

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026300 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026300 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026308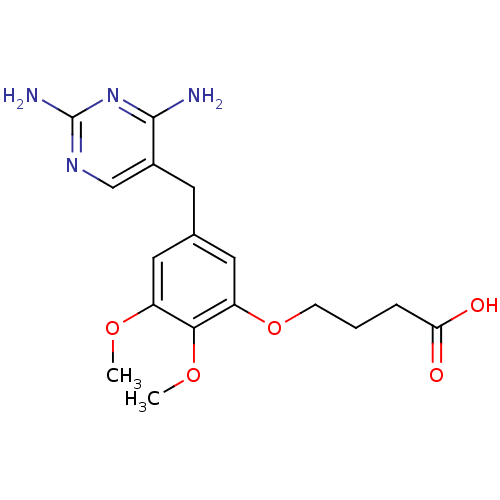 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026308 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026318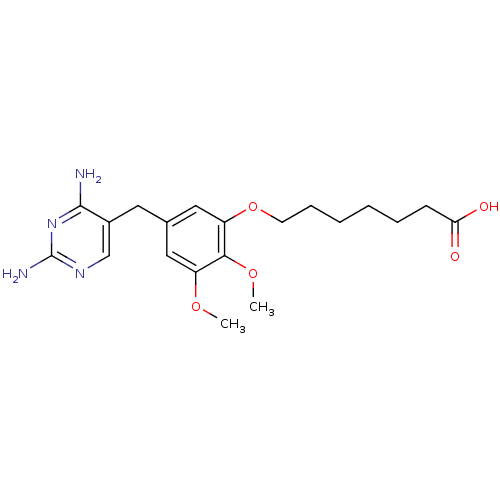 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026318 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026314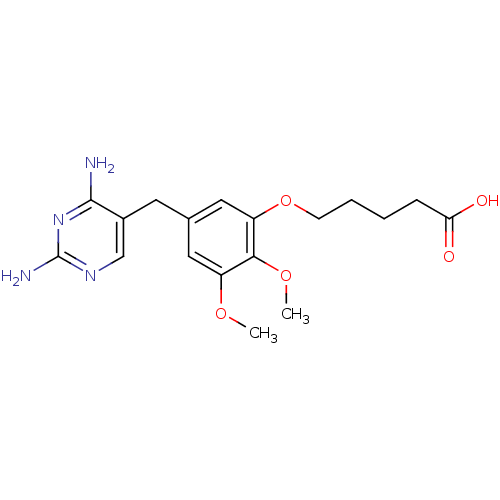 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026314 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026307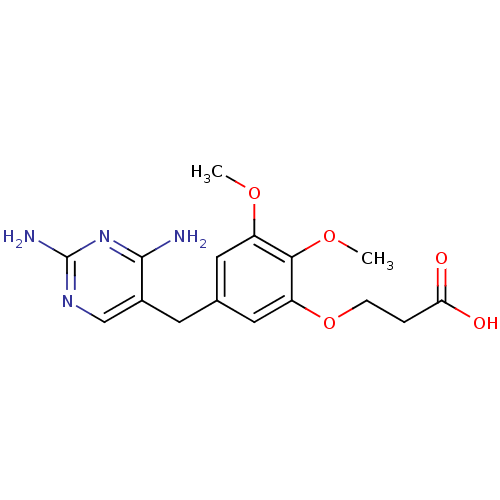 (3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026307 (3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026317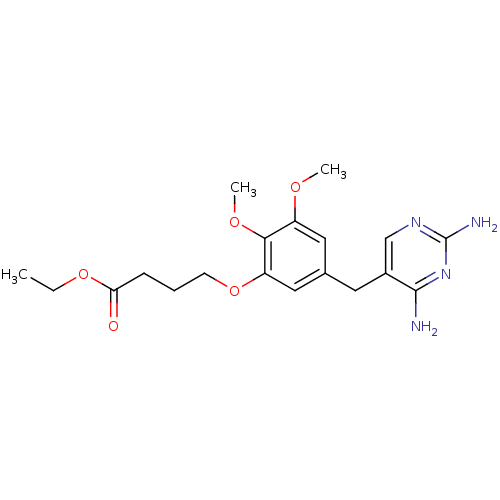 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026317 (4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026316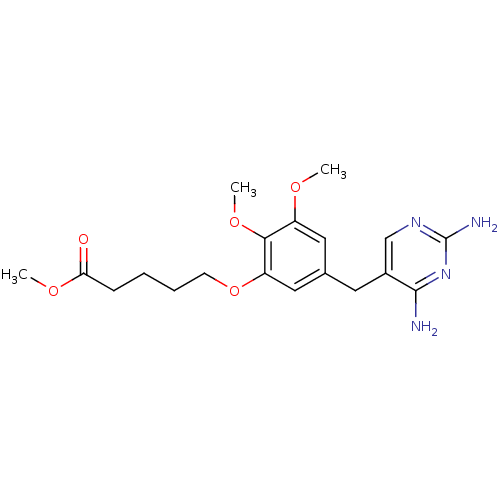 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026316 (5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026306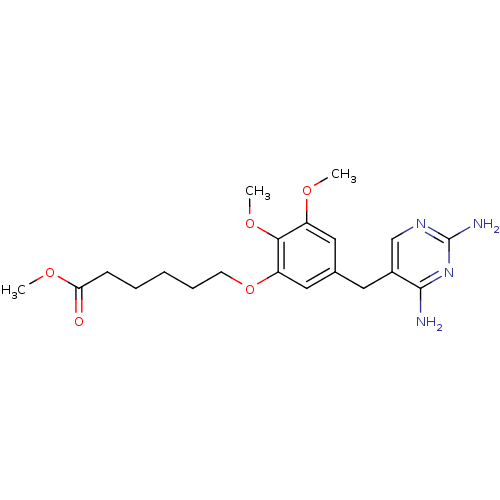 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026306 (6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase Inhibitor of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026304 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026304 (7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026313 (5-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026309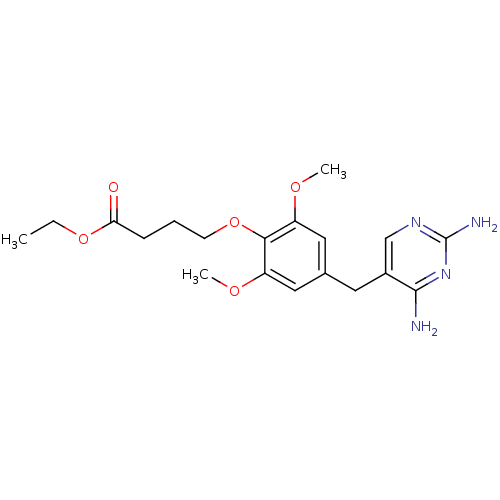 (4-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026302 (CHEMBL14201 | [5-(2,4-Diamino-pyrimidin-5-ylmethyl...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026302 (CHEMBL14201 | [5-(2,4-Diamino-pyrimidin-5-ylmethyl...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026305 (CHEMBL322001 | [4-(2,4-Diamino-pyrimidin-5-ylmethy...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026310 (6-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026301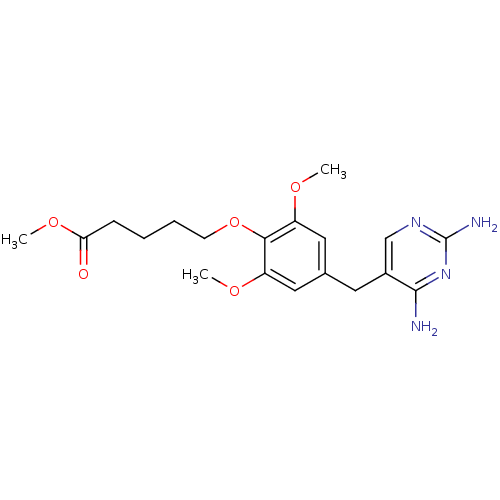 (5-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026311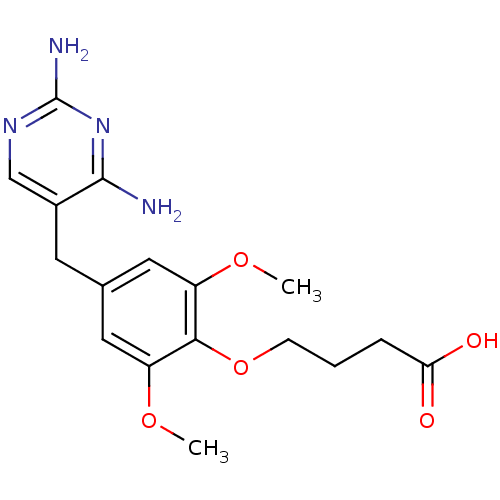 (4-[4-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,6-dimeth...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Relative Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090249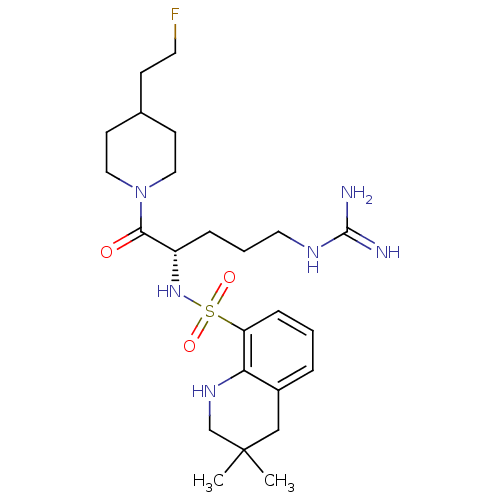 (3,3-Dimethyl-1,2,3,4-tetrahydro-quinoline-8-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50367239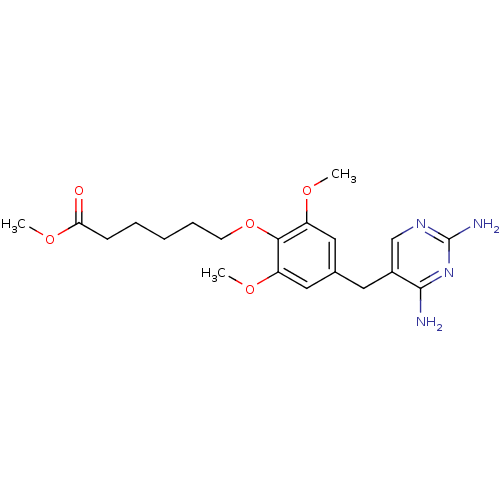 (CHEMBL318078) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026315 (CHEMBL266611 | [5-(2,4-Diamino-pyrimidin-5-ylmethy...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for E. coli Dihydrofolate reductase | J Med Chem 25: 1120-2 (1983) BindingDB Entry DOI: 10.7270/Q28G8JRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50026315 (CHEMBL266611 | [5-(2,4-Diamino-pyrimidin-5-ylmethy...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase Inhibitor of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50367240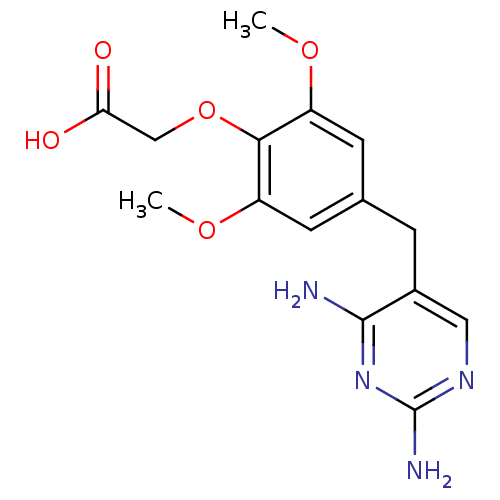 (CHEMBL104675) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of Escherichia coli | J Med Chem 28: 303-11 (1985) BindingDB Entry DOI: 10.7270/Q2J966Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090222 (CHEMBL39086 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM24498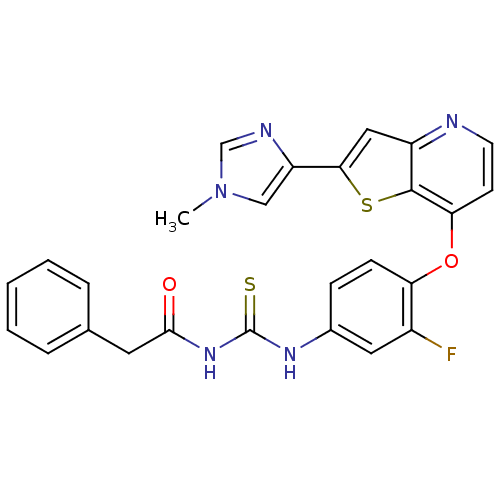 (3-(3-fluoro-4-{[2-(1-methyl-1H-imidazol-4-yl)thien...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oncologia Medica, Dipartimento Medico-Chirurgico di Internistica Clinica e Sperimentale"F. Magrassi e A. Lanzara", UniversitÓ della Campania"Luigi Vanvitelli" , Via Pansini 6, 801 Curated by ChEMBL | Assay Description Displacement of [3H]-cyclopamine from human wild-type SMO receptor expressed in HEK293T cell membranes by liquid scintillation spectrometry | J Med Chem 60: 7447-7458 (2017) Article DOI: 10.1021/acs.jmedchem.7b00794 BindingDB Entry DOI: 10.7270/Q22B91GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50399540 (FORETINIB | US10464902, Foretinib | US10882853, Co...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oncologia Medica, Dipartimento Medico-Chirurgico di Internistica Clinica e Sperimentale"F. Magrassi e A. Lanzara", UniversitÓ della Campania"Luigi Vanvitelli" , Via Pansini 6, 801 Curated by ChEMBL | Assay Description Displacement of [3H]-cyclopamine from human wild-type SMO receptor expressed in HEK293T cell membranes by liquid scintillation spectrometry | J Med Chem 60: 7447-7458 (2017) Article DOI: 10.1021/acs.jmedchem.7b00794 BindingDB Entry DOI: 10.7270/Q22B91GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090226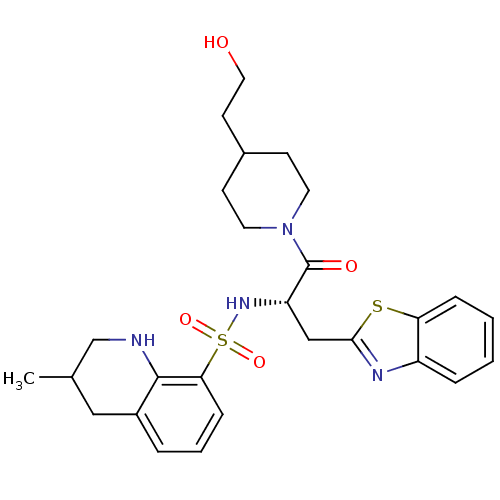 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090226 (3-Methyl-1,2,3,4-tetrahydro-quinoline-8-sulfonic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366643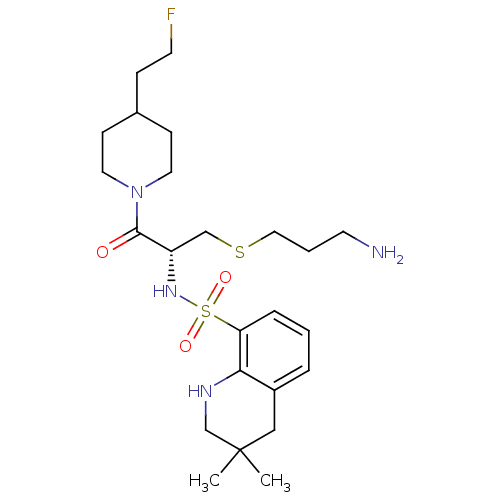 (CHEMBL1907778) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090231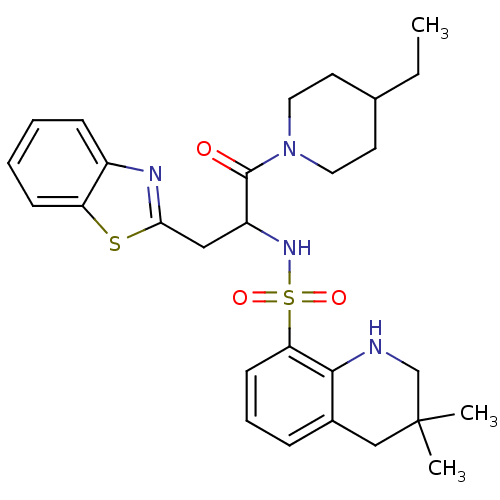 (CHEMBL39375 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366642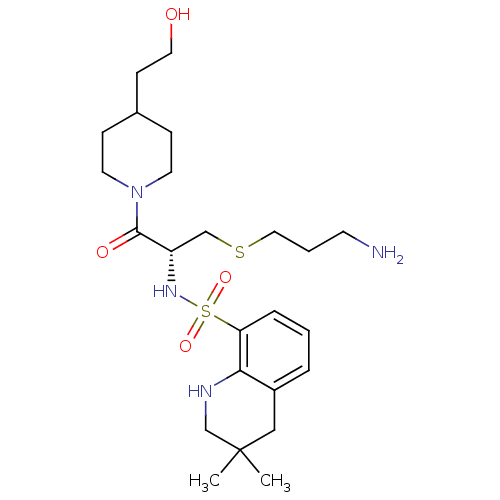 (CHEMBL1907779) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090236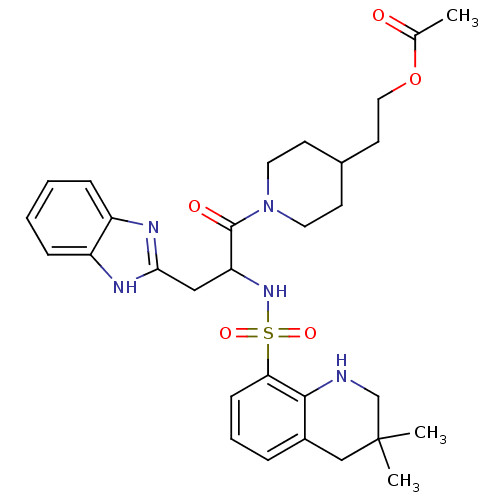 (CHEMBL418248 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090235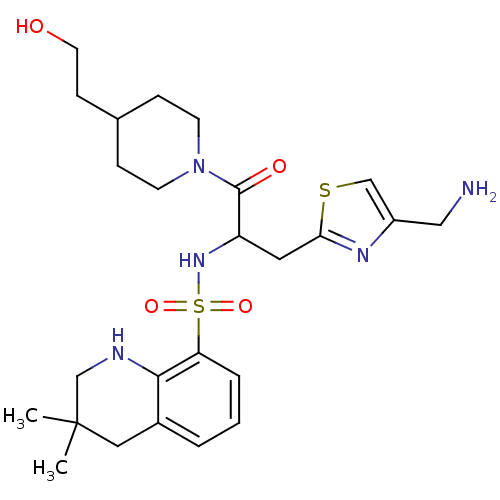 (CHEMBL38907 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090237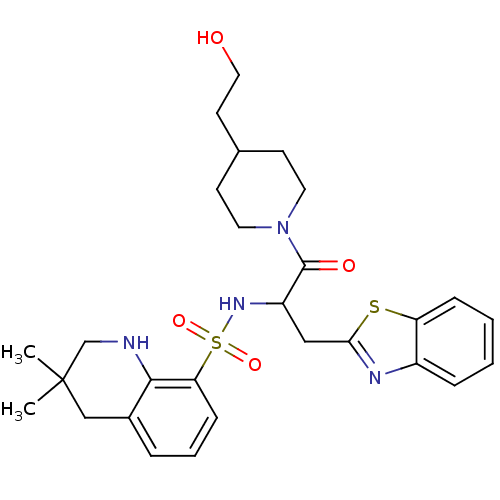 (CHEMBL43074 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090220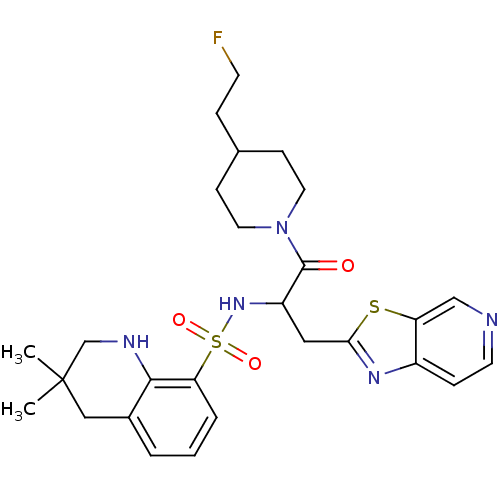 (CHEMBL289623 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50274638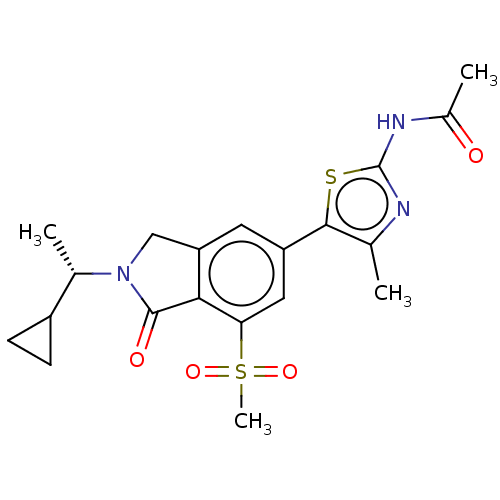 (CHEMBL4126156 | US10858355, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human P2Y1 receptor | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50366643 (CHEMBL1907778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Trypsin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50090234 (CHEMBL40904 | MD805 Analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Thrombin | Bioorg Med Chem Lett 10: 1563-6 (2000) BindingDB Entry DOI: 10.7270/Q2VX0H1B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1546 total ) | Next | Last >> |