

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587,V571I] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0520 | -59.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L565M] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.114 | -57.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785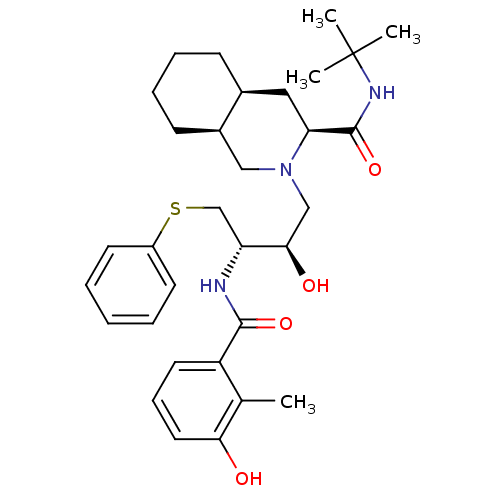 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50006828 (2-(2-{[1-(3-{2-[2-(2-Amino-3-hydroxy-propionylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of HIV-Protease, using a peptide hydrolysis assay | J Med Chem 35: 2525-33 (1992) BindingDB Entry DOI: 10.7270/Q2PC31C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of Wild-type protease | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I539L] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.323 | -55.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.358 | -54.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L512I] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.585 | -53.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,V521I] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.64 | -49.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,L512V] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.81 | -49.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I573V] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.11 | -49.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.32 | -49.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,I536L] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6.62 | -47.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Proc Natl Acad Sci U S A 91: 4096-100 (1994) Article DOI: 10.1073/pnas.91.9.4096 BindingDB Entry DOI: 10.7270/Q23F4MS9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50366785 (NELFINAVIR) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (K-60) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (A-44) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Dissociation constant obtained by inhibition of mutant HIV-protease (V-18) | J Med Chem 43: 3386-99 (2000) BindingDB Entry DOI: 10.7270/Q23J3DP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037527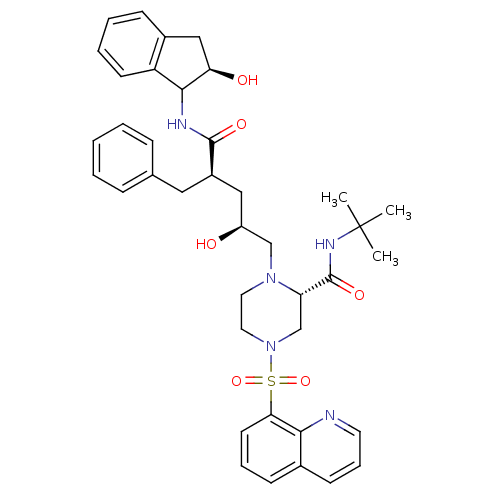 ((S)-1-[(2S,4R)-2-Hydroxy-4-((R)-(R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the inhibition of HIV protease | J Med Chem 37: 3443-51 (1994) BindingDB Entry DOI: 10.7270/Q24J0D5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283729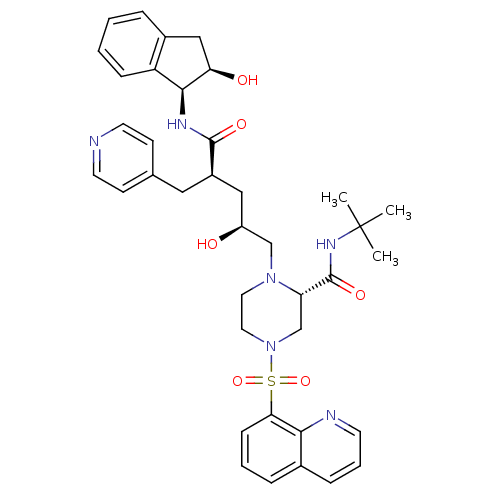 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283726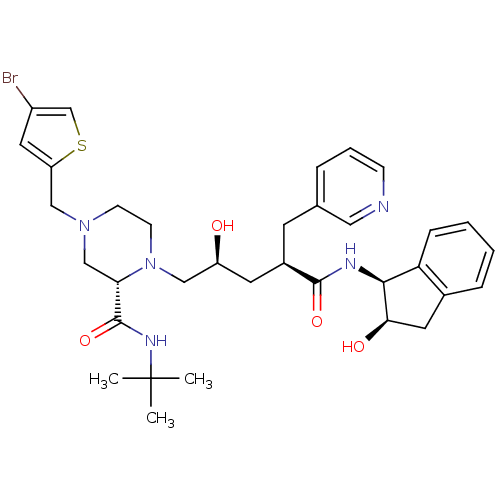 ((S)-4-(4-Bromo-thiophen-2-ylmethyl)-1-[(2S,4R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283732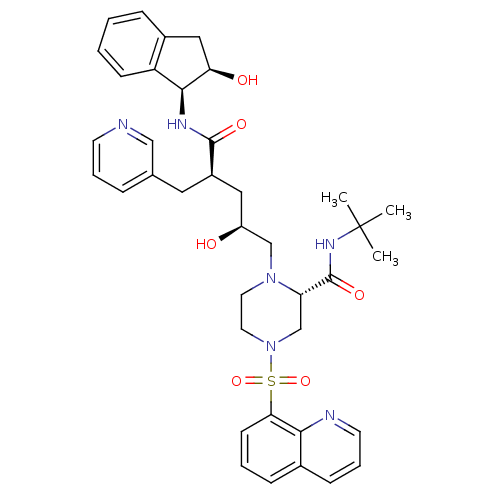 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383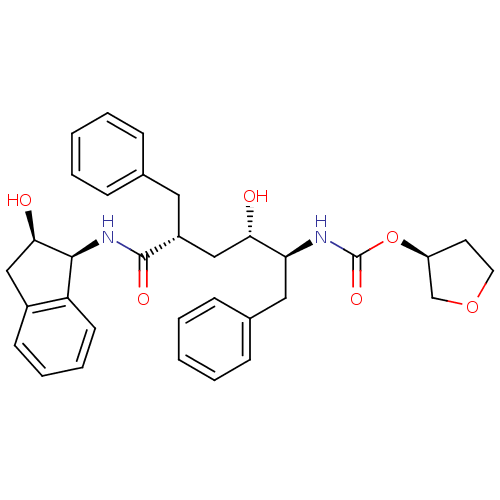 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 40: 2440-4 (1997) Article DOI: 10.1021/jm970195u BindingDB Entry DOI: 10.7270/Q2WD417W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 36: 292-4 (1993) Article DOI: 10.1021/jm00054a015 BindingDB Entry DOI: 10.7270/Q25D8Q1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM384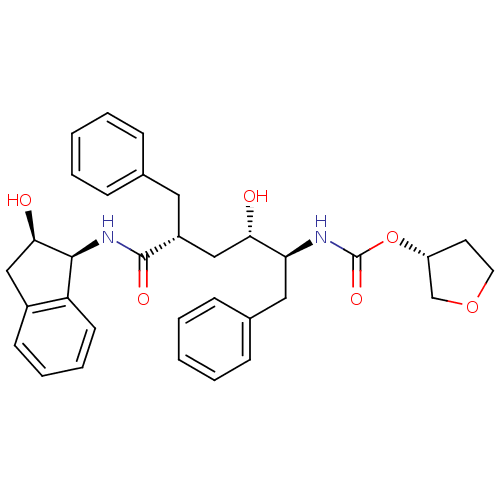 ((3R)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 36: 292-4 (1993) Article DOI: 10.1021/jm00054a015 BindingDB Entry DOI: 10.7270/Q25D8Q1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM386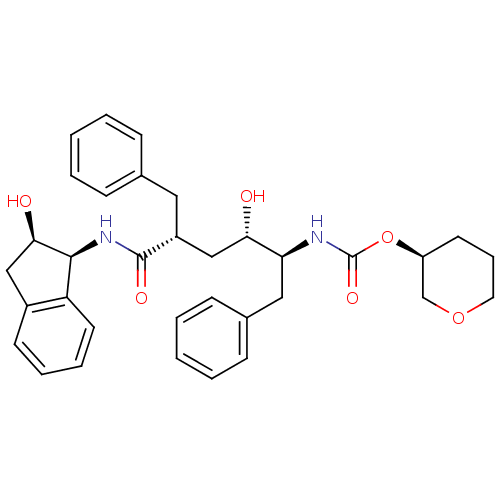 ((3S)-oxan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 36: 292-4 (1993) Article DOI: 10.1021/jm00054a015 BindingDB Entry DOI: 10.7270/Q25D8Q1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM387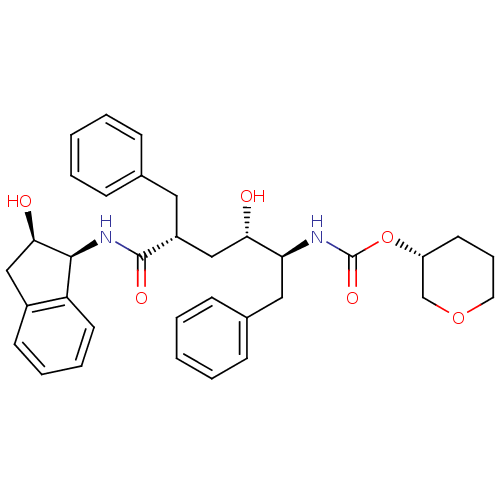 ((3R)-oxan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 36: 292-4 (1993) Article DOI: 10.1021/jm00054a015 BindingDB Entry DOI: 10.7270/Q25D8Q1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM762 (BocPhe[CHOH(CH2)3CH=CHPhCO]IleAMBI | L-687,908 | t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 1225-8 (1991) Article DOI: 10.1021/jm00107a050 BindingDB Entry DOI: 10.7270/Q2765CH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285450 (CHEMBL300140 | [(1S,2R)-1-Benzyl-3-((S)-4-[2,2']bi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV-1 Protease | Bioorg Med Chem Lett 5: 185-190 (1995) Article DOI: 10.1016/0960-894X(95)00005-E BindingDB Entry DOI: 10.7270/Q2JS9QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM894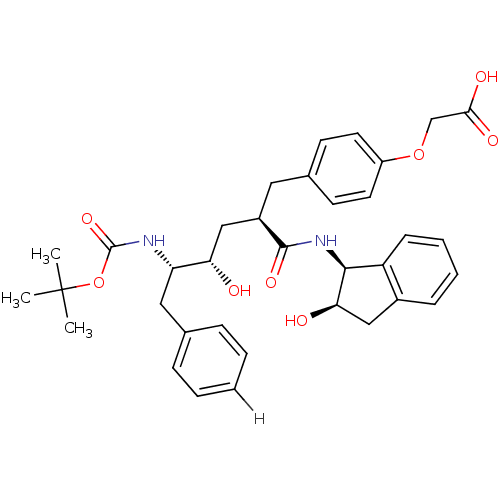 (2-{4-[(2R)-2-[(2S,3S)-3-{[(tert-butoxy)carbonyl]am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283723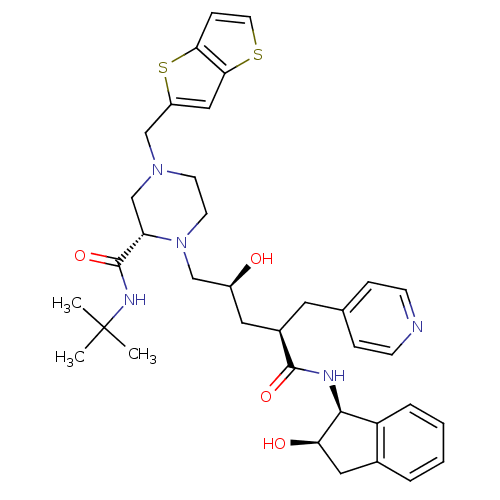 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1277 (L-682, 679 analog 36 | tert-butyl N-[(2S,3S,5R)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Sharp and Dohme Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 2852-7 (1991) Article DOI: 10.1021/jm00113a025 BindingDB Entry DOI: 10.7270/Q2M906TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9291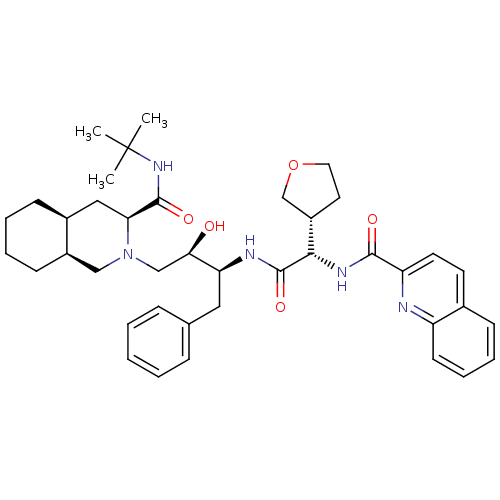 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. The products were analyzed by usi... | J Med Chem 36: 2300-10 (1993) Article DOI: 10.1021/jm00068a006 BindingDB Entry DOI: 10.7270/Q28G8HX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283722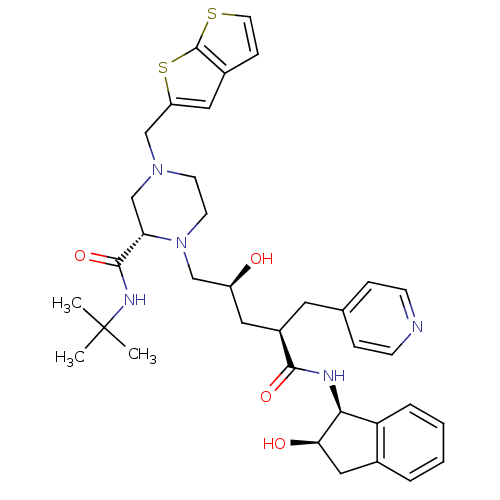 ((S)-1-[(2S,4R)-2-Hydroxy-4-((1S,2R)-2-hydroxy-inda...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Human Immunodeficiency virus-1 protease | Bioorg Med Chem Lett 4: 2769-2774 (1994) Article DOI: 10.1016/S0960-894X(01)80592-8 BindingDB Entry DOI: 10.7270/Q25B02F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9305 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-2-{[6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. The products were analyzed by usi... | J Med Chem 36: 2300-10 (1993) Article DOI: 10.1021/jm00068a006 BindingDB Entry DOI: 10.7270/Q28G8HX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037530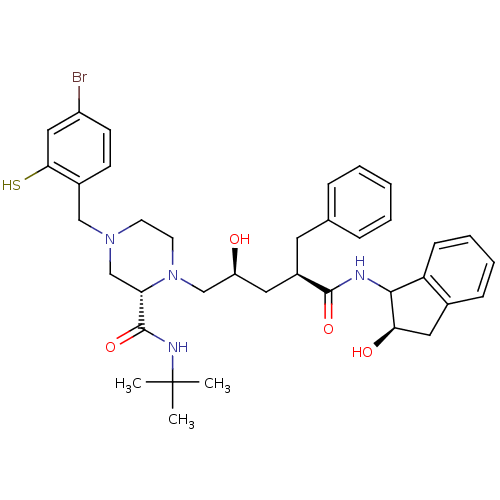 ((S)-4-(4-Bromo-2-mercapto-benzyl)-1-[(2S,4R)-2-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the inhibition of HIV protease | J Med Chem 37: 3443-51 (1994) BindingDB Entry DOI: 10.7270/Q24J0D5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against Human immunodeficiency virus (HIV-1) protease | Bioorg Med Chem Lett 4: 499-504 (1994) Article DOI: 10.1016/0960-894X(94)80025-1 BindingDB Entry DOI: 10.7270/Q2GM8777 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM761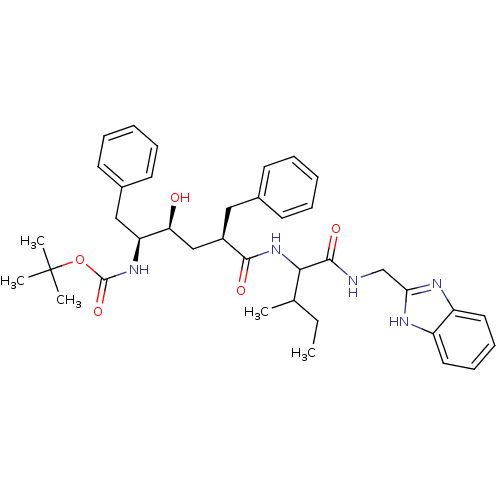 (Hydroxyethylene deriv. 12 | tert-butyl N-[(2S,3S,5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 34: 1225-8 (1991) Article DOI: 10.1021/jm00107a050 BindingDB Entry DOI: 10.7270/Q2765CH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1051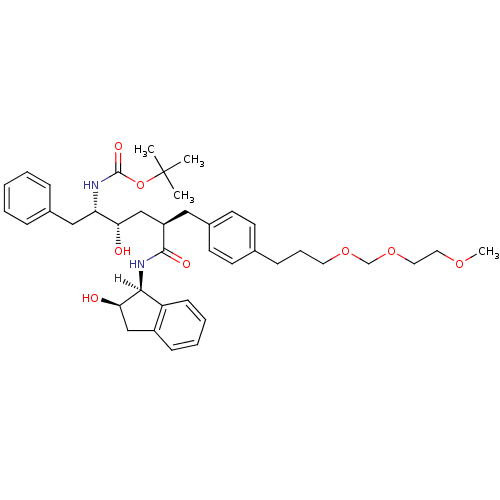 (Hydroxyethylene dipeptide isostere 20 | tert-butyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | J Med Chem 35: 1702-9 (1992) Article DOI: 10.1021/jm00088a004 BindingDB Entry DOI: 10.7270/Q2N877ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM891 (L-685,434 deriv. 39 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9300 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-3-[(2S)-2-{[6...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. The products were analyzed by usi... | J Med Chem 36: 2300-10 (1993) Article DOI: 10.1021/jm00068a006 BindingDB Entry DOI: 10.7270/Q28G8HX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 406 total ) | Next | Last >> |