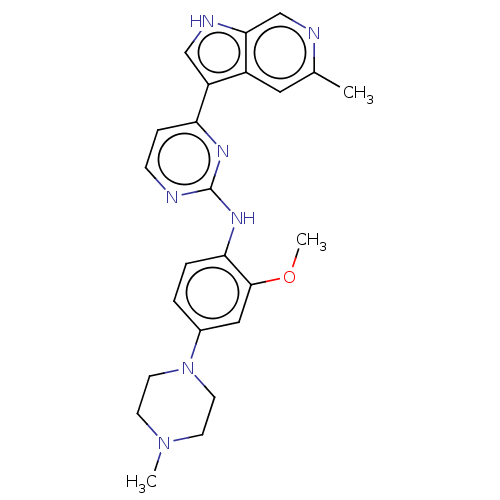
(CHEMBL3421969)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c[nH]c2cnc(C)cc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-16-12-18-19(14-27-22(18)15-26-16)20-6-7-25-24(28-20)29-21-5-4-17(13-23(21)32-3)31-10-8-30(2)9-11-31/h4-7,12-15,27H,8-11H2,1-3H3,(H,25,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081181

(CHEMBL3421967)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c[nH]c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C23H25N7O/c1-29-9-11-30(12-10-29)16-3-4-20(22(13-16)31-2)28-23-25-8-6-19(27-23)18-14-26-21-15-24-7-5-17(18)21/h3-8,13-15,26H,9-12H2,1-2H3,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082117
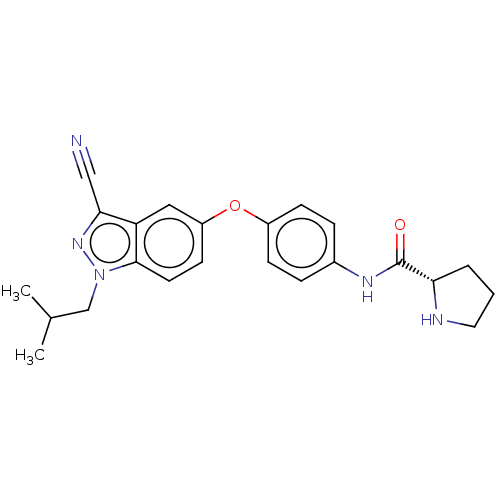
(CHEMBL3422677)Show SMILES CC(C)Cn1nc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C23H25N5O2/c1-15(2)14-28-22-10-9-18(12-19(22)21(13-24)27-28)30-17-7-5-16(6-8-17)26-23(29)20-4-3-11-25-20/h5-10,12,15,20,25H,3-4,11,14H2,1-2H3,(H,26,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081179

(CHEMBL3421970)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c[nH]c2c(C)nccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-16-23-18(6-8-25-16)19(15-27-23)20-7-9-26-24(28-20)29-21-5-4-17(14-22(21)32-3)31-12-10-30(2)11-13-31/h4-9,14-15,27H,10-13H2,1-3H3,(H,26,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601781
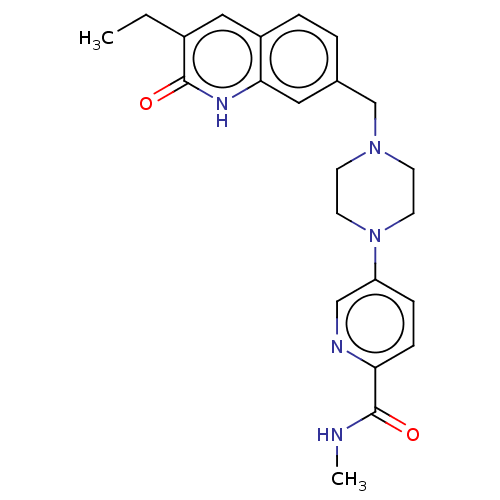
(CHEMBL5197101)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM553947
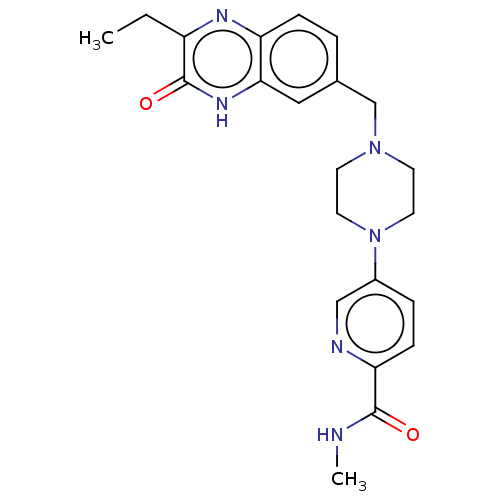
(US11325906, Example 11)Show SMILES CCc1nc2ccc(CN3CCN(CC3)c3ccc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601767
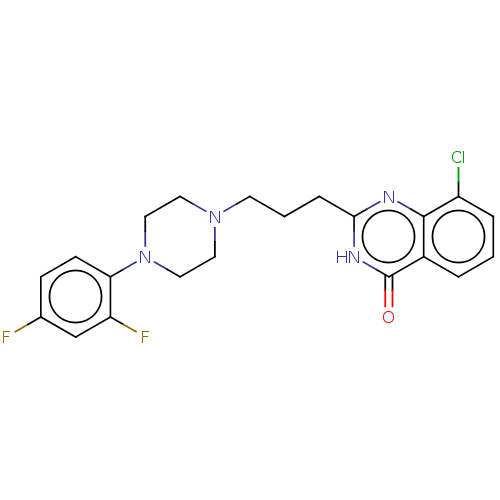
(CHEMBL5190481)Show SMILES Fc1ccc(N2CCN(CCCc3nc4c(Cl)cccc4c(=O)[nH]3)CC2)c(F)c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514979
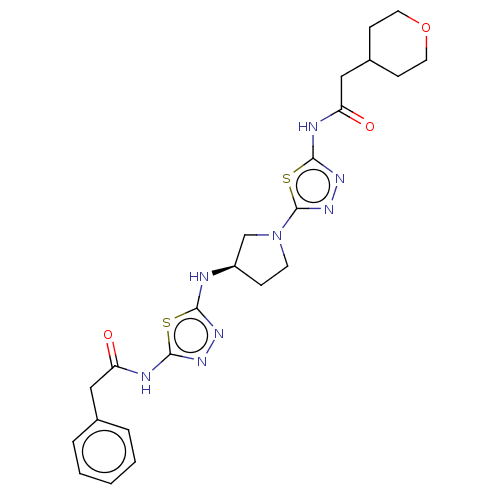
(CHEMBL4457936)Show SMILES O=C(CC1CCOCC1)Nc1nnc(s1)N1CC[C@H](C1)Nc1nnc(NC(=O)Cc2ccccc2)s1 |r| Show InChI InChI=1S/C23H28N8O3S2/c32-18(12-15-4-2-1-3-5-15)25-21-28-27-20(35-21)24-17-6-9-31(14-17)23-30-29-22(36-23)26-19(33)13-16-7-10-34-11-8-16/h1-5,16-17H,6-14H2,(H,24,27)(H,25,28,32)(H,26,29,33)/t17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM109086

(US10793535, Cmpd ID 727 | US8604016, 670 | US99382...)Show SMILES FC(F)(F)Oc1cccc(CC(=O)Nc2ccc(CCCCc3nnc(NC(=O)Cc4ccccn4)s3)nn2)c1 Show InChI InChI=1S/C26H24F3N7O3S/c27-26(28,29)39-20-9-5-6-17(14-20)15-22(37)31-21-12-11-18(33-34-21)7-1-2-10-24-35-36-25(40-24)32-23(38)16-19-8-3-4-13-30-19/h3-6,8-9,11-14H,1-2,7,10,15-16H2,(H,31,34,37)(H,32,36,38) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081191

(CHEMBL3421978)Show InChI InChI=1S/C19H14N4/c1-2-4-14(5-3-1)6-7-19-21-11-9-17(23-19)16-12-22-18-13-20-10-8-15(16)18/h1-13,22H/b7-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081189
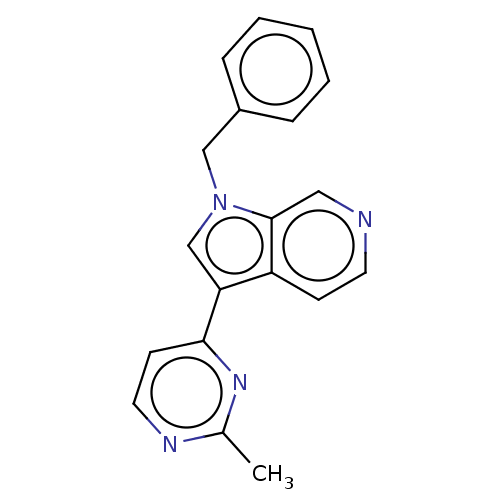
(CHEMBL3421980)Show InChI InChI=1S/C19H16N4/c1-14-21-10-8-18(22-14)17-13-23(12-15-5-3-2-4-6-15)19-11-20-9-7-16(17)19/h2-11,13H,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514986
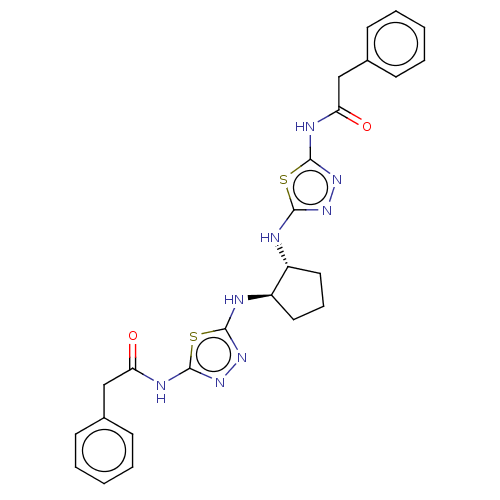
(CHEMBL4437956)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCC[C@H]2Nc2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C25H26N8O2S2/c34-20(14-16-8-3-1-4-9-16)28-24-32-30-22(36-24)26-18-12-7-13-19(18)27-23-31-33-25(37-23)29-21(35)15-17-10-5-2-6-11-17/h1-6,8-11,18-19H,7,12-15H2,(H,26,30)(H,27,31)(H,28,32,34)(H,29,33,35)/t18-,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601779
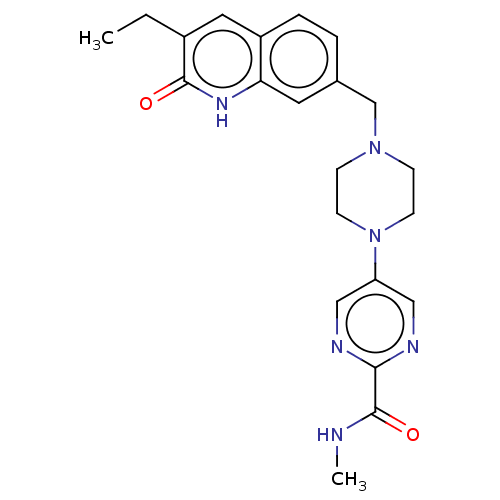
(CHEMBL5183769)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3cnc(nc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082114
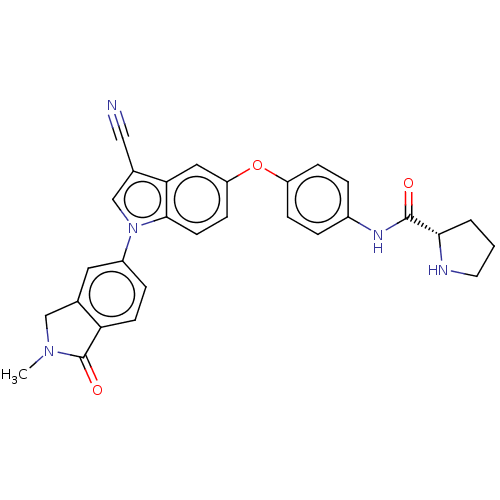
(CHEMBL3422674)Show SMILES CN1Cc2cc(ccc2C1=O)-n1cc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C29H25N5O3/c1-33-16-18-13-21(6-10-24(18)29(33)36)34-17-19(15-30)25-14-23(9-11-27(25)34)37-22-7-4-20(5-8-22)32-28(35)26-3-2-12-31-26/h4-11,13-14,17,26,31H,2-3,12,16H2,1H3,(H,32,35)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601776
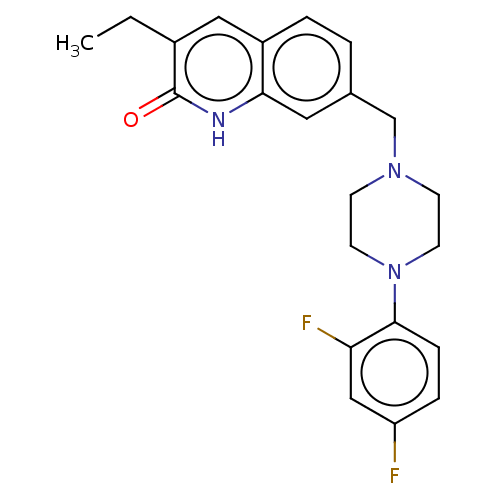
(CHEMBL5196187)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(F)cc3F)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601768
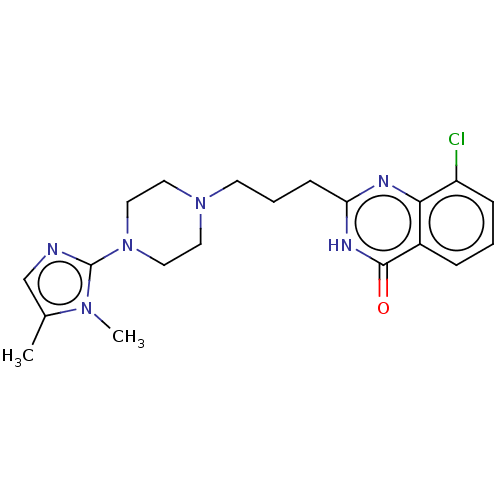
(CHEMBL5193246)Show SMILES Cc1cnc(N2CCN(CCCc3nc4c(Cl)cccc4c(=O)[nH]3)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50084621
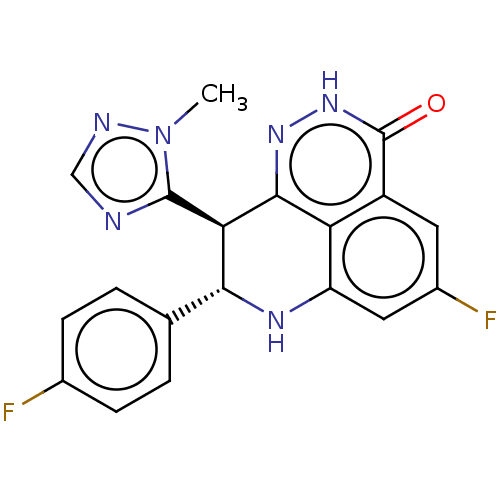
(BMN 673 | Talazoparib)Show SMILES Cn1ncnc1[C@@H]1[C@H](Nc2cc(F)cc3c2c1n[nH]c3=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081182

(CHEMBL3421966)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c[nH]c2cnccc12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H25N7O2/c1-16(32)30-9-11-31(12-10-30)17-3-4-21(23(13-17)33-2)29-24-26-8-6-20(28-24)19-14-27-22-15-25-7-5-18(19)22/h3-8,13-15,27H,9-12H2,1-2H3,(H,26,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601769
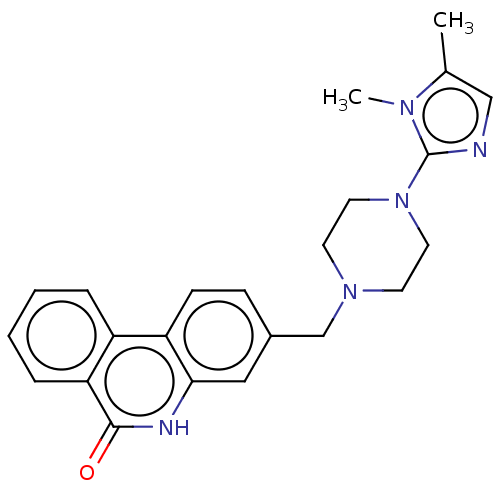
(CHEMBL5191396)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c(c3)[nH]c(=O)c3ccccc43)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
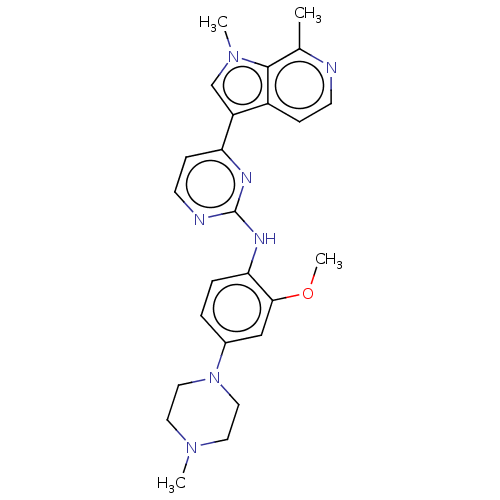
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081178
(CHEMBL3421971)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2c(C)nccc12)N1CCN(C)CC1 Show InChI InChI=1S/C25H29N7O/c1-17-24-19(7-9-26-17)20(16-31(24)3)21-8-10-27-25(28-21)29-22-6-5-18(15-23(22)33-4)32-13-11-30(2)12-14-32/h5-10,15-16H,11-14H2,1-4H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601772
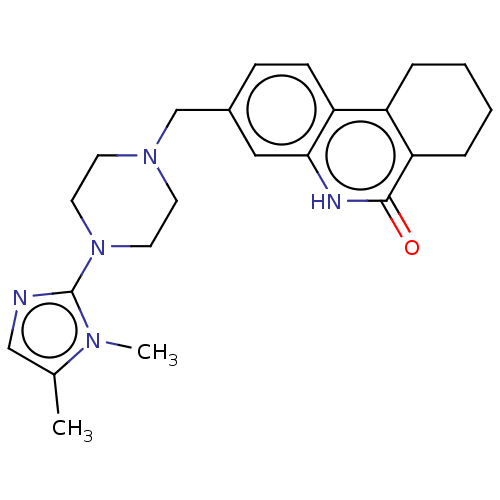
(CHEMBL5177596)Show SMILES Cc1cnc(N2CCN(Cc3ccc4c5CCCCc5c(=O)[nH]c4c3)CC2)n1C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514985

(CHEMBL4573635)Show SMILES C[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7OS/c1-13(14-6-3-2-4-7-14)17(27)22-19-25-24-18(28-19)21-15-9-11-26(12-15)16-8-5-10-20-23-16/h2-8,10,13,15H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t13-,15+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082116
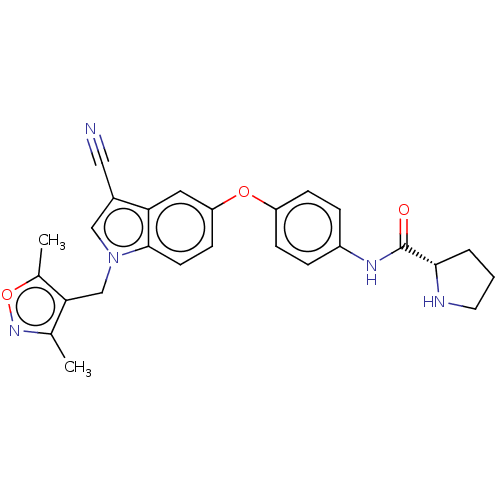
(CHEMBL3422676)Show SMILES Cc1noc(C)c1Cn1cc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C26H25N5O3/c1-16-23(17(2)34-30-16)15-31-14-18(13-27)22-12-21(9-10-25(22)31)33-20-7-5-19(6-8-20)29-26(32)24-4-3-11-28-24/h5-10,12,14,24,28H,3-4,11,15H2,1-2H3,(H,29,32)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081193

(CHEMBL3421976)Show SMILES Cn1cc(-c2ccnc(n2)-c2ccc(cc2)S(C)(=O)=O)c2ccncc12 Show InChI InChI=1S/C19H16N4O2S/c1-23-12-16(15-7-9-20-11-18(15)23)17-8-10-21-19(22-17)13-3-5-14(6-4-13)26(2,24)25/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM209932
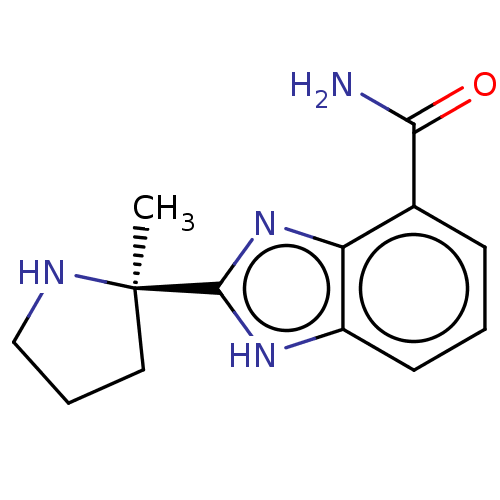
(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081192

(CHEMBL3421977)Show SMILES Cc1nccc2c(c[nH]c12)-c1ccnc(n1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H16N4O2S/c1-12-18-15(7-9-20-12)16(11-22-18)17-8-10-21-19(23-17)13-3-5-14(6-4-13)26(2,24)25/h3-11,22H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514975

(CHEMBL4473143)Show SMILES O=C(Cc1ccccc1)Nc1nnc(NC[C@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C25H26N8O2S2/c34-20(13-17-7-3-1-4-8-17)27-23-30-29-22(36-23)26-15-19-11-12-33(16-19)25-32-31-24(37-25)28-21(35)14-18-9-5-2-6-10-18/h1-10,19H,11-16H2,(H,26,29)(H,27,30,34)(H,28,31,35)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50150109
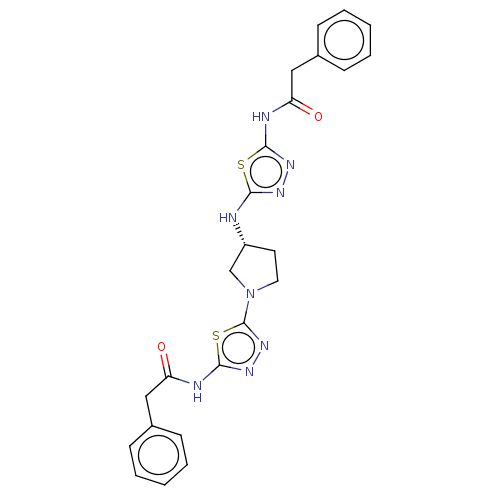
(CHEMBL3770355)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C24H24N8O2S2/c33-19(13-16-7-3-1-4-8-16)26-22-29-28-21(35-22)25-18-11-12-32(15-18)24-31-30-23(36-24)27-20(34)14-17-9-5-2-6-10-17/h1-10,18H,11-15H2,(H,25,28)(H,26,29,33)(H,27,30,34)/t18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2
(Homo sapiens (Human)) | BDBM50082117

(CHEMBL3422677)Show SMILES CC(C)Cn1nc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C23H25N5O2/c1-15(2)14-28-22-10-9-18(12-19(22)21(13-24)27-28)30-17-7-5-16(6-8-17)26-23(29)20-4-3-11-25-20/h5-10,12,15,20,25H,3-4,11,14H2,1-2H3,(H,26,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB2 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081184

(CHEMBL3421964)Show SMILES COc1cc(ccc1Nc1cc(ncn1)-c1c[nH]c2cnccc12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H25N7O2/c1-16(32)30-7-9-31(10-8-30)17-3-4-20(23(11-17)33-2)29-24-12-21(27-15-28-24)19-13-26-22-14-25-6-5-18(19)22/h3-6,11-15,26H,7-10H2,1-2H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514977
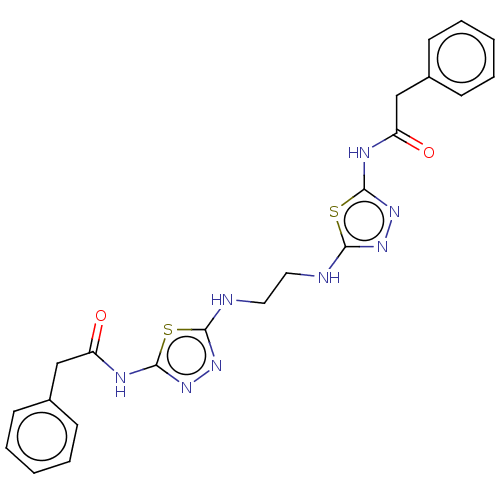
(CHEMBL4461749)Show SMILES O=C(Cc1ccccc1)Nc1nnc(NCCNc2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C22H22N8O2S2/c31-17(13-15-7-3-1-4-8-15)25-21-29-27-19(33-21)23-11-12-24-20-28-30-22(34-20)26-18(32)14-16-9-5-2-6-10-16/h1-10H,11-14H2,(H,23,27)(H,24,28)(H,25,29,31)(H,26,30,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514982

(CHEMBL4469711)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1 |r| Show InChI InChI=1S/C18H19N7OS/c26-16(11-13-5-2-1-3-6-13)21-18-24-23-17(27-18)20-14-8-10-25(12-14)15-7-4-9-19-22-15/h1-7,9,14H,8,10-12H2,(H,20,23)(H,21,24,26)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081190
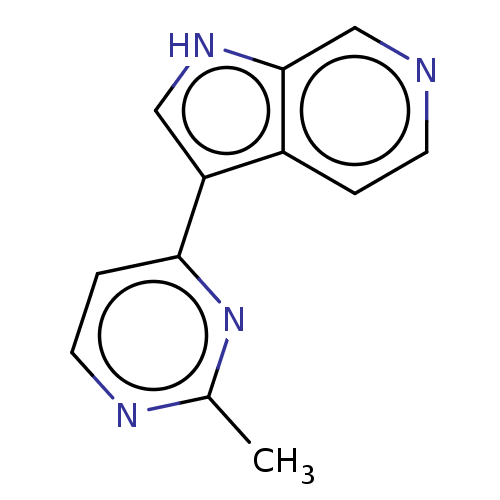
(CHEMBL3421979)Show InChI InChI=1S/C12H10N4/c1-8-14-5-3-11(16-8)10-6-15-12-7-13-4-2-9(10)12/h2-7,15H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50601777
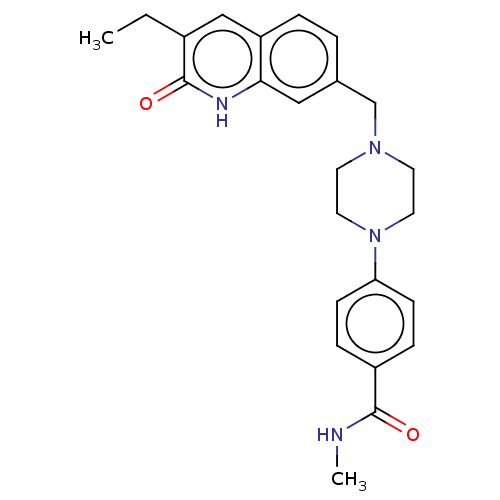
(CHEMBL5200723)Show SMILES CCc1cc2ccc(CN3CCN(CC3)c3ccc(cc3)C(=O)NC)cc2[nH]c1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01012
BindingDB Entry DOI: 10.7270/Q2Z03D7J |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50081177
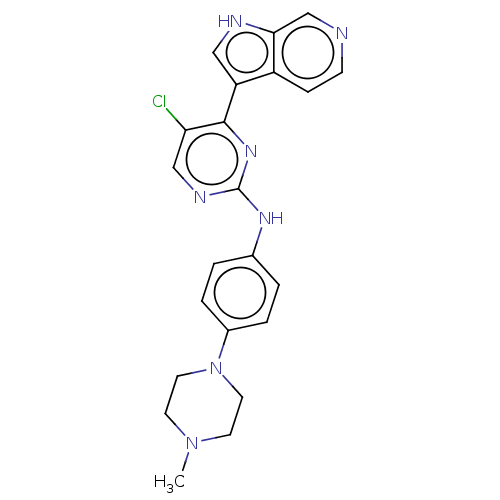
(CHEMBL3421973)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(n2)-c2c[nH]c3cnccc23)cc1 Show InChI InChI=1S/C22H22ClN7/c1-29-8-10-30(11-9-29)16-4-2-15(3-5-16)27-22-26-13-19(23)21(28-22)18-12-25-20-14-24-7-6-17(18)20/h2-7,12-14,25H,8-11H2,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length C-terminal His6-tagged CDK2 expressed in baculovirus infected Sf21 insect cells using fluorescence substr... |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082115
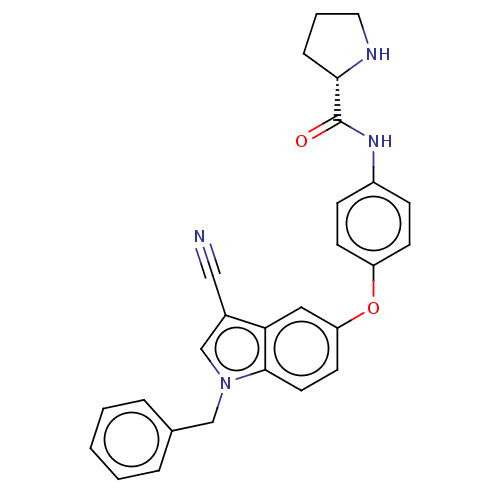
(CHEMBL3422675)Show SMILES O=C(Nc1ccc(Oc2ccc3n(Cc4ccccc4)cc(C#N)c3c2)cc1)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C27H24N4O2/c28-16-20-18-31(17-19-5-2-1-3-6-19)26-13-12-23(15-24(20)26)33-22-10-8-21(9-11-22)30-27(32)25-7-4-14-29-25/h1-3,5-6,8-13,15,18,25,29H,4,7,14,17H2,(H,30,32)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50081173
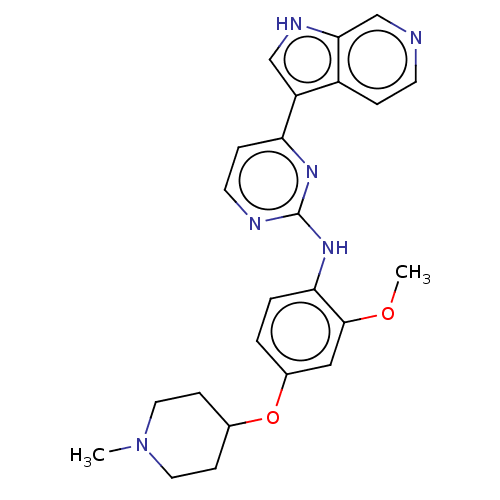
(CHEMBL3421972)Show SMILES COc1cc(OC2CCN(C)CC2)ccc1Nc1nccc(n1)-c1c[nH]c2cnccc12 Show InChI InChI=1S/C24H26N6O2/c1-30-11-7-16(8-12-30)32-17-3-4-21(23(13-17)31-2)29-24-26-10-6-20(28-24)19-14-27-22-15-25-9-5-18(19)22/h3-6,9-10,13-16,27H,7-8,11-12H2,1-2H3,(H,26,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human wild-type TTK (514 to 795) expressed in insect sf9 cells |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM278400
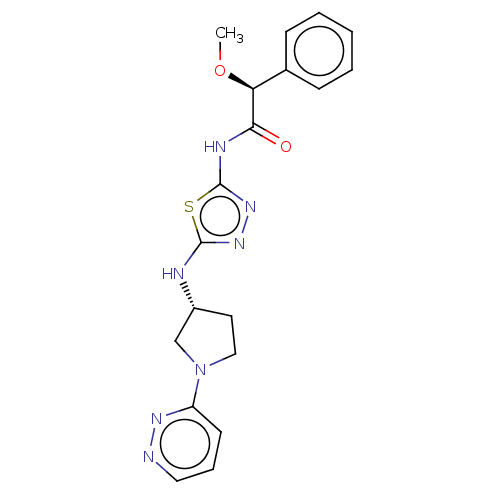
((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...)Show SMILES CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7O2S/c1-28-16(13-6-3-2-4-7-13)17(27)22-19-25-24-18(29-19)21-14-9-11-26(12-14)15-8-5-10-20-23-15/h2-8,10,14,16H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t14-,16+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081175
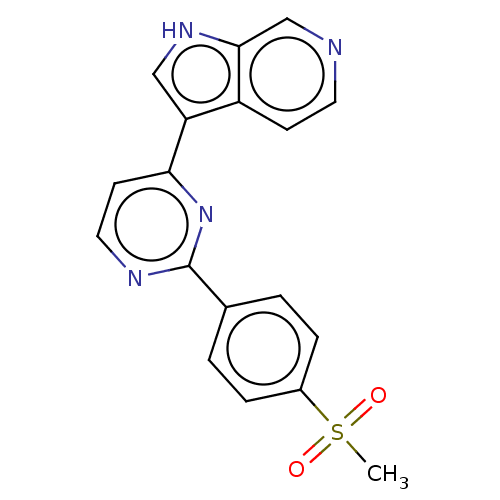
(CHEMBL3421975)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nccc(n1)-c1c[nH]c2cnccc12 Show InChI InChI=1S/C18H14N4O2S/c1-25(23,24)13-4-2-12(3-5-13)18-20-9-7-16(22-18)15-10-21-17-11-19-8-6-14(15)17/h2-11,21H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data