

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50292202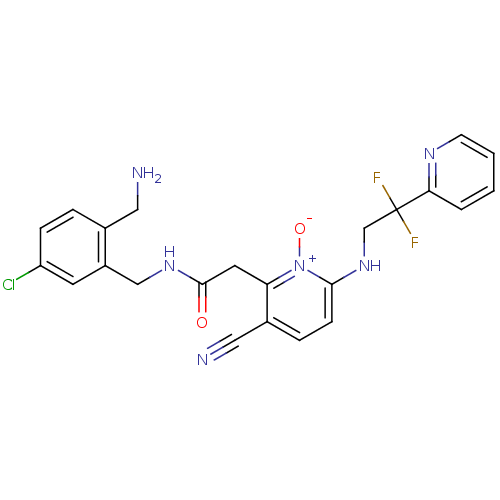 (CHEMBL382542 | N-(2-Aminomethyl-5-chloro-benzyl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against thrombin in human plasma | Bioorg Med Chem Lett 15: 2771-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.110 BindingDB Entry DOI: 10.7270/Q2PN96C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50292203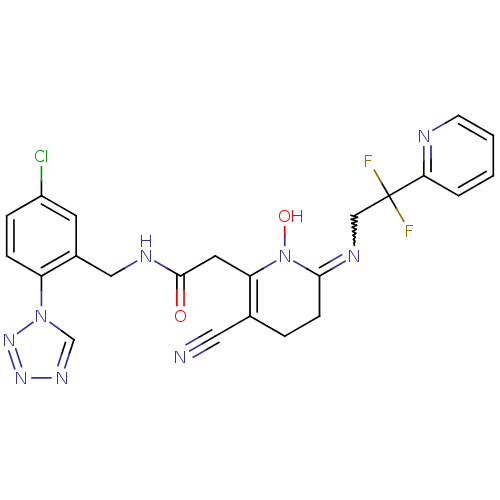 (CHEMBL196030 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against thrombin in human plasma | Bioorg Med Chem Lett 15: 2771-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.110 BindingDB Entry DOI: 10.7270/Q2PN96C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50049912 (7-(1,1-Dimethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase enzyme from Candida albicans | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant Dihydrofolate reductase enzyme | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50049905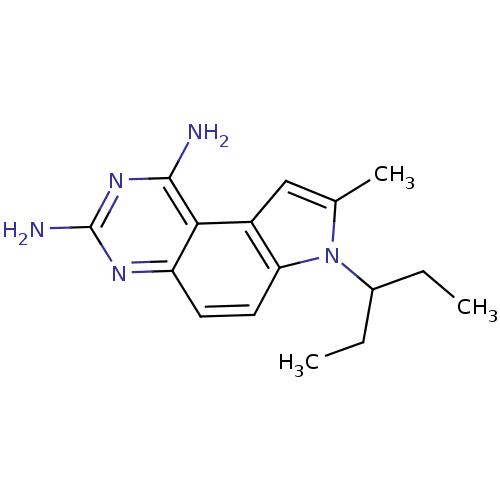 (7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase enzyme from Candida albicans | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50292196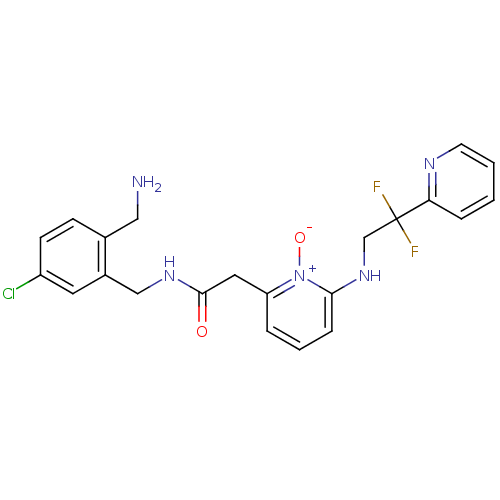 (CHEMBL195366 | N-(2-Aminomethyl-5-chloro-benzyl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against thrombin in human plasma | Bioorg Med Chem Lett 15: 2771-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.110 BindingDB Entry DOI: 10.7270/Q2PN96C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123490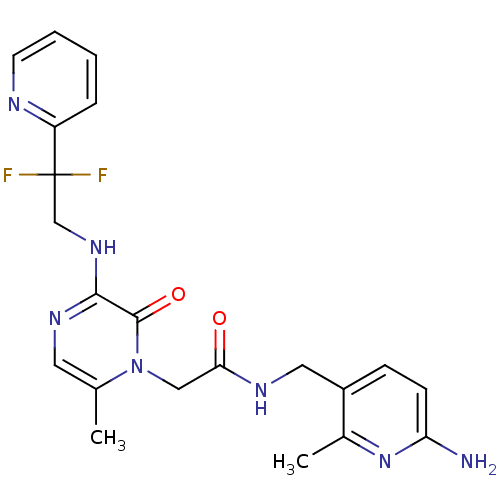 (CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50292200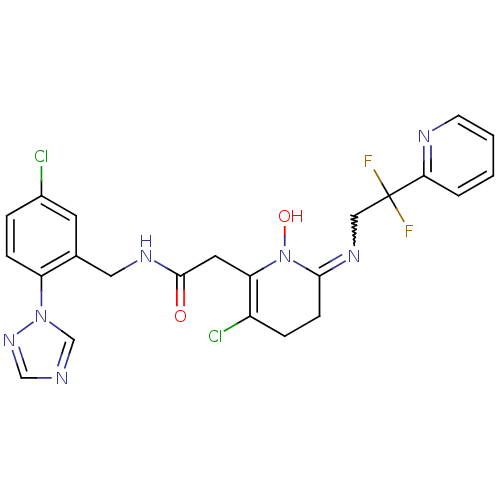 (2-[3-Chloro-6-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against thrombin in human plasma | Bioorg Med Chem Lett 15: 2771-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.110 BindingDB Entry DOI: 10.7270/Q2PN96C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50057826 ((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | J Med Chem 40: 1565-9 (1997) Article DOI: 10.1021/jm970140s BindingDB Entry DOI: 10.7270/Q2PR7V29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q] (Homo sapiens (Human)) | BDBM26526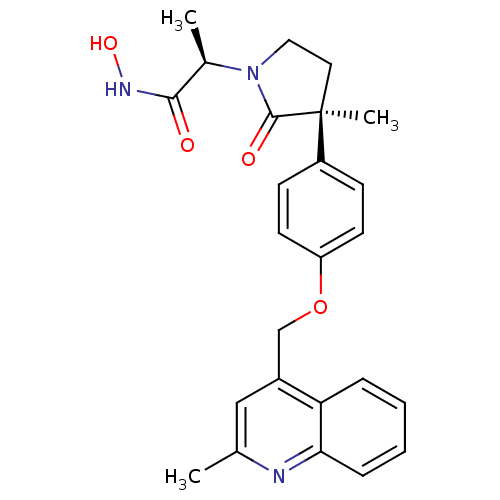 ((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute | Assay Description Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... | Bioorg Med Chem Lett 19: 54-7 (2009) Article DOI: 10.1016/j.bmcl.2008.11.034 BindingDB Entry DOI: 10.7270/Q2JH3JH7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50292189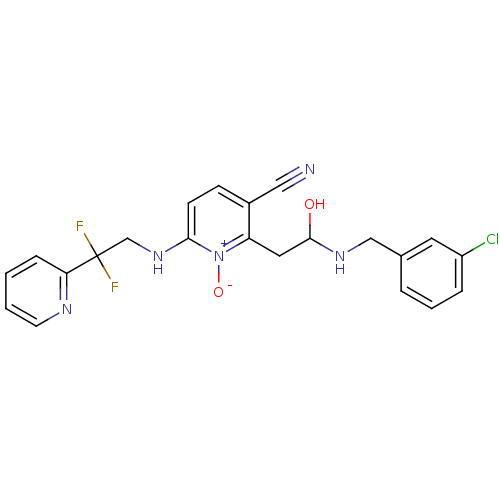 (2-[2-(3-Chloro-benzylamino)-2-hydroxy-ethyl]-6-(2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against thrombin in human plasma | Bioorg Med Chem Lett 15: 2771-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.110 BindingDB Entry DOI: 10.7270/Q2PN96C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126304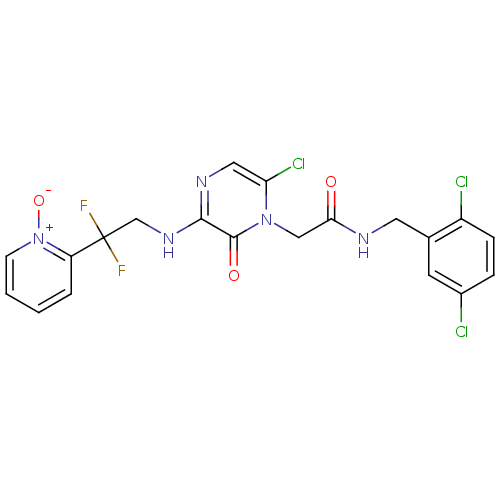 (2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | Bioorg Med Chem Lett 13: 1353-7 (2003) BindingDB Entry DOI: 10.7270/Q2833RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50056772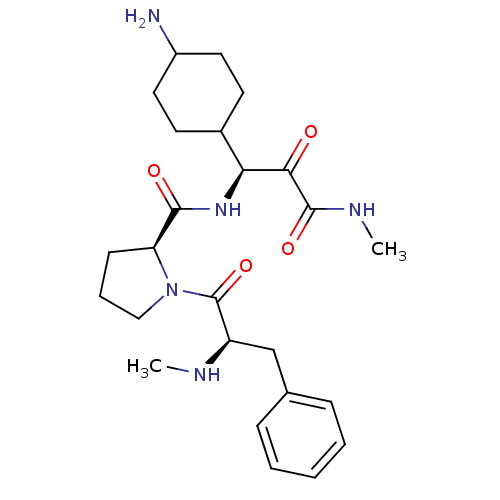 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of the compound against human thrombin was determined | Bioorg Med Chem Lett 7: 67-72 (1997) Article DOI: 10.1016/S0960-894X(96)00583-5 BindingDB Entry DOI: 10.7270/Q2639PQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50454822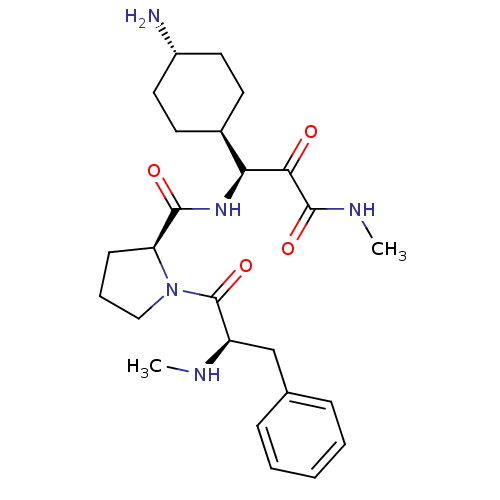 (CHEMBL2062141 | L-370518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11873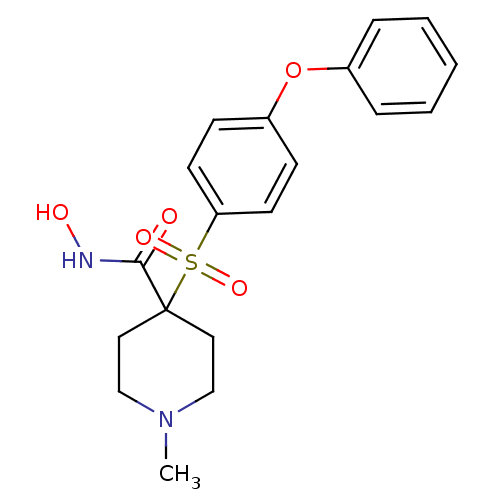 (N-Hydroxy-1-methyl-4-{[4-(phenoxyphenyl]sulfonyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50325003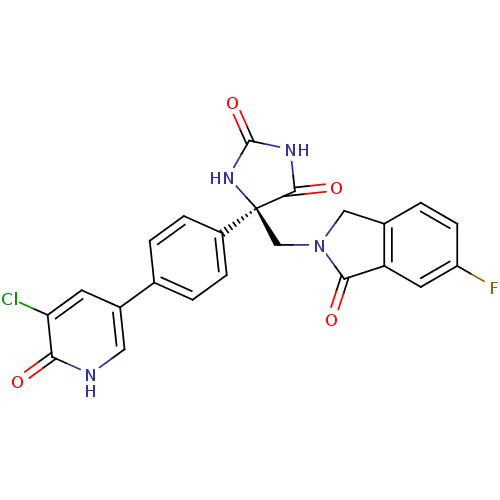 ((R)-5-(4-(5-chloro-6-oxo-1,6-dihydropyridin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage | Bioorg Med Chem Lett 20: 5286-9 (2010) Article DOI: 10.1016/j.bmcl.2010.06.134 BindingDB Entry DOI: 10.7270/Q26W9B8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11876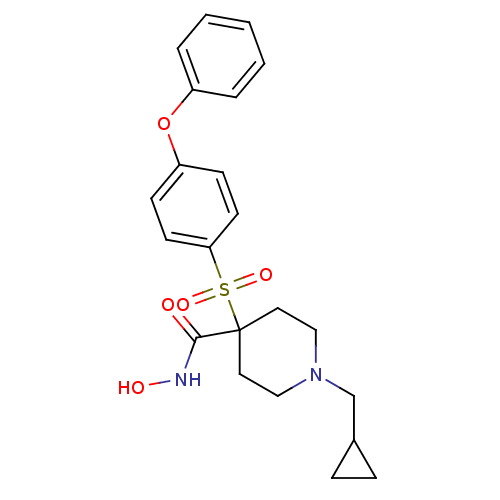 (1-(Cyclopropylmethyl)-N-hydroxy-4-[(4-phenoxypheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11878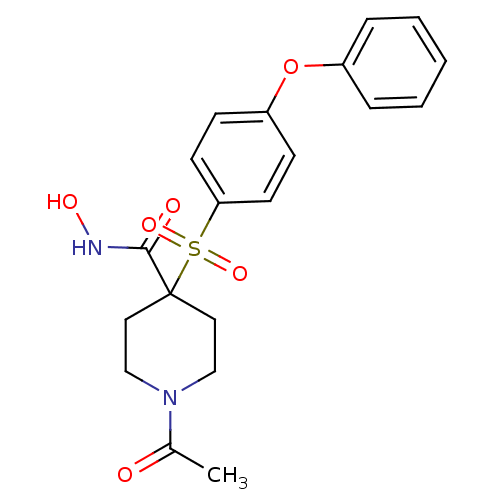 (1-Acetyl-N-hydroxy-4-{[4-(phenoxyphenyl]sulfonyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11870 (4-{[4-(4-Chlorophenoxy)phenyl]sulfonyl}-N-hydroxy ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11873 (N-Hydroxy-1-methyl-4-{[4-(phenoxyphenyl]sulfonyl}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11874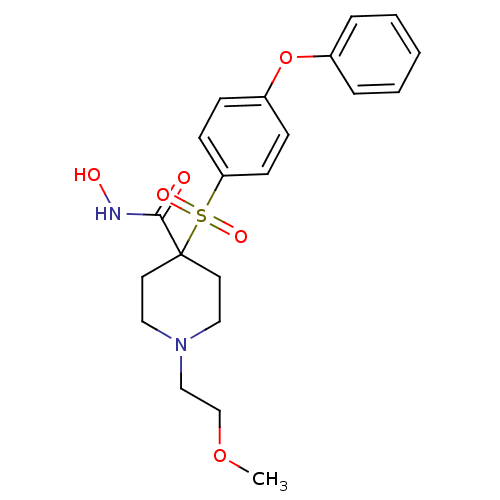 (N-Hydroxy-1-(2-methoxyethyl)-4-{[4-(phenoxyphenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11876 (1-(Cyclopropylmethyl)-N-hydroxy-4-[(4-phenoxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11878 (1-Acetyl-N-hydroxy-4-{[4-(phenoxyphenyl]sulfonyl}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11883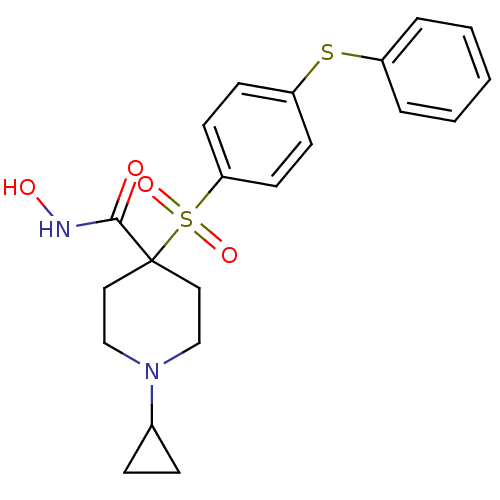 (1-Cyclopropyl-N-hydroxy-4-{[4-(phenylthio)phenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11889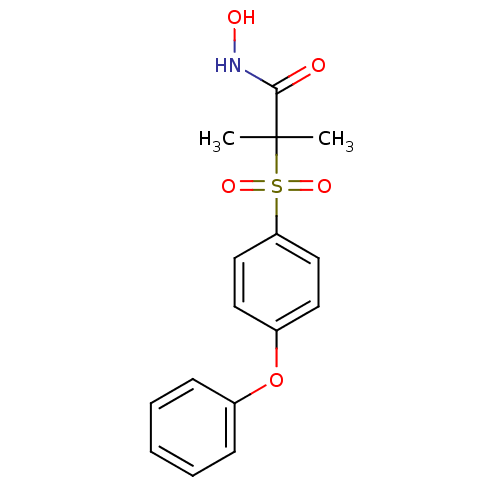 (N-Hydroxy-2-methyl-2-[(4-phenoxyphenyl)sulfonyl]-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123504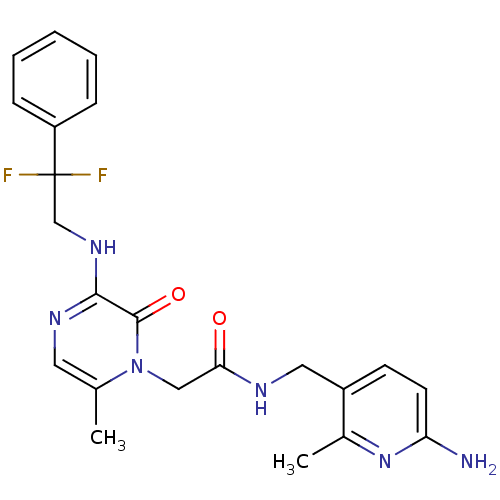 (CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory constant against thrombin (IIa) | J Med Chem 46: 461-73 (2003) Article DOI: 10.1021/jm020311f BindingDB Entry DOI: 10.7270/Q2W958J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049906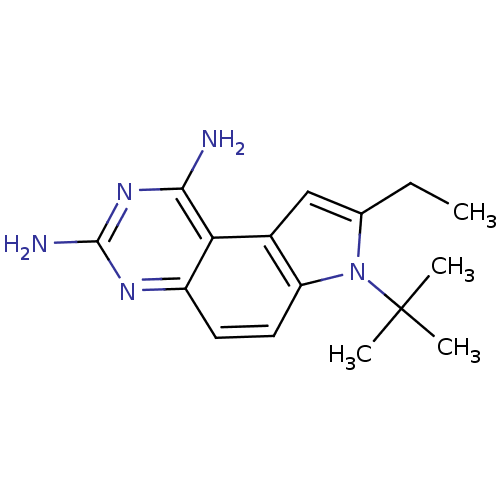 (7-tert-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant Dihydrofolate reductase enzyme | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049901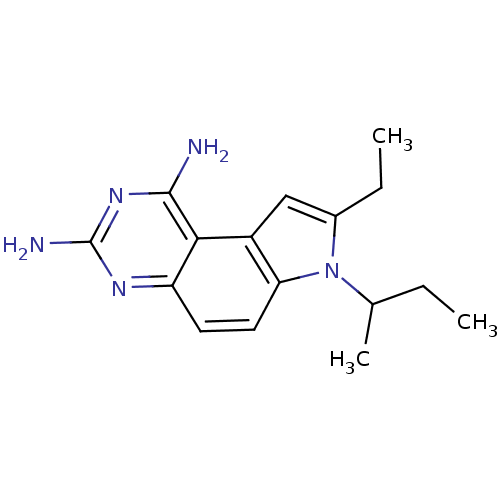 (7-sec-Butyl-8-ethyl-7H-pyrrolo[3,2-f]quinazoline-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant Dihydrofolate reductase enzyme | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049907 (7-(1-Ethyl-propyl)-8-isopropyl-7H-pyrrolo[3,2-f]qu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant Dihydrofolate reductase enzyme | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50057827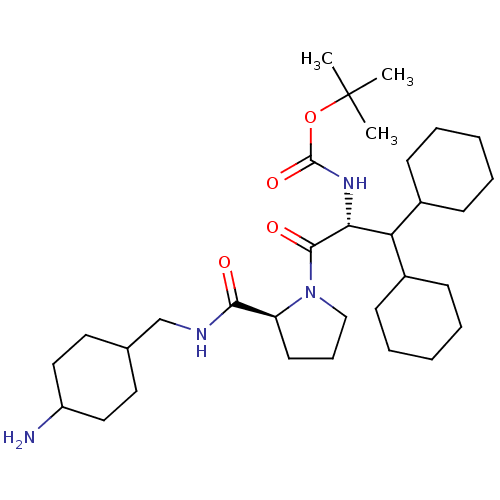 (((R)-2-{(S)-2-[(4-Amino-cyclohexylmethyl)-carbamoy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | J Med Chem 40: 1565-9 (1997) Article DOI: 10.1021/jm970140s BindingDB Entry DOI: 10.7270/Q2PR7V29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50057828 ((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | J Med Chem 40: 1565-9 (1997) Article DOI: 10.1021/jm970140s BindingDB Entry DOI: 10.7270/Q2PR7V29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50057828 ((S)-1-((R)-2-Amino-3,3-diphenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards thrombin was determined | J Med Chem 41: 401-6 (1998) Article DOI: 10.1021/jm9705014 BindingDB Entry DOI: 10.7270/Q2H995V2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11874 (N-Hydroxy-1-(2-methoxyethyl)-4-{[4-(phenoxyphenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50049911 (8-tert-Butyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase enzyme from Candida albicans | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070824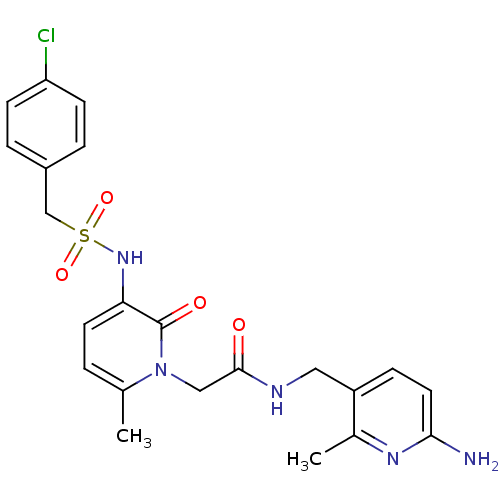 (CHEMBL47920 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50343081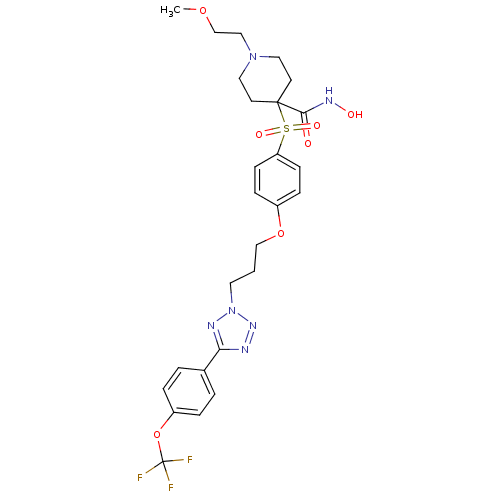 (CHEMBL1771216 | N-hydroxy-1-(2-methoxyethyl)-4-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human MMP-13 | Bioorg Med Chem Lett 21: 2820-2 (2011) Article DOI: 10.1016/j.bmcl.2011.03.099 BindingDB Entry DOI: 10.7270/Q2416XCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11877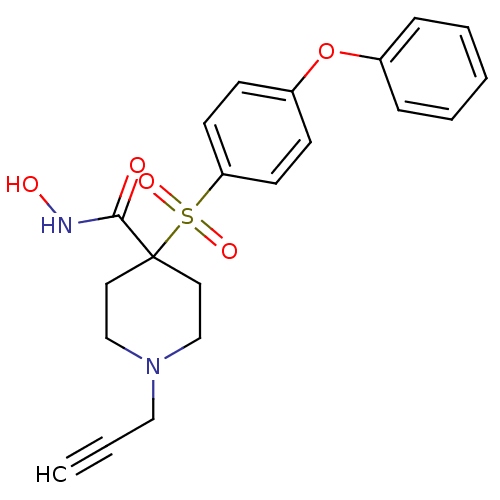 (N-Hydroxy-4-{[4-(phenoxyphenyl]sulfonyl}-1- (2-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50292201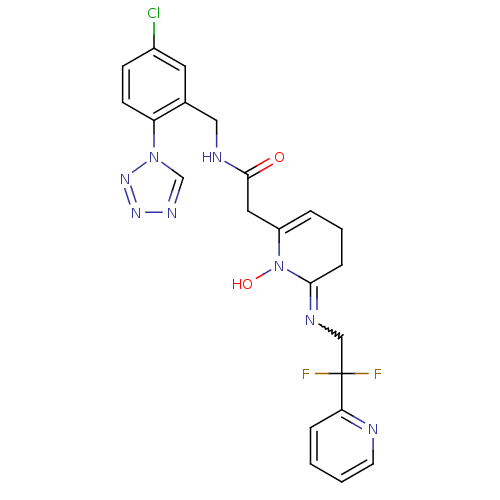 (CHEMBL372367 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against thrombin in human plasma | Bioorg Med Chem Lett 15: 2771-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.110 BindingDB Entry DOI: 10.7270/Q2PN96C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q] (Homo sapiens (Human)) | BDBM26524 ((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute | Assay Description Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... | Bioorg Med Chem Lett 19: 54-7 (2009) Article DOI: 10.1016/j.bmcl.2008.11.034 BindingDB Entry DOI: 10.7270/Q2JH3JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11870 (4-{[4-(4-Chlorophenoxy)phenyl]sulfonyl}-N-hydroxy ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11884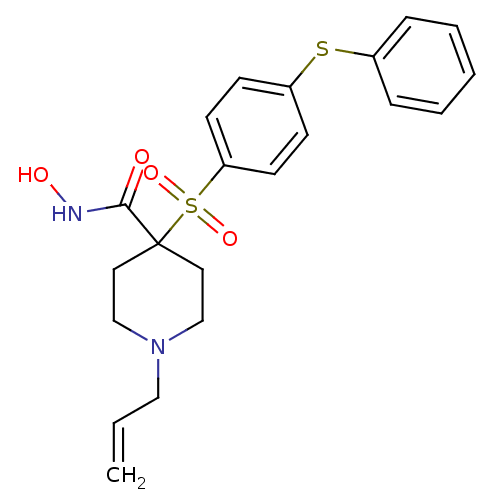 (N-Hydroxy-4-{[4-(phenylthio)phenyl]sulfonyl}-1-(vi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126303 (2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | Bioorg Med Chem Lett 13: 1353-7 (2003) BindingDB Entry DOI: 10.7270/Q2833RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50049898 (8-Isopropyl-7H-pyrrolo[3,2-f]quinazoline-1,3-diami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase enzyme from Candida albicans | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q] (Homo sapiens (Human)) | BDBM26525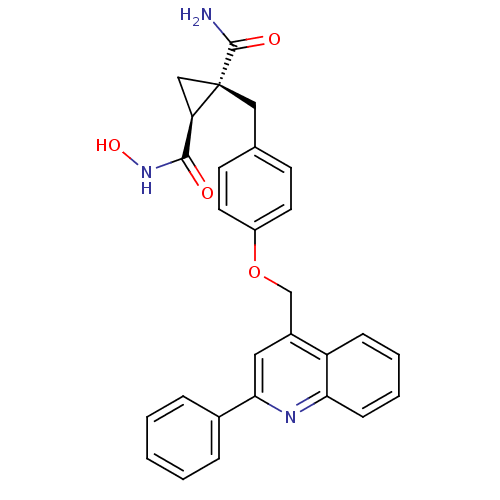 ((1S,2R)-1-N-hydroxy-2-({4-[(2-phenylquinolin-4-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute | Assay Description Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... | Bioorg Med Chem Lett 19: 54-7 (2009) Article DOI: 10.1016/j.bmcl.2008.11.034 BindingDB Entry DOI: 10.7270/Q2JH3JH7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50126309 (CHEMBL29744 | N-(3-Bromo-benzyl)-2-{6-chloro-3-[2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin | Bioorg Med Chem Lett 13: 1353-7 (2003) BindingDB Entry DOI: 10.7270/Q2833RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11883 (1-Cyclopropyl-N-hydroxy-4-{[4-(phenylthio)phenyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11866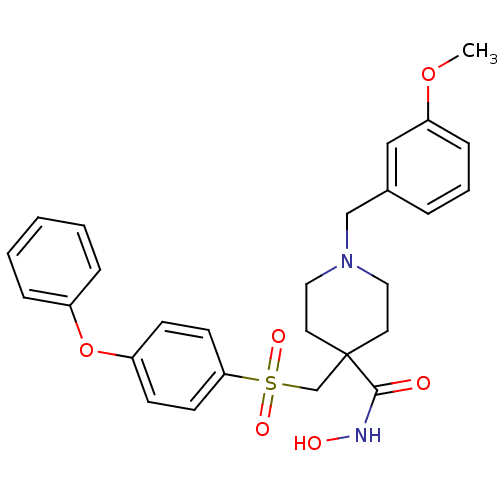 (N-Hydroxy-1-(3-methoxybenzyl)-4-{[(4-phenoxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11871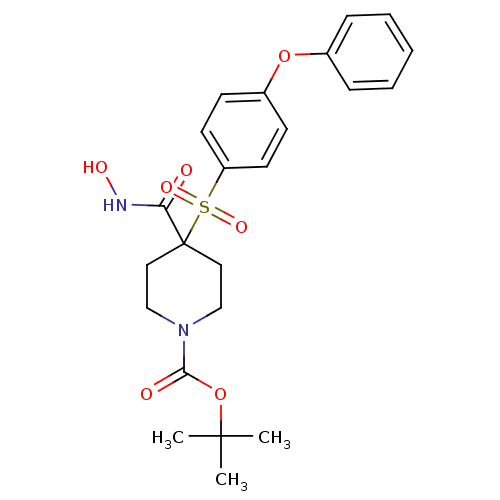 (1-tert-Butyl 4-[(Hydroxyamino)carbonyl]-4-[(4-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11875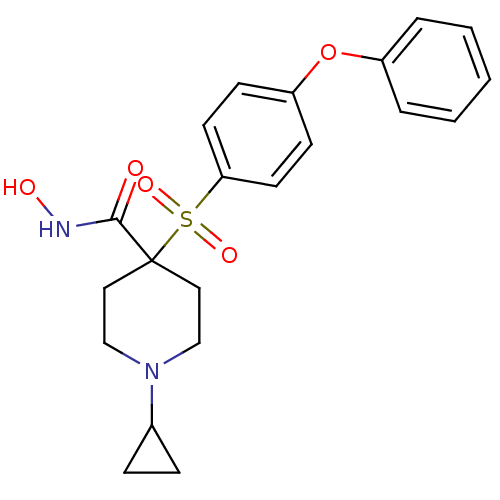 (1-Cyclopropyl-N-hydroxy-4-{[4-(phenoxyphenyl]-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... | J Med Chem 48: 6713-30 (2005) Article DOI: 10.1021/jm0500875 BindingDB Entry DOI: 10.7270/Q2N58JMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049897 (8-Ethyl-7-(1-ethyl-propyl)-7H-pyrrolo[3,2-f]quinaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant Dihydrofolate reductase enzyme | J Med Chem 39: 892-903 (1996) Article DOI: 10.1021/jm9505122 BindingDB Entry DOI: 10.7270/Q2XD10RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5530 total ) | Next | Last >> |